Introduction
Mycobacterium (M.)
tuberculosis, the causative agent of tuberculosis (TB), was
responsible for ~8.7 million novel cases of TB worldwide in 2007
(1). Tuberculosis is considered to
be a sustained immune response, which is induced by chronic,
persistent antigen stimulation, results in immune suppression and
generally fails to eradicate the M. tuberculosis infection
in part. The host response, which involves several branches of the
cellular immune system, predominantly consists of M.
tuberculosis-specific T helper type 1 (Th)1 interferon
(IFN)-γ-secreting CD4+ and CD8+ effector T
cells (2–4). CD4+ CD25+
regulatory T cells (Treg), expressing the lineage marker
forkhead box P3 (FOXP3), are important in controlling the immune
response and in maintaining T-cell homeostasis (5,6).
Several previous studies have found that the number of
FOXP3+ Treg cells is increased in patients
with active TB and are expanded at sites of active disease, where
containment of inflammation and immune-mediated pathology is
required most (7,8). A previous study by our group
demonstrated that elevated levels of FOXP3+
Treg cells decreased following pulmonary resection in
patients with pulmonary multiplicitous drug resistant tuberculosis
(MDR-TB) (9). This suggested that
FOXP3+ cells may be essential during TB development;
however, the mechanism underlying the increased levels of
FOXP3+ cells in active TB patients remains to be
elucidated. A variety of mycobacterial proteins and lipids are
secreted into the cytoplasm of M. tuberculosis-infected
macrophages, where they may be important in inhibiting the ability
of macrophages to eradicate the bacterium (10–12).
M. tuberculosis infection or treatment with mycobacterial
proteins alone induces the secretion of several cytokines,
including interleukin (IL)-1, -2, -10 and -12 and tumor necrosis
factor-alpha (TNF-α), by monocytes/macrophages (13–15).
The 6 kDa early secreted antigenic target 6 (ESAT-6) is among the
proteins secreted by M. tuberculosis and is encoded by
region of difference 1 (RD1). Comparative genomics of the M.
tuberculosis family have revealed that overlapping portions of
RD1 are absent from the attenuated or avirulent strain M.
bovis Bacillus Calmette-Guerin (BCG) and environmental
mycobacteria (16,17). Antigen 85 complex B (Ag85B), a
fibronectin-binding protein with mycolyl transferase activity, is
the major secretory protein in actively replicating M.
tuberculosis (18). Ag85B is
highly immunogenic, as demonstrated by the ease of detection of
specific humoral and cell-mediated immune responses in latently and
actively infected TB patients (19,20).
Based on the immunogenicity of proteins secreted by
M. tuberculosis, the present study hypothesized that
Treg elevation may be induced by these proteins,
including ESAT-6 and Ag85B, and that Treg activation may
be important in the failure of the host immune response to
eradicate M. tuberculosis.
Materials and methods
Study population
The study procedure was approved by the ethics
committee of Shantou University Medical College (Shantou, China).
Written informed consent was obtained from all individuals involved
in the present study. Peripheral blood was obtained from 18
patients (10 males) with a median age of 51 years (range, 16–79
years), who had been diagnosed with MDR-TB at The Third People’s
Hospital of Shantou City (Shantou, China). The TB diagnosis was
based on smear positivity and/or M. tuberculosis culture.
The indication (therapeutic vs. diagnostic) and the main clinical
pathologies are listed in Table I
and the bacterial susceptibility assessment results are shown in
Table II. A total of 18
uninfected volunteers (10 males) with a median age of 47 years
(range, 25–73 years) were also included, who were tuberculin skin
test-negative, had not been vaccinated and had no known exposure to
M. tuberculosis. In addition, 18 patients with latent
tuberculosis (TB) infection (LTBI; 9 males) with a median
age of 35 years (range, 23–53 years) were selected according to the
following recommended criteria (21): Tuberculin skin test scores between
2+ and 3+, including a risk-stratified induration 72 h after
intradermal injection of 2 units tuberculin (PPD-RT23 SST; Statens
Serum Institute, Copenhagen, Denmark), chest radiographs were
normal and the patients exhibited no clinical signs of active TB.
All individuals were human immunodeficiency virus seronegative. No
patients suffered from an immunodepressive illness or received
immunosuppressive treatment.
 | Table IDiagnostic indicators and main
clinical therapeutical pathologic features. |
Table I
Diagnostic indicators and main
clinical therapeutical pathologic features.
| Subject | Gender | Age | Chest CT | TST | Sputum | Clinical
features | Antitubercular drug
therapy (years) |
|---|
|
|
|---|
| Smear | Culture | Fever (°C) | Cough (years) | Hemoptysis |
|---|
| 1 | M | 32 | LLL cavity
concurrent infection; both side pleural thickening accretio | 2+ | 3+ | + | 38.0–40.0 | 3
Intermittently | − | 2.5 |
| 2 | M | 79 | Both pulmonary
tuberculosis, concurrent crinosity cavity form Both U. local
pleural thickening accretio | 3+ | 1+ | + | 37.5–39.0 | 10
Intermittently | + | 9.5 |
| 3 | M | 48 | L.apex of lung
tuberculosis; RLL cavity concurrent aspergilloma | 3+ | 1+ | + | 38.5–39.5 | 4
Intermittently | − | 3 |
| 4 | F | 59 | Both pulmonary
tuberculosis; both side pleural thickening accretio | 3+ | 1+ | + | 37.2–39.5 | 2.5
Intermittently | − | 2 |
| 5 | M | 69 | L.apex of lung
tuberculosis LUL cavity concurrent aspergilloma | 2+ | 3+ | + | 37.0–38.5 | 3
Intermittently | − | 2.5 |
| 6 | F | 52 | RUL cavity
concurrent aspergilloma; both U. local pleural thickening
accretio | 2+ | Q | + | 37.6–39.2 | 3.8
Intermittently | + | 3.5 |
| 7 | F | 42 | Both lobus superior
pulmonis tuberculosis; Both side pleural thickening accretio | 2+ | 1+ | + | 37.0–39.0 | 5
Intermittently | − | 4.5 |
| 8 | F | 34 | R.apex of lung
tuberculosis RUL cavity concurrent aspergilloma | 3+ | 1+ | + | 37.8–38.9 | 2
Intermittently | − | 1.5 |
| 9 | M | 29 | LUL cavity
concurrent aspergilloma; RCW abscess | 3+ | Q | + | 37.0–39.5 | 2
Intermittently | − | 2 |
| 10 | M | 38 | L.apex of lung
tuberculosis LLL tuberculoma | 3+ | Q | + | 37.5–39.0 | 3
Intermittently | − | 2.5 |
| 11 | M | 72 | RLL cavity
concurrent infection; both side pleural thickening accretio | 2+ | 3+ | + | 37.2–39.0 | 7
Intermittently | − | 7 |
| 12 | F | 59 | Both pulmonary
tuberculosis; RUL cavity concurrent infection | 2+ | 2+ | + | 37.1–38.8 | 4
Intermittently | + | 3.5 |
| 13 | M | 67 | LUL cavity
concurrent aspergilloma; LCW abscess | 2+ | 1+ | + | 37.1–39.2 | 6
Intermittently | + | 6 |
| 14 | M | 16 | Both lobus superior
pulmonis tuberculosis; both side pleural; thickening accretio | 3+ | 2+ | + | 37.5–39.0 | 1
Intermittently | − | 0.5 |
| 15 | F | 46 | R. apex of lung
tuberculosis; RLL cavity concurrent aspergilloma | 3+ | 3+ | + | 37.2–39.0 | 3
Intermittently | − | 3 |
| 16 | F | 72 | Both pulmonary
tuberculosis; RUL cavity concurrent aspergilloma | 2+ | Q | + | 37.0–38.8 | 6
Intermittently | + | 5.5 |
| 17 | F | 59 | Both pulmonary
tuberculosis; both side pleural thickening accretio | 3+ | 1+ | + | 37.4–39.2 | 3
Intermittently | − | 3 |
| 18 | M | 43 | Both pulmonary
tuberculosis; RUL cavity concurrent aspergilloma; RCW abscess | 2+ | 1+ | + | 37.1–39.5 | 5
Intermittently | − | 4.5 |
 | Table IIPreoperative bacterial susceptibility
assessment results of patients. |
Table II
Preoperative bacterial susceptibility
assessment results of patients.
| | |
Susceptibility assessment results | Strain
appraisement |
|---|
| | |
|
|
|---|
| Subject | Gender | Age | INH | RFP | SM | EMB | RFT | CPM | OFX | Th1321 | KM | PAS | PNB | TCH |
|---|
| 1 | M | 32 | R | R | S | S | R | S | R | S | S | S | S | R |
| 2 | M | 79 | R | R | S | R | R | S | R | S | S | S | S | R |
| 3 | M | 48 | R | R | S | S | R | S | S | S | S | S | S | R |
| 4 | F | 59 | R | R | R | S | R | R | R | S | R | S | S | R |
| 5 | M | 69 | R | R | R | R | R | S | R | S | S | S | S | R |
| 6 | F | 52 | R | R | R | S | R | S | R | S | S | S | S | R |
| 7 | F | 42 | S | S | R | S | S | S | R | S | S | S | S | R |
| 8 | F | 34 | R | R | R | R | R | R | R | S | S | S | S | R |
| 9 | M | 29 | R | R | R | S | R | S | R | R | R | S | S | R |
| 10 | M | 38 | R | R | R | S | R | S | S | S | S | R | S | R |
| 11 | M | 72 | R | R | R | R | R | R | R | R | R | R | R | R |
| 12 | F | 59 | R | R | R | R | R | R | R | R | R | R | R | R |
| 13 | M | 67 | R | R | R | R | R | S | R | S | S | S | S | R |
| 14 | M | 16 | S | R | R | S | R | R | R | S | R | S | S | R |
| 15 | F | 46 | R | R | S | S | R | R | R | S | R | S | S | R |
| 16 | F | 72 | R | R | S | R | R | S | R | S | S | S | S | R |
| 17 | F | 59 | S | R | R | S | R | S | R | R | R | S | S | R |
| 18 | M | 43 | R | R | R | S | R | S | R | R | R | S | S | R |
Cell preparation and culture
Heparinized peripheral blood (600 μl) was suspended
in 200 μl RPMI-1640 (HyClone, Thermo Fisher Scientific, Logan, UT,
USA) containing 100 U/ml penicillin, 100 μg/ml streptomycin and 10%
heat-inactivated fetal calf serum (Invitrogen Life Technologies,
Carlsbad, CA, USA), termed complete medium, and was stimulated
in vitro with recombinant ESAT-6 (cat. no. PRO-291;
ProSpec-Tany, TechnoGene, Ltd., Rehovot, Israel), Ag85B (cat. no.
PRO-589; ProSpec-Tany, Israel) or BCG (dead strain; cat. no
2009110102; Shanghai Institute of Biological Products, Shanghai,
China). The final culture concentration of the secretory proteins
was 4 μg/ml. The positive control used was phytohemagglutinin (PHA)
and pure medium was used for the negative control. The blood was
then incubated at 37°C in 5% CO2 for 72 h to separate
the culture supernatant fluid from the cells. In the blood samples
from six individuals (n=2 from each group), multiple different
antigen concentrations (0, 1 and 4 μg/ml) were used.
Flow cytometry
The whole blood was aliquoted (100 μl/tube) with 20
μl of the appropriate test antibody (1:5) or respective isotype
control for three staining procedures: Fluorescein isothiocyanate
(FITC), monoclonal anti-FOXP3 (clone PCH101; cat.no. 11-4766;
eBioscience, San Diego, CA, USA); phycoerythrin peridinin
chlorophyll protein (PerCP), mouse monoclonal anti-CD4 (clone
RPA-T4; cat.no. 300528; Biolegend, San Diego, CA, USA); and
phycoerythrin (PE), monoclonal anti-CD25 (clone B1.49.9; cat. no.
IM0479u, Beckman Coulter, Los Angeles, CA, USA). The following
isotype control antibodies were used: FITC, rat monoclonal
immunoglobulin (Ig)G2b (clone κ; cat.no. 11-4031; eBioscience);
PerCP, mouse monoclonal IgG1 (clone MOPC-21; cat.no. 400145;
Biolegend); and PE, mouse monoclonal IgG1 (clone 679.1 Mc7; cat.no.
IM06700u; Beckman Coulter). Following surface staining for 30 min
in the dark at room temperature, the erythrocytes were lysed and
the cells were fix with ImmunoPrep Reagents Systmem (PN7546946;
Beckman Coulter) using a Q-prep Immunology workstation (Beckman
Coulter). The surface-stained cells then underwent intracellular
FOXP3 staining using the anti-FOXP3 staining kit according to the
manufacturer’s instructions.
The listmode data were acquired using an Epics XL
flow cytometer (Beckman Coulter) and were analyzed using EXPO32 ADC
analysis system (Expo 32v1.2 Analysis 1.1C; Beckman Coulter). The
lymphocyte gate was generated by use of forward and side-angle
scattered light window leukogating to analyze the CD4 and CD25
cell-surface antigens and to determine the proportion of
Treg cells. The CD4+ T cells were gated by
plotting forward, vs. side scatter to analyze FOXP3.
Reverse transcription quantitative
polymerase chain reaction (RT-qPCR)
For RT-qPCR analysis of the mRNA expression of the
forkhead transcription factor FOXP3, total RNA was isolated from
leukocytes using a total RNA extraction kit for mammalian RNA
(TRIzol reagent; cat.no. 15596-026; Invitrogen Life Technologies)
according to the manufacturer’s instructions. RT-PCR was performed
in duplicate in a total volume of 20 μl in a LightCycler
(Prism® 7500; Applied Biosystems Life Technologies,
Foster City, CA, USA), according to the manufacturer’s
instructions, using the following cycling conditions: 30 sec at
95°C and 5 sec at 95°C for 40 cycles followed by 31 sec at 60°C.
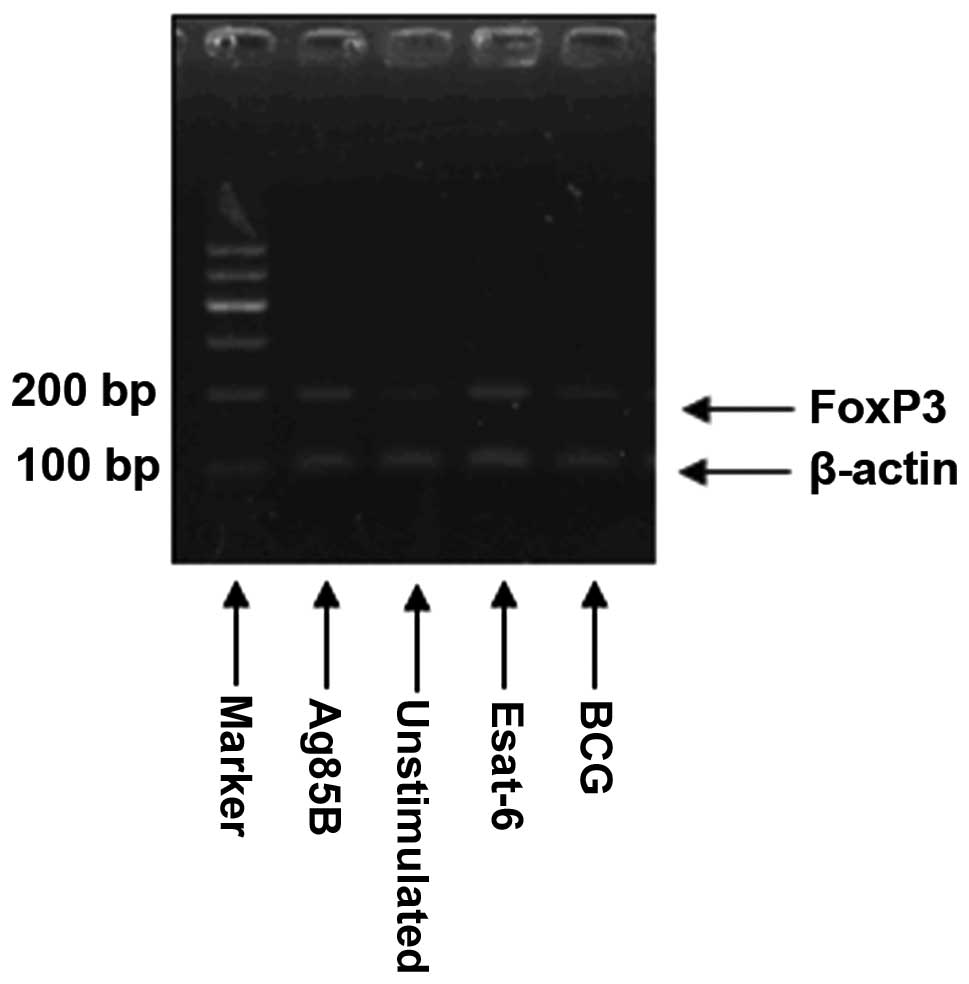
The normalized expression data were obtained by dividing the
relative expression level for each sample by the relative
expression level of β-actin for the same sample. The primer
sequences used were as follows: β-actin forward,
5′-GGCGGCACCACCATGTACCCT-3′ and reverse,
5′-AGGGGCCGGACTCGTCATACT-3′; and FOXP3 forward,
5′-ACCTGGAAGAACGCCATC-3′ and reverse, 5′-TGTTCGTCCATCCTCCTTTC-3′.
The results (FOXP3/β-actin =2−(CTFOXP3 − CTβ-actin) were
presented as the relative expression level for each gene (Fig. 1).
Statistical analysis
All values are expressed as the mean ± standard
deviation and all results were analyzed using SPSS 13.0 software
(SPSS Inc., Chicago, IL, USA). Differences in the mean values
between the patients and the controls were analyzed by one-way
analysis of variance. P<0.05 was considered to indicate a
statistically significant difference.
Results
Percentage of CD4+
CD25+ FOXP3+ Treg cells and the
mRNA expression levels of FOXP3 in the peripheral blood of MDR-TB
patients are higher than those in uninfected controls (CON) and
LTBI patients
The percentage of CD4+ CD25+
FOXP3+ Treg cells in the MDR-TB patients were
significantly increased (P<0.01) compared with those in the
controls (CON) and the LTBI patients, whereas no significant
difference (P=0.093) was observed between those in the CON and LTBI
patients (MDR-TB, 0.93±0.57%; CON, 0.24±0.14% and LTBI,
0.52±0.35%). The mRNA expression levels of FOXP3 were increased in
the MDR-TB patients (0.0093±0.0027) compared with those in the CON
(0.0043±0.0014) and LTBI (0.0066±0.0017) groups (P<0.05);
however, the levels were similar in the LTBI and control patients
(P=0.080) as shown in Fig. 2.
Proportion of CD4+
CD25+ FOXP3+ Treg cells and the
mRNA expression levels of FOXP3 increase depending on the
concentration of ESAT-6, Ag85B or BCG
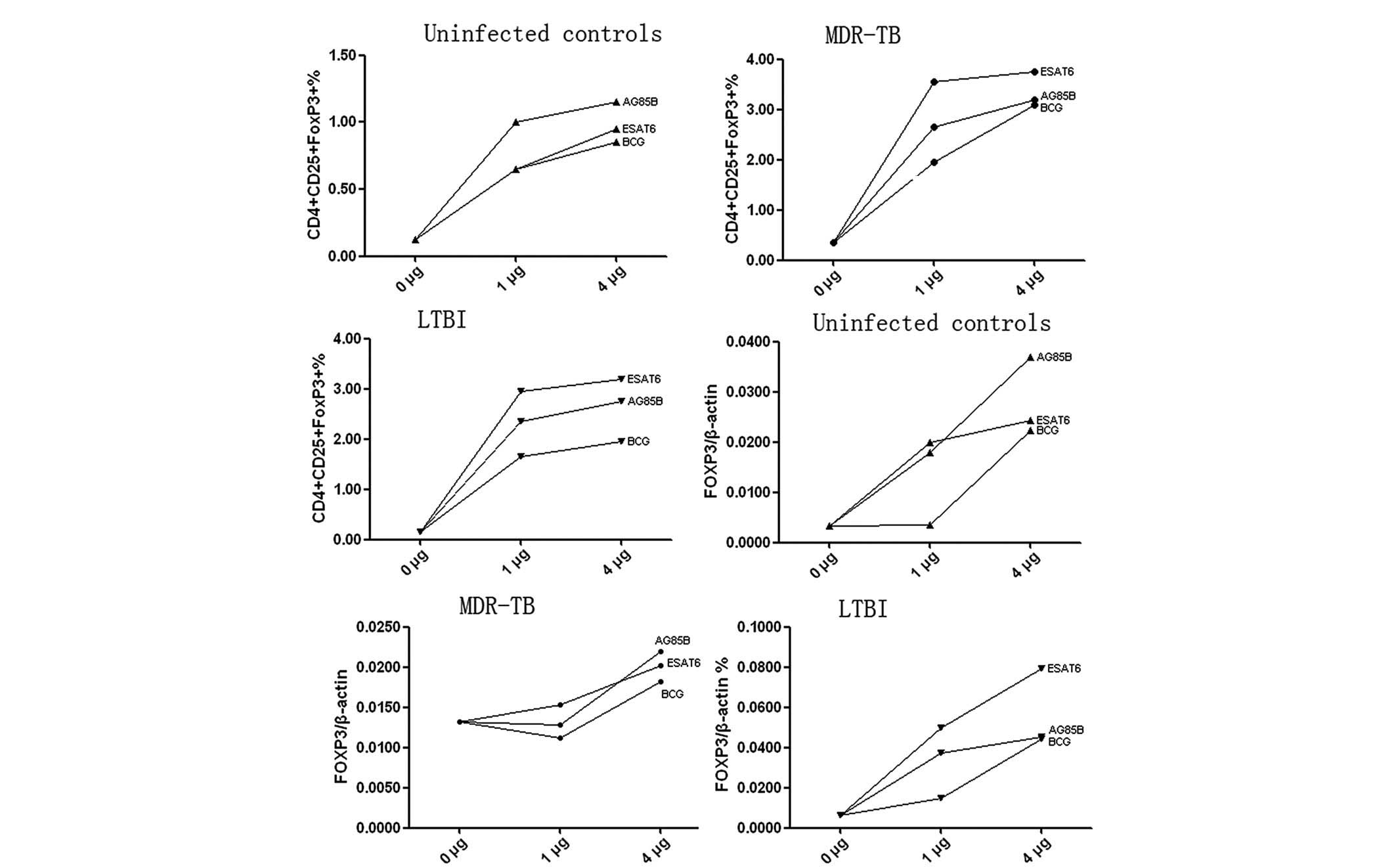
Following cell culture with different concentrations
(0, 1 and 4 μg/ml) of ESAT-6, Ag85B or BCG, the proportion of
CD4+ CD25+ FOXP3+ Treg
cells and the mRNA levels of FOXP3 increased with increasing
concentrations of the mycobacterial antigens (Fig. 3). However, due to the small sample
number, no statistical analyses were performed.
In healthy controls, the proportion of
CD4+ CD25+ FOXP3+ Treg
cells and the mRNA expression levels of FOXP3 increases in vitro by
ESAT-6 and Ag85B
The proportion of CD4+ CD25+
FOXP3+ Treg cells increased by 2.7- and
3.2-fold following stimulation with ESAT-6 or Ag85B, respectively
(ESAT-6, 0.644±0.22%; Ag85B, 0.76±0.33% and unstimulated,
0.24±0.14%; P<0.001), whereas BCG did not significantly increase
the percentage of CD4+ CD25+
FOXP3+ Treg cells (P=0.104) (Fig. 4A and B). Similar results were found
for the mRNA expression levels of FOXP3 following stimulation with
ESAT-6 (0.0251±0.0052; P<0.01), Ag85B (0.0261±0.0115; P<0.01)
or BCG (0.0174±0.0193; P=0.130) compared with those in the
unstimulated controls (0.0043±0.0014). As with the expansion of the
CD4+ CD25+ FOXP3+ cells, the
highest FOXP3 induction was observed following stimulation with
Ag85B; however, the difference when compared with ESAT-6 or BCG was
not statistically significant (P>0.05) (Fig. 4A and B).
 | Figure 4Proportion of CD4+
CD25+ FOXP3+ cells among total lymphocytes in
the unstimulated (n=18), BCG (n=18), ESAT-6 (n=18) and Ag85B (n=18)
and the mRNA expression level of FOXP3 in the peripheral blood of
unstimulated (n=8), BCG(n=8), ESAT-6 (n=8) and Ag85B (n=8) patients
prior to and following stimulation with different antigens (4
μg/ml) for 72 h. The positive control was PHA (n=6) and the
negative control was medium (n=8). (A and B) show the results in
uninfected controls; (C and D) show the results in the MDR-TB
patients; (E and F) show the results in the LTBI patients. LTBI,
latent tuberculosis infection; Ag85B, antigen 85 complex B; Esat-6,
early secreted antigenic target 6; BCG, Bacillus Calmette-Guerin;
PHA, phytohemagglutinin; FOXP3, forkhead box P3. |
ESAT-6 and Ag85B can expand the
proportion of CD4+ CD25+ FOXP3+
Treg cells and elevate the mRNA expression levels of
FOXP3 in vitro in MDR-TB
The proportion of CD4+ CD25+
FOXP3+ Treg cells increased following
stimulation with ESAT-6 (3.04±0.80%) and Ag85B (2.52±0.46%) in
MDR-TB (BCG, 2.19±0.70%; unstimulated, 0.93±0.57%) (Fig. 4C and D). The mRNA expression levels
of FOXP3 increased 2.2-fold following Ag85B stimulation
(0.0205±0.0087) compared with those in the unstimulated control
(0.0093±0.0027). No statistically significant changes were observed
in the mRNA expression levels of FOXP3 following stimulation with
ESAT-6 (0.0182±0.0062) or BCG (0.0113±0.0079) (Fig. 4C and D).
ESAT-6 and Ag85B expand the proportion of
CD4+ CD25+ FOXP3+ Treg
cells and elevate the mRNA expression levels of FOXP3 in vitro in
LTBI
Following cell culture for 72 h in the presence of
ESAT-6, Ag85B or BCG, the proportion of CD4+
CD25+ FOXP3+ Treg cells and the
mRNA expression levels of FOXP3 increased significantly. There was
a significant difference in the proportion of CD4+
CD25+ FOXP3+ Treg cells between
the simulated and unstimulated groups (P<0.001) (Fig. 4E and F). Increases of 5.0- and
4.2-fold were obtained following stimulation with ESAT-6
(2.62±0.64) or Ag85B (2.16±0.52%), respectively (BCG 1.42±0.66%;
unstimulated 0.52±0.35%). As shown in Fig. 4E and F, the mRNA expression levels
of FOXP3 increased following stimulation with ESAT-6
(0.0486±0.0353) or Ag85B (0.0468±0.0254) only (unstimulated
0.0066±0.0017). No statistically significant difference in mRNA
levels was observed between the BCG-stimulated group and the
unstimulated control (P=0.830). When comparing BCG, ESAT-6 and
Ag85B, the effects of ESAT6 and Ag85B were greater compared with
those of BCG (P<0.05), although no statistically significant
difference was observed between ESAT-6 and Ag85B (P=0.998)
(Fig. 4E and F).
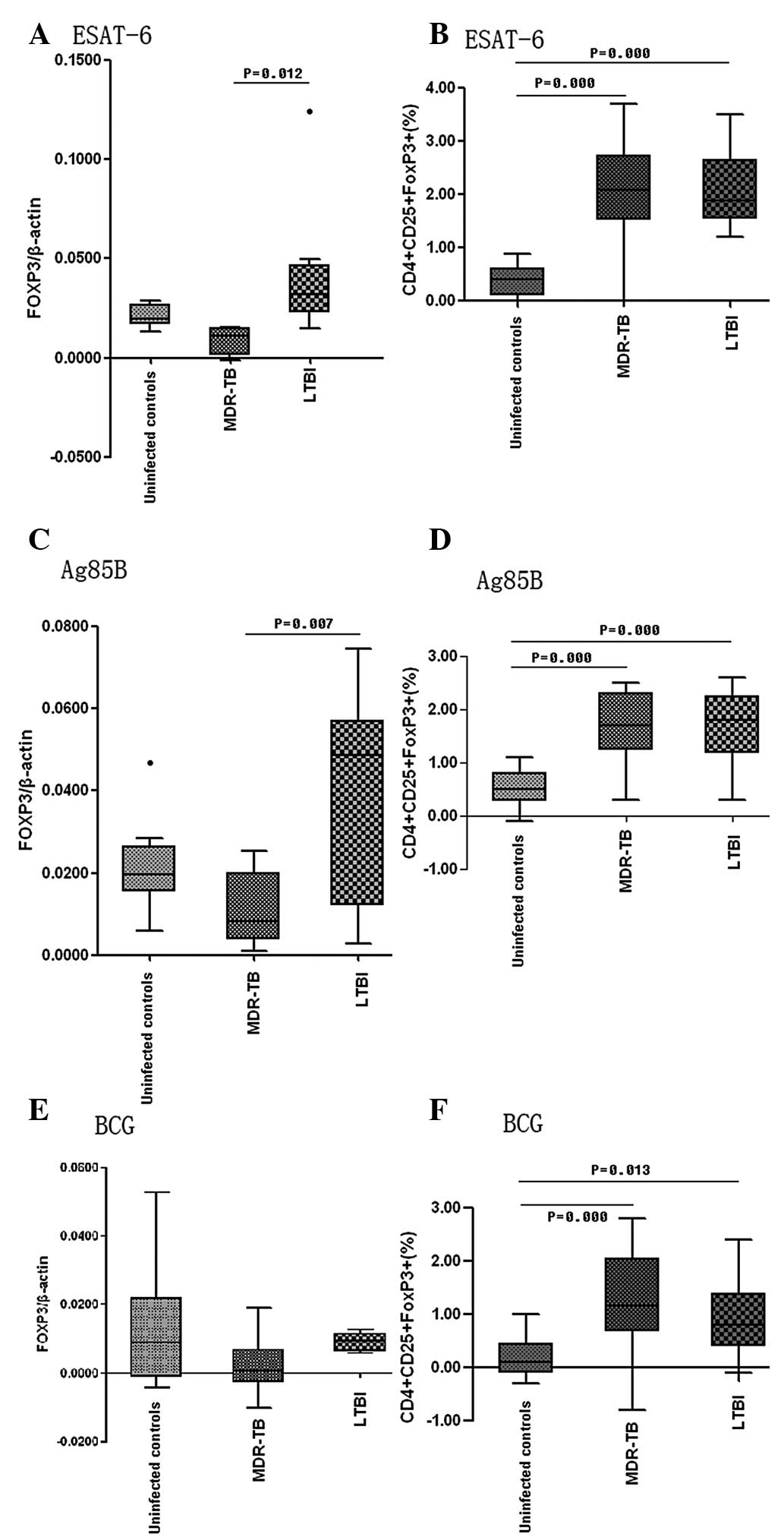
Increased FOXP3 mRNA levels and
proportion of CD4+ CD25+ FOXP3+
cells following stimulation with ESAT-6, Ag85B or BCG
In the LTBI group, culture with ESAT-6 resulted in a
six-fold increase in the mRNA expression of FOXP3 (Fig. 5A), which was higher compared with
those in the MDR-TB group (P=0.012); however, there was no
significant difference from levels in healthy controls (P=0.151).
In the cultures stimulated with Ag85B, the mRNA expression of FOXP3
increased six-fold in the LTBI group (Fig. 5C), which was higher compared with
that in the MDR-TB group (P=0.007), but not significantly different
from that in the healthy controls (P=0.102). BCG increased the mRNA
expression of FOXP3 in the healthy controls only (MDR-TB,
0.22-fold; control, three-fold, LTBI, 1.4-fold; Fig. 5E). The proportion of
CD4+ CD25+ FOXP3+ Treg
cells increased following stimulation and the increment was higher
in the MDT-TB and LTBI groups following culture with ESAT-6, Ag85B
and BCG compared with that in the healthy controls (P<0.01;
Fig. 5B, D and F).
Discussion
Tuberculosis remains to be a challenging medical,
social, and economic problem worldwide, especially in the third
world (1). The host response to
infection with M. tuberculosis involves the cellular immune
system, specifically the Th1-type interferon-γ-secreting
CD4+ and CD8+ effector T cells (4). This response assists in limiting
bacterial replication and dissemination in vivo and results
in important immunopathological features, including inflammation;
however, it does not eradicate the infection (22). The present study hypothesized that
the immune system may possess regulatory mechanisms that suppress
the response to persistent antigens. It has been previously
reported that patients with TB have a high proportion of
circulating Treg cells of the CD4+
CD25+ FOXP3+ phenotype compared with patients
with LTBI or uninfected controls (7,8). A
previous study by our group revealed that the elevated numbers of
CD4+ CD25+ FOXP3+ cells observed
in MDR-TB were found to decrease following removal of the M.
tuberculosis burden by pulmonary resection (9), which demonstrated that the increase
in FOXP3+ Treg cells is a potential mechanism
by which M. tuberculosis evades immune eradication. In
individuals with tuberculosis, these cells have been observed to
suppress the T-cell response to mycobacterial antigens, whereas the
CD4+ CD25+ FOXP3+ cells from
non-healthy individuals do not suppress the secretion of IFN-γ
induced by protective mycobacterial antigens (8,23).
The increased levels of functional CD4+ CD25+
FOXP3+ Treg cells in the peripheral blood of
patients with TB may be either caused by or a result of the
disease, which suggests that they are either generated or expanded
during latent infection prior to the onset of disease. Although
CD4+ CD25+ FOXP3+ Treg
cells are naturally generated in the thymus (24), they are induced in the periphery in
mice (25) and humans (26), which suggests that peripheral
Treg cells may arise from antigenic challenge during the
immune response.
In the present study, intracellular staining
combined with flow cytometry and RT-qPCR analysis was performed to
detect the expression of the most accurate available
Treg markers, CD25+ and FOXP3. The results
demonstrated that there was a higher frequency of CD4+
CD25+ FOXP3+ Treg cells and
elevated mRNA levels of FOXP3 in MDR-TB patients compared with the
LTBI patients or the uninfected controls. No difference was
observed between the LTBI patients and the uninfected controls
prior to stimulation with ESAT-6 and Ag85B, the proteins secreted
by M. tuberculosis, in vitro. However, when the
peripheral blood from individual patients was cultured with either
ESAT-6 or Ag85B in vitro, the results demonstrated that
circulating CD4+ CD25+ FOXP3+
Treg cells were expanded by stimulation with ESAT-6 or
Ag85B. This observation supported the hypothesis of the present
study that the elevation of circulating CD4+
CD25+ FOXP3+ Treg cells in active
TB patients was induced by the proteins secreted by M.
tuberculosis, which may ultimately decrease anti-tuberculosis
immunity and may be partly responsible for failure of the host to
eradicate M. tuberculosis. Comparison of ESAT-6, Ag85B and
BCG revealed that CD4+ CD25+
FOXP3+ Treg expansion and elevation of mRNA
levels of FOXP3 was caused by ESAT-6 or Ag85B to a greater extent
than by BCG. In addition, exposure to BCG did not increase the
proportion of CD4+ CD25+ FOXP3+
Treg cells or the mRNA expression levels of FOXP3, which
suggested that the higher levels of circulating CD4+
CD25+ FOXP3+ Treg cells in active
TB patients may be associated with M. tuberculosis
pathogenicity.
In the present study, the highest levels of elevated
FOXP3 mRNA were observed in the LTBI patients compared with those
in the uninfected controls or the MDR-TB patients. Therefore, the
peripheral CD4+ CD25+ FOXP3+
Treg cells may be involved in the early stages of TB
pathogenesis. They may be generated and expanded from the
peripheral blood mononuclear cells in LTBI and have been
demonstrated to depress cellular immune responses in non-anergic
patients with pulmonary TB (7,8).
These results indicated that, in the majority of M.
tuberculosis infections, a cell-mediated protective immune
response controls the pathogen over several years and often for a
lifetime without clinical consequences. Upon exposure of the
infected individual to mycobacterial antigens, latent infection,
re-exposure to M. tuberculosis or post-exposure BCG
vaccination causes marked generation and expansion of
CD4+ CD25+ FOXP3+ Treg
cells. These cells then suppress the effective immune response,
allowing mycobacteria to evade the control of the immune system and
to proliferate and colonize vulnerable tissue, including the lungs
(8). This may be the reason why
patients with LTBI reacquire the disease more easily upon
re-exposure to M. tuberculosis.
Antigen-specific γδ T cells may be involved in
antimycobacterial immunity and complex patterns of γδT-cell immune
responses have been observed in humans and animal models during
early mycobacterial infections and chronic tuberculosis (27,28).
ESAT-6, a protein secreted by the M. tuberculosis ESX-1
system, inhibits human T-cell immune responses and the secretion of
IFN-γ, and CD4+ CD25+ Treg cells
inhibit the production of IFN-γ by human memory γδ T cells in
response to ESAT-6 (29,30). The results of the present study
suggested that CD4+ CD25+ FOXP3+
Treg cells are important in immunity against M.
tuberculosis by expanding in response to proteins secreted by
M. tuberculosis, including ESAT-6 and Ag85B, and reducing
the ability of the host to eradicate M. tuberculosis.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (no. 81302540) and the Natural Science
Foundation of Guangdong Province, China (no. s2012010009081).
References
|
1
|
World Health Organization. Global
tuberculosis report. 2012, Available at: http://www.who.int/tb/publications/global_report/en/index.html.
|
|
2
|
Newport MJ, Huxley CM, Huston S, et al: A
mutation in the interferon-gamma-receptor gene and susceptibility
to mycobacterial infection. N Engl J Med. 335:1941–1949. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ottenhoff TH, Kumararatne D and Casanova
JL: Novel human immuno-deficiencies reveal the essential role of
type-I cytokines in immunity to intracellular bacteria. Immunol
Today. 19:491–494. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Stenger S and Modlin RL: T cell mediated
immunity to Mycobacterium tuberculosis. Curr Opin Microbiol.
2:89–93. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Namita M, Jagadeesh B, Sébastien LD,
Michel DK and Srini VK: Cutting edge: human CD4+
CD25+ T cells restrain the maturation and
antigen-presenting function of dendritic cells. J Immunol.
172:4676–4680. 2004. View Article : Google Scholar
|
|
6
|
Ethan MS, Richard AD, John A, et al: The
lifestyle of naturally occurring CD4+ CD25+
FOXP3+ regulatory T cells. Immunol Rev. 212:60–73. 2006.
View Article : Google Scholar
|
|
7
|
Valerie GR, John AI, Sarah H, Tim H and
Ajit L: Regulatory T cells are expanded in blood and disease sites
in tuberculosis patients. Am J Respir Crit Care Med. 173:803–810.
2006. View Article : Google Scholar
|
|
8
|
Hougardy JM, Place S, Hildebrand M, et al:
Regulatory T cells depress immune responses to protective antigens
in active tuberculosis. Am J Respir Crit Care Med. 176:409–416.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wu YE, Peng WG, Cai YM, et al: Decrease in
CD4+CD25+FOXP3+ Treg
cells after pulmonary resection in the treatment of cavity
multidrug-resistant tuberculosis. Int J Infect Dis. 14:e815–e822.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wandy LB and David GR: Identification of
mycobacterial surface proteins released into subcellular
compartments of infected macrophages. Infect Immun. 68:6997–7002.
2000. View Article : Google Scholar
|
|
11
|
Beatty WL, Ullrich HJ and Russell DG:
Mycobacterial surface moieties are released from infected
macrophages by a constitutive exocytic event. Eur J Cell Biol.
80:31–40. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rhoades E, Hsu FF, Torrelles JB, et al:
Identification and macrophage-activating activity of glycolipids
released from intracellular Mycobacterium bovis BCG. Mol Microbiol.
48:875–888. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Barnes PF, Chatterjee D, Abrams JS, et al:
Cytokine production induced by Mycobacterium tuberculosis
lipoarabinoman-nan. Relationship to chemical structure. J Immunol.
149:541–547. 1992.PubMed/NCBI
|
|
14
|
Fulton SA, Johnsen JM, Wolf SF, Sieburth
DS and Boom WH: Interleukin-12 production by human monocytes
infected with Mycobacterium tuberculosis: role of phagocytosis.
Infect Immun. 64:2523–2531. 1996.PubMed/NCBI
|
|
15
|
Lee JS, Song CH, Kim CH, et al: Depressed
interleukin-12 production by peripheral blood mononuclear cells
after in vitro stimulation with the 30-kDa antigen in recurrent
pulmonary tuberculosis patients. Med Microbiol Immunol. 192:61–69.
2003.PubMed/NCBI
|
|
16
|
Mahairas GG, Sabo PJ, Hickey MJ, Singh DC
and Stover CK: Molecular analysis of genetic differences between
Mycobacterium bovis BCG and virulent M. bovis. J Bacteriol.
178:1274–1282. 1996.PubMed/NCBI
|
|
17
|
Sorensen AL, Nagai S, Houen G, Andersen P
and Andersen AB: Purification and characterization of a
low-molecular-mass T-cell antigen secreted by Mycobacterium
tuberculosis. Infect Immun. 63:1710–1717. 1995.PubMed/NCBI
|
|
18
|
Belisle JT, Vissa VD, Todd S, et al: Role
of the major antigen of Mycobacterium tuberculosis in cell wall
biosynthesis. Science. 276:1420–1422. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Boesen H, Jensen BN, Wilcke T and Andersen
P: Human T-cell responses to secreted antigen fractions of
Mycobacterium tuberculosis. Infect Immun. 63:1491–1497.
1995.PubMed/NCBI
|
|
20
|
Smith SM, Klein MR, Malin AS, et al: Human
CD8+ T cells specific for Mycobacterium tuberculosis secreted
antigens in tuberculosis patients and healthy BCG-vaccinated
controls in The Gambia. Infect Immun. 68:7144–7148. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
No authors listed. Targeted tuberculin
testing and treatment of latent tuberculosis infection. This
official statement of the American Thoracic Society was adopted by
the ATS Board of Directors, July 1999 This is a Joint Statement of
the American Thoracic Society (ATS) and the Centers for Disease
Control and Prevention (CDC) This statement was endorsed by the
Council of the Infectious Diseases Society of America (IDSA),
September 1999, and the sections of this statement. Am J Respir
Crit Care Med. 161:S221–S247. 2000.
|
|
22
|
Schwander S and Dheda K: Human lung
immunity against Mycobacterium tuberculosis: insights into
pathogenesis and protection. Am J Respir Crit Care Med.
183:696–707. 2011. View Article : Google Scholar :
|
|
23
|
Ribeiro-Rodrigues R, Co TR, Rojas R, et
al: A role for CD4+CD25+ T cells in
regulation of the immune response during human tuberculosis. Clin
Exp Immunol. 144:25–34. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Apostolou I, Sarukhan A, Klein L and
Boehmer HV: Origin of regulatory T cells with known specificity for
antigen. Nat Immunol. 3:756–763. 2002.PubMed/NCBI
|
|
25
|
Chen WJ, Jin WW, Hardegen N, et al:
Conversion of peripheral CD4+CD25− naive T
cells to CD4+CD25+ regulatory T cells by
TGF-β induction of transcription factor FOXP3. J Exp Med.
198:1875–1886. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mindi RW, Deborah JK, Vivian HG, et al:
Induction of FOXP3 and acquisition of T regulatory activity by
stimulated human CD4+CD25+ T cells. J Clin
Invest. 112:1437–1443. 2003. View Article : Google Scholar
|
|
27
|
Euan L, Angela MG and JoAnne LF: IL-17
production is dominated by gammadelta T cells rather than CD4 T
cells during Mycobacterium tuberculosis infection. J Immunol.
177:4662–4669. 2006. View Article : Google Scholar
|
|
28
|
Stefan HEK, Stewart TC, Valerie M, Eric R
and Carl N: Mycobacterium tuberculosis and the host response. J Exp
Med. 201:1693–1697. 2005. View Article : Google Scholar
|
|
29
|
Samtena B, Wang X and Barnes PF:
Mycobacterium tuberculosis ESX-1 system-secreted protein ESAT-6 but
not CFP10 inhibits human T-cell immune responses. Tuberculosis.
89:S74–S76. 2009. View Article : Google Scholar
|
|
30
|
Li L and Wu CY:
CD4+CD25+ Treg cells inhibit human
memory γδT cells to produce IFN-γ in response to M. tuberculosis
antigen ESAT-6. Blood. 111:5629–5636. 2008. View Article : Google Scholar : PubMed/NCBI
|