Introduction
Hepatocellular carcinoma (HCC) is one of the primary
causes of cancer-related mortality, with a global incidence of
>500,000 cases per year (1).
The incidence and mortality of this disease have increased amongst
all races and in the two genders over the past two decades. HCC has
a poor prognosis, and there are few treatment options. Curative
surgery and liver transplantation are only available to a small
minority of patients with early-stage HCC. The majority of cases
are detected at an advanced stage. Other commonly-used therapies
are predominantly palliative (2,3).
α-fetoprotein (AFP) is an oncofetal antigen.
Suppression of AFP synthesis occurs shortly subsequent to birth.
However, 50–80% of adults with HCC exhibit re-expression of AFP
during tumor progression (2,3).
Human T cell repertoires are able to recognize AFP-derived peptide
epitopes in the context of major histocompatibility complex (MHC)
class I molecules and to induce AFP-specific protection (2,3).
Therefore, AFP, as a tumor-associated antigen, may be a suitable
target for dendritic cell-based cytotoxic T lymphocyte
(CTL)-mediated antigen-targeted immunotherapy in HCC.
Pulsing dendritic cells (DCs) with tumor antigen is
a versatile approach by which to generate cancer vaccines (4–8). DCs
are the most effective type of antigen-presenting cells and are
able to stimulate naive T lymphocytes to initiate a primary immune
response (4). DCs are scarce,
representing only 0.2% of total white blood cells. Therefore, a
number of protocols in order to procure DCs from peripheral blood
mononuclear cells (PBMCs) in vitro have been developed
(9). These protocols allow the
in vitro manipulation of DCs for clinical and
laboratory-based studies (10).
Techniques for generating antigen-loaded DCs include pulsing or
incubating DCs with cancer cell lysate, utilizing cancer cells
undergoing apoptosis, tumor antigen peptide and specific tumor
protein, and the delivery of tumor antigen gene into DCs using
viral vectors (5–7,11–15).
A phase II clinical trial demonstrated that
vaccination with mature autologous DCs pulsed ex vivo with
tumor cell line lysate in patients with advanced HCC was safe and
well-tolerated, with evidence of antitumor efficacy assessed
radiologically and serologically (8). The results from a separate study
supported the superiority of lentivirus-AFP-engineered DC vaccine
over AFP peptide-pulsed DC vaccine (16). Recombinant adeno-associated virus
(rAAV) is one of the safest virus vectors used in gene therapy, as
the wild-type virus has never been shown to cause human disease. A
further advantage of rAAV is the absence of viral coding sequences,
which may diminish the elimination of transduced DCs by
virus-specific CTL (17–19). In addition, rAAV is able to
transduce dividing and nondividing cells, which may allow the
transduction of DCs in various states of activation and maturation,
from a broad range of sources (19–21).
For these reasons above, rAAV rather than a lentivirus was selected
as the vector-carrying AFP gene for pulsing DCs in the present
study, and was compared with cancer lysate-pulsed DCs.
The HPV-16 E7 antigen gene has been successfully
transduced into DCs using rAAV, which resulted in the induction of
a CTL response against cervical cancer cells (22). This result indicated that AFP gene
delivery into DCs by rAAV may also be an effective approach for
priming CTL response against HCC. The aim of the present study was
to demonstrate whether rAAV was able to transduce the AFP gene into
DCs effectively and prime an AFP-specific MHC-class I-restricted
CTL response.
Materials and methods
Cell lines
The HepG2 and SK-HEP-1 HCC cell lines, and the K562
and HEK293 myelogenous leukemia cell lines were obtained from the
American Type Culture Collection (Manassas, VA, USA). The BEL7402
and SMMC7721 HCC cell lines were purchased from the Cancer Research
Department of the China Medical Science Institute (Beijing, China).
Cells were cultured in complete Dulbecco’s modified Eagle’s medium
or RPMI-1640 medium (Invitrogen Life Technologies, Carlsbad, CA,
USA) supplemented with 5 or 10% fetal bovine serum (Invitrogen Life
Technologies). PBMCs obtained from healthy donors were separated
using the Ficoll gradient method and cultured in AIM-V medium (Life
Technologies, Carlsbad, CA). All blood donors were enrolled by the
Provincial Hospital Affiliated to Shandong University (Jinan,
China) and provided written informed consent. The study protocol
received a priori approval by the Human Research Internal
Review Board of the Provincial Hospital affiliated to Shandong
University, indicating that the protocol conformed to the ethical
guidelines of the 1975 Declaration of Helsinki. The human leukocyte
antigen (HLA) haplotype of all donors was HLA-A2.
Construction of the rAAV vector
The wild-type AAV type 2 genome, pSM620, was
digested in order to delete the internal AAV sequences from map
units 3–97, including the p5 promoter, and a specially designed
polylinker was ligated in place, resulting in the rAAV vector
plasmid, dl3–97 (23). The
cytomegalovirus (CMV) enhancer and the SV40 early mRNA
polyadenylation signal DNA were derived from the p-enhanced green
fluorescent protein (EGFP)-N1 plasmid (Clontech, Mountain View, CA,
USA) and inserted into the dl3–97 vector. Subsequently, the CMV
immediate early promoter was inserted to the dl3–97 vector
(dl3–97/CMVp), which was derived from pEGFP-N1. Human AFP cDNA was
amplified using reverse transcription-polymerase chain reaction
(RT-PCR). Total mRNA was isolated from the HepG2 cells using an
Oligotex mRNA isolation kit (Qiagen, Valencia, CA, USA). Once the
first-strand cDNA was generated, PCR amplification of AFP sequence
from nucleotides 12 to 1902 was conducted using the following
primers: Forward: 5′-CTTCCACCACTGCCAATAAC-3′ and reverse:
5′-TTGTCTTCTCTTCCCCTG-3′ (24).
The AFP cDNA was then inserted into the dl3–97 vectors for 8 h at
4°C in order to generate the rAAV/AFP vector.
Generation of rAAV virus
The pSH3 plasmid is able to express the AAV type 2
rep and cap genes and the adenovirus type 5 E2A, VA1 and E4 genes,
to allow rAAV DNA replication and packaging into viral particles
without contaminating the wild-type AAV and adenovirus (25). The rAAV vector was co-lipofected
into HEK293 cells with the pSH3 plasmid, and the rAAV was harvested
after four days. A one-step column purification technique, which
used gravity flow based on its affinity to heparin, without
ultracentrifugation, was performed in order to generate the
purified rAAV (26). The rAAV was
titered by dot blot hybridization as described previously (22).
Generation and pulsing of
monocyte-derived DCs
PBMCs were obtained from HLA-A2-positive healthy
volunteers, separated by Ficoll density-gradient centrifugation at
400 × g for 20 min and incubated in six-well culture plates at 37°C
for 2 h in AIM-V medium. Following incubation, nonadherent cells
were removed, and adherent PBMCs were cultured in AIM-V medium
containing 800 units/ml granulocyte macrophage colony-stimulating
factor (GM-CSF; R&D Systems, Minneapolis, MN, USA). Adherent
PBMCs were infected with 1010 eg/ml rAAV/AFP. Following
incubation for 8 h, the medium/virus solution was removed, the
cells were washed and fresh AIM-V medium was added to the cultures.
Throughout the culture period, 800 IU/ml GM-CSF was included in the
medium. In order to induce the maturation of DCs, 1,000 IU/ml of
human interleukin-4 (IL-4; R&D Systems) was added at 24 h. This
permitted a brief period of monocyte proliferation, which promoted
a higher level of rAAV transduction.
Cancer cell lysates were generated by four rapid
freeze-thaw cycles of HepG2 cells. Cancer cell lysate-loading of
DCs was performed by incubation of DCs with cancer cell lysate in
the absence of transfection reagents. In brief, adherent PBMCs were
cultured in AIM-V medium containing 800 units/ml GM-CSF and 1000
IU/ml IL-4. On day three, cancer cell lysates were added (100 μg
lysate/5×105 DCs) and incubated with immature DCs. Every
two days, the culture medium was replaced with fresh medium
containing the cytokines. At day four, 50 IU/ml of human tumor
necrosis factor-α (R&D Systems) was added into the culture.
After six days of culturing, monocyte-derived dendritic cells were
harvested from nonadherent and loosely adherent cells. The survival
of DCs upon viral infection was tested by trypan blue stain
(Sigma-Aldrich, St. Louis, MO, USA).
AFP expression of DCs and cell lines
DCs were harvested at six days post-transduction,
and intracellular staining was performed in order to analyze AFP
expression in rAAV/AFP-pulsed DCs. In brief, the cells were fixed,
permeabilized and incubated with phycoerythrin (PE)-conjugated
mouse anti-human AFP monoclonal antibody (1:100; 563002; BD
Pharmingen, San Diego, CA, USA). A FACSCalibur flow cytometer (BD
Biosciences, San Jose, CA, USA) was used for data acquisitions. For
each sample ≥10,000 cells were counted. Lysate-pulsed or untreated
DCs were used as a control. AFP expression of the cell lines was
tested using the same procedure.
Cell surface marker analysis of DCs and
cell lines
For analysis of the phenotype of DCs, the
non-adherent and loosely adherent cells were collected by gentle
pipetting following six days in culture. Cells were incubated with
fluorescin isothiocynate (FITC)-conjugated monoclonal mouse
anti-human antibodies directed against MHC class I (HLA-ABC; 1:100;
555552), MHC class II (HLA-DR; 1:100; 555811), CD80 (1:100;
555683), CD83 (1:100; 555910) and CD86 (1:100; 555657), which were
all purchased from BD Pharmingen. All samples were then analyzed
using a FACSCalibur flow cytometer. HLA-A2 expression of the cell
lines were tested using FITC-conjugated mouse anti-human monoclonal
antibodies against HLA-A2 (1:100; 551285; BD Pharmingen).
T-cell proliferation assay
CD3+ T cells were isolated from PBMCs using a Pan T
Cell Isolation kit II (Miltenyi Biotec, Auburn, CA, USA) according
to the manufacturer’s instructions. The rAAV/AFP-pulsed or
lysate-pulsed DCs were co-cultured with CD3+ T cells at a ratio of
1:20 in the presence of 20 units/ml recombinant human IL-2 and 20
ng/ml IL-7 (R&D Systems). The wild type AAV-pulsed and
untreated DCs were used as controls. The DC-T cell culture medium
was replaced by fresh medium plus the cytokines every two days. On
day eight, T cell proliferation was assessed using the Cell Titer
96 AQueous One Solution Cell Proliferation Assay kit (Promega
Corporation, Madison, WI, USA) and 3H-thymidine uptake
assay. For the Cell Titer 96 AQueous One Solution Cell
Proliferation Assay, dye solution was added to each well and
incubated for 4 h. A microplate imaging system was used to measure
the absorbance of the samples at 490 nm. For
3H-thymidine uptake assay, the cultures were pulsed with
1 μCi 3H-thymidine (New England Nuclear, Boston, MA,
USA) per well for 12 h. 3H-thymidine uptake was counted
using an LS 6000SE liquid scintillation counter (Beckman Coulter,
Brea, CA, USA).
Cytokine expression level analysis in
primed T cells
T cells were harvested eight days after priming. The
intracellular staining assay described above was performed to
analyze interferon-γ (IFN-γ) and IL-4 expression using
FITC-conjugated mouse anti-human IFN-γ (1:100; 552882) and
PE-conjugated mouse anti-human IL-4 (1:100; 559333) monoclonal
antibodies (BD Pharmingen).
Cytotoxicity assays
After eight days of the DC-T cell culture, 6 h
chromium-51 (51Cr) release assays were used to analyze
the killing activity of the CTL, which had been elicited by the
rAAV/AFP-pulsed, lysate-pulsed or untreated DCs against the target
cells. The targets included HepG2, BEL7402, SMMC7721, Sk-Hep-1 and
natural killer cell (NK)-sensitive K562 tumor cells. The
51Cr-labeled target cells were mixed with the CTL (1:20)
and incubated for 6 h at 37°C with 5% CO2. In order to
determine the structures on the target cells, the mouse anti-human
HLA-A2 monoclonal antibody (1:100; 551230; BD Pharmingen) was used
to block cytotoxicity. The 51Cr-labeled targets were
pre-incubated with mouse anti-human HLA-A2 antibody for 2 h prior
to performance of the 51Cr-release assay was. In order
to demonstrate the AFP-specific killing activity of the CTL, a
series of AFP-negative cells were also tested. K562 cells were used
as targets to observe NK activity.
Statistical analysis
All data are expressed as the mean ± standard
deviation and differences between groups were analyzed using the
Student’s t-test with SPSS 15.0 software (SPSS, Inc., Chicago, IL,
USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
Construction and generation of rAAV
To construct the rAAV vectors for this study, the
CMV immediate early promoters were successfully inserted into the
dl3–97 vectors. AFP mRNA was isolated from HepG2 cells and
amplified by RT-PCR (data not shown). cDNA was successfully cloned
into the rAAV vectors, sequenced and determined to be identical to
the published sequence (24,27).
The viral stocks of rAAV were generated and titered (data not
shown). The viral titer was 1011 eg/ml.
Levels of antigen-positive DCs following
rAAV delivery
In order to assess the efficiency of rAAV pulsing of
DCs, the level of AFP protein in rAAV/AFP pulsed DCs was analyzed
using intracellular staining at six days post-transduction. The
results, shown in Fig. 1A,
demonstrated that rAAV/AFP infection of DCs results in a high
percentage of cells containing intracellular AFP protein
(82.8%).
Characterization of DCs
DCs were generated from healthy volunteers. The
phenotypes of DCs were examined by flow cytometric analysis in
order to determine whether significant differences in phenotype
were discernible among untreated, lysate-pulsed and rAAV/AFP-pulsed
DC populations. The results demonstrated that the DCs generated
from all three techniques displayed a characteristic phenotype,
with expression of MHC class I (HLA-ABC), MHC class II (HLA-DR),
CD80, CD83 and CD86 (P>0.05; Fig.
1B). The survival of DCs following viral infection, was
measured using trypan blue staining. The results showed that
rAAV/AFP-pulsing or wild type AAV-pulsing has no significant effect
on the viability of DCs (data not shown).
Proliferation of T cells stimulated by
rAAV/AFP-pulsed DCs
In order to assess the stimulatory capacity of DCs,
rAAV/AFP-pulsed, lysate-pulsed, wild type AAV-pulsed and untreated
DCs were co-cultured for eight days with T cells from the donors
from which the DCs had been obtained. The formation of large T cell
clusters was observed in T cells co-cultured with rAAV/AFP-pulsed
DCs. Smaller clusters were observed in T cells that had been
co-cultured with lysate-pulsed and untreated DCs (Fig. 2A). T cell proliferation was induced
by rAAV/AFP-pulsed, lysate-pulsed, wild type AAV-pulsed and
untreated DCs. However, proliferation levels were lower for the
lysate-pulsed, wild type AAV-pulsed and untreated groups that for
the rAAV/AFP-pulsed groups (P<0.05; Fig. 2B and C).
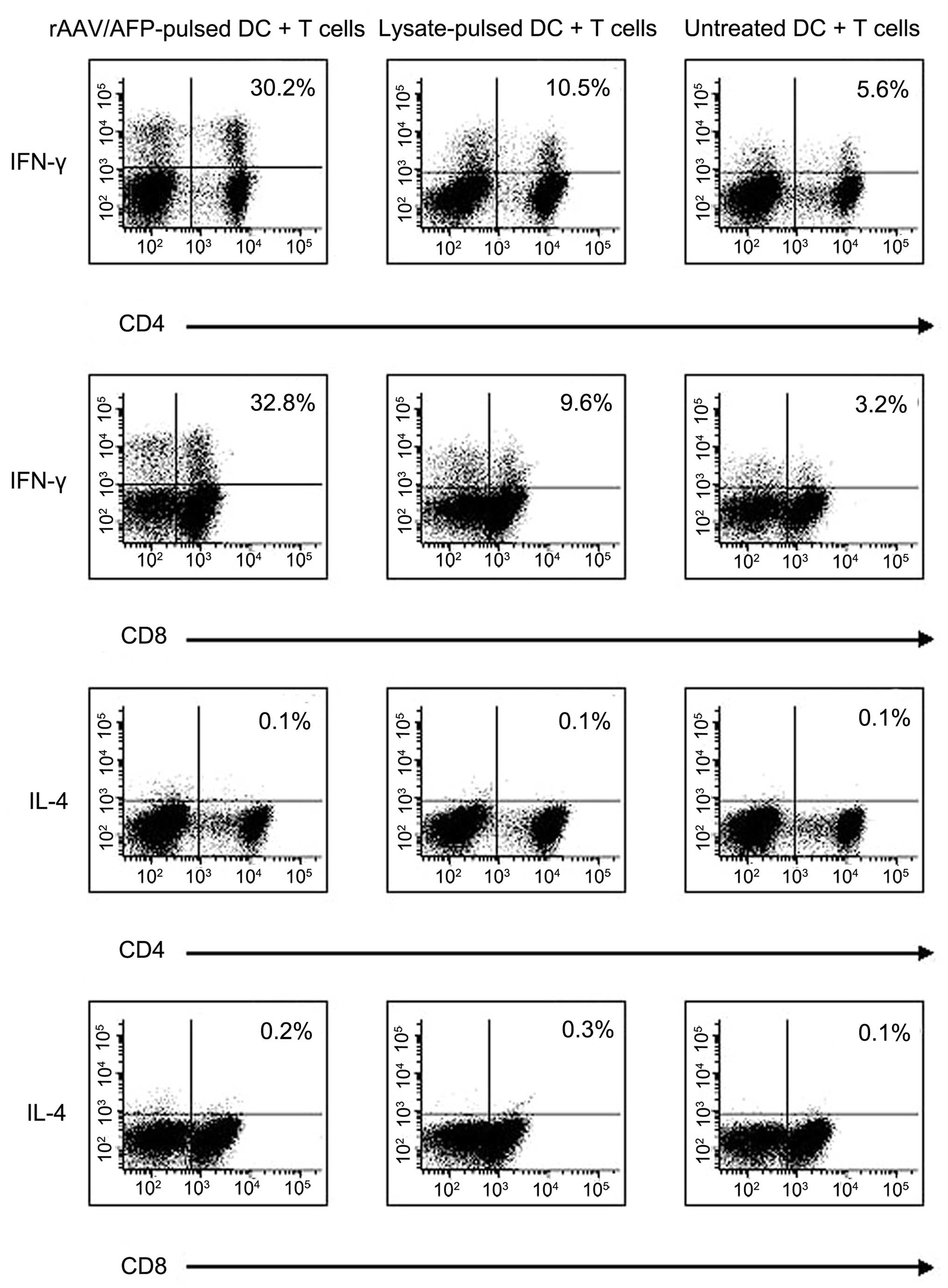
Cytokine production of CD4+ and CD8+ T
cells
The cytokine production of T cells was measured
using intracellular staining in order to determine T cell
activation. T cells co-cultured with DCs were collected on day
eight and analyzed for expression of IFN-γ and IL-4. A relatively
high level of IFN-γ production was detected in CD4+ (30.2%) and
CD8+ (32.8%) T cells stimulated by rAAV/AFP-pulsed DCs. A smaller
proportion of IFN-γ producing T cells were observed in the T cell
populations co-cultured with either cancer cell lysate-pulsed DCs
(10.5% of CD4-positive cells and 9.6% of CD8-positive cells) or
untreated DCs (5.6 and 3.2% of CD4 and CD8-positive cells,
respectively; P<0.05). Minimal production of IL-4 was detected
in CD4+ and CD8+ T cells (Fig.
3).
rAAV/AFP-pulsed DCs primed and propagated
AFP-specific and HLA class I-restricted CTLs
T cells were co-cultured with rAAV/AFP-pulsed,
lysate-pulsed or untreated DCs at a ratio of 20:1 for eight days. A
6 h chromium-51 (51Cr) release assay was used to assess the
induction of the CTL response. In order to determine
antigen-specific and HLA class I-restricted tumor lysis by CTLs,
multiple tumor targets were used (Fig.
4A). The T cells stimulated by rAAV/AFP-pulsed DCs exhibited
42.9% lysis of HepG2 (HLA-A2+, AFP+) cells and 48.5% lysis of
BEL7402 (HLA-A2+, AFP+) cells. By contrast, there was reduced CTL
activity against SMMC-7721 (HLA-A2+, AFP−), SK-Hep1 (HLA-A2+, AFP−)
and K562 cells (HLA-A2-, AFP-; P<0.05). Furthermore, lysis of
HepG2 and BEL7402 cells was significantly reduced by preincubation
of the tumor cells with anti-HLA-A2 mAb (Fig. 4B). As shown in Fig. 4C, only 22.4% lysis of HepG2 cells
was observed when using T cells stimulated by HepG2-lysate-pulsed
DCs, which was significantly lower than that induced by T cells
stimulated by rAAV/AFP-pulsed DCs (P<0.05). The killing activity
of CTLs stimulated by lysate-pulsed DCs against BEL7402, SMMC-7721
and SK-Hep1 cells was observed. Cell death was not observed using
the untreated DC-stimulated T cells (Fig. 4D).
Discussion
DCs are potent antigen-presenting cells that are
involved in regulating immune responses (28–30).
In human tumors, the presence of functional immunogenic DCs is
rare. Defective DC recruitment, differentiation, maturation and
survival may contribute to the low levels of functional DCs,
observed in human cancer (31). An
approach which may be effective in combatting these low levels is
to generate functional DCs in vitro and transplant the
genetically manipulated DCs into patients. Immunization of patients
with ex vivo-generated DCs is feasible and enhances
antigen-specific immune responses in humans.
In the present study, DCs transduced with
antigen-expressing rAAV were compared with control lysate-pulsed
DCs. The results demonstrated that direct transduction of DCs with
antigen-expressing rAAV was more efficient than loading DCs with
tumor lysate. There are a number of possible reasons that may
explain this result. Due to protein degradation and MHC molecule
cycling, protein pulsing of DCs may be an inefficient way to
deliver an antigen to target cells. By contrast, gene transfer
ensures long-lasting expression of the target antigen and may
provide an opportunity for the repeated stimulation of CTLs. Viral
entry into DCs is often more efficient than other approaches for
the introduction of antigen. Viral delivery of antigens may result
in the production of an entire array of epitopes presented by the
autologous MHC class I and MHC class II molecules, which results in
a more efficient activation of multiclonal T cells (32–34).
Viral gene delivery results in the production and endogenous
expression of a number of epitopes in DCs, which are theoretically
more representative and immunoreactive than that produced by
alternative approaches.
AFP is an ideal antigen target for immunotherapy in
HCC, due to the high level of expression in the majority of HCCs,
and the low level of expression in healthy liver and other cell
types. The present study demonstrated that the rAAV/AFP virus
effectively loaded freshly adherent DC precursors with the AFP
gene. In addition, the transduction of the AFP gene into DCs
resulted in functionally active AFP-epitope-presenting DCs, without
impacting upon the phenotype, viability and functions of these
cells. One explanation for this result may be that rAAV is one of
the safest virus vectors used in gene therapy. The wild-type virus
has never been shown to cause human disease.
The rAAV/AFP-pulsed DCs were able to prime and
propagate AFP antigen-specific CTLs in an efficient manner. A
notable phenomenon observed during the priming process was the
formation of large DC-T cell clusters two days after addition of T
cells, which may suggest enhanced priming in this group (35–37).
T cell proliferation were also induced by wild type AAV-pulsed DCs.
However, the proliferation level was significantly lower in this
group, which indicates that proliferation is specific for AFP, but
not for the cytokines produced by DCs in response to a viral
infection.
The T cells primed by rAAV/AFP-pulsed DCs exhibited
low levels of IL-4, a characteristic cytokine in the Th2 response,
and increased levels of IFN-γ, a typical Th1 cytokine. These data
suggest that the use of rAAV/AFP-pulsed DCs is effective in
generating a significant Th1 response.
Marked CTL activity, induced by rAAV/AFP-pulsed DCs
against MHC class I-matched, AFP-positive HCC cell lines was
observed after eight days of priming. The majority of DC
loading/priming protocols require a far longer period in order to
demonstrate CTL activity. The underlying reason for this is
unknown. However, there are a number of possible explanations.
Firstly, the high transduction efficiency of the rAAV vector.
Secondly, the effective formation of the DC-T cell clusters and
proliferation of T cells. Thirdly, the high levels of CD80, CD83
and CD86 expression. Finally, higher IFN-γ secretion with no
detectable IL-4 secretion, which is associated with the desirable
Th1 response. Blockage of HLA-A2 inhibited cytotoxity, which was in
accordance with HLA-A2-restriction. No significant cytotoxicity was
observed against AFP-negative target cells or NK-sensitive K562
cells, indicating that the cytotoxicity is antigen-specific and MHC
class I restricted.
In conclusion, rAAV/AFP pulsing of DCs represents a
promising technique for effectively introducing the AFP antigen
gene into DCs in order to stimulate an AFP-specific CTL response
against HCC. The protocol described in the current study may in
future be used to develop an adjuvant immunotherapy for the
treatment of HCC. In addition, the present study provides a
foundation for future studies, involving transduction of
alternative tumor antigen genes into DCs using rAAV in order to
elicit a CTL response against the transduced antigen.
References
|
1
|
Liu Y, Daley S, Evdokimova VN, Zdobinski
DD, Potter DM and Butterfield LH: Hierarchy of alpha fetoprotein
(AFP)-specific T cell responses in subjects with AFP-positive
hepatocellular cancer. J Immunol. 177:712–721. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Butterfield LH, Koh A, Meng W, et al:
Generation of human T cell responses to an HLA-A2.1-restricted
peptide epitope from alpha-fetoprotein. Cancer Res. 59:3134–3142.
1999.PubMed/NCBI
|
|
3
|
Butterfield LH, Meng WS, Koh A, et al: T
cell responses To HLA-A*0201-restricted peptides derived from human
alpha fetoprotein. J Immunol. 166:5300–5308. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Steinman RM: The dendritic cell system and
its role in immunogenicity. Ann Rev Immunol. 9:271–296. 1991.
View Article : Google Scholar
|
|
5
|
Zitvogel L, Mayordomo JI, Tjandrawan T,
DeLeo AB, Clarke MR, Lotze MT and Storkus WJ: Therapy of murine
tumors with tumor peptide-pulsed dendritic cells: dependence on T
cells, B7 costimulation and T helper cell I-associated cytokines. J
Exp Med. 183:183–187. 1996. View Article : Google Scholar
|
|
6
|
Philip R, Brunette E, Ashton J, et al:
Tansgene expression in dendritic cells to induce antigen-specific
cytotoxic T cells in healthy donors. Cancer Gene Ther. 5:236–246.
1998.PubMed/NCBI
|
|
7
|
McArthur JG and Mulligan RC: Induction of
protective anti-tumor immunity by gene-modified dendritic cells. J
Immunother. 21:41–47. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Palmer DH, Midgley RS, Mirza N, et al: A
phase II study of adoptive immunotherapy using dendritic cells
pulsed with tumor lysate in patients with hepatocellular carcinoma.
Hepatology. 49:124–132. 2009. View Article : Google Scholar
|
|
9
|
Romani N, Gruner S, Brang D, et al:
Proliferating dendritic cell progenitors in human blood. J Exp Med.
180:83–93. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sallusto F and Lanzavecchia A: Efficient
presentation of soluble antigen by cultured human dendritic cells
is maintained by granulocyte/macrophage colony stimulating factor
plus interleukin 4 and down regulated by tumor necrosis factor
alpha. J Exp Med. 179:1109–1118. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Young JW and Inaba K: Dendritic cells as
adjuvants for class I major histocompatibility complex-restricted
antitumor immunity. J Exp Med. 183:7–11. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Paglia P, Chiodoni C, Rodolfo M and
Colombo MP: Murine dendritic cells loaded in vitro with soluable
protein prime cytotoxic T lymphocytes against tumor antigen in
vivo. J Exp Med. 183:317–322. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Alexander M, Salgaller ML, Celis E, Sette
A, Barnes WA, Rosenberg SA and Steller MA: Generation of
tumor-specific cytotoxic T lymphocytes from peripheral blood of
cervical cancer patients by in vitro stimulation with a synthetic
human papillomavirus type 16 E7 epitope. Am J Obstet Gynecol.
175:1586–1593. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sonderbye L, Feng S, Yacoubian S, Buehler
H, Ahsan N, Mulligan R and Langhoff E: In vivo and in vitro
modulation of immune stimulatory capacity of primary dendritic
cells by adenovirus-mediated gene transduction. Exp Clin
Immunogenet. 15:100–111. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kim CJ, Prevette T, Cormier J, et al:
Dendritic cells infected with poxviruses encoding MART-1/Melan A
sensitize T lymphocyte in vitro. J Immunother. 20:276–286. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu Y, Butterfield LH, Fu X, et al:
Lentivirally Engineered DC activate AFP-specific T cells which
Inhibit Hepatocellular Carcinoma Growth in vitro and in vivo. Int J
Oncol. 39:245–253. 2011.PubMed/NCBI
|
|
17
|
Jooss K, Yang Y, Fisher KJ and Wilson JM:
Transduction of dendritic cells by DNA viral vectors directs the
immune response to transgene products in muscle fibers. J Virol.
72:4212–4223. 1998.PubMed/NCBI
|
|
18
|
Yang Y, Su Q and Wilson JM: Role of viral
antigens in destructive cellular immune responses to adenovirus
vector-transduced cells in mouse lungs. J Virol. 70:7209–7212.
1996.PubMed/NCBI
|
|
19
|
Veron P, Allo V, Rivière C, Bernard J,
Douar AM and Masurier C: Major subsets of human dendritic cells are
efficiently transduced by self-complementary adeno-associated virus
vectors 1 and 2. J Virol. 81:5385–5394. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dullaers M and Thielemans K: From pathogen
to medicine: HIV-1-derived lentiviral vectors as vehicles for
dendritic cell based cancer immunotherapy. J Gene Med. 8:3–17.
2006. View
Article : Google Scholar
|
|
21
|
Warrington KH Jr and Herzog RW: Treatment
of human disease by adeno-associated viral gene transfer. Hum
Genet. 119:571–603. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chiriva-Internati M, Liu Y, Salati E, et
al: Efficient generation of cytotoxic T lymphocytes against
cervical cancer cells by adeno-associated virus/human
papillomavirus type 16 E7 antigen gene transduction into dendritic
cells. Eur J Immunol. 32:30–38. 2002. View Article : Google Scholar
|
|
23
|
Samulski RJ, Berns KI, Tan M and Muzyczka
N: Cloning of adeno-associate d virus into pBR322: rescue of intact
virus from the recombinant plasmid in human cells. Proc Natl Acad
Sci. 79:2077–2081. 1982. View Article : Google Scholar
|
|
24
|
Morinaga T, Sakai M, Wegmann TG and
Tamaoki T: Primary structures of human alpha-fetoprotein and its
mRNA. Proc Natl Acad Sci. 80:4604–4608. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Collaco RF, Cao X and Trempe JP: A helper
virus-free packaging system for recombinant adeno-associated virus
vectors. Gene. 238:397–405. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Auricchio A, Hildinger M, O’Connor E, Gao
GP and Wilson JM: Isolation of highly infectious and pure
adeno-associated virus type 2 vectors with a single-step
gravity-flow column. Hum Gene Ther. 12:71–76. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Devos R, Cheroutre H, Taya Y, Degrave W,
Van Heuverswyn H and Fiers W: Molecular cloning of human immune
interferon cDNA and its expression in eukaryotic cells. Nucleic
Acids Res. 10:2487–2501. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Steinman RM: Dendritic cells: from the
fabric of immunology. Clin Invest Med. 27:231–236. 2004.PubMed/NCBI
|
|
29
|
Bender A, Sapp M, Feldman M, et al:
Dendritic cells as immunogens for human CTL responses. Adv Exp Med
Biol. 417:383–387. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sheng KC, Pietersz GA, Wright MD and
Apostolopoulos V: Dendritic cells: activation and
maturation-applications for cancer immunotherapy. Curr Med Chem.
12:1783–1800. 2005. View Article : Google Scholar
|
|
31
|
Zou W: Immunosuppressive networks in the
tumor environment and their therapeutic relevance. Nat Rev Cancer.
5:263–274. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
32
|
Nonacs R1, Humborg C, Tam JP and Steinman
RM: Mechanisms of mouse spleen dendritic cell function in the
generation of influenza-specific cytolytic T lymphocytes. J Exp
Med. 176:519–529. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Banchereau J, Briere F, Caux C, et al:
Immunobiology of dendritic cells. Annu Rev Immunol. 18:767–811.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Jaraquemada D, Marti M and Long EO: An
endogenous processing pathway in vaccinia virus-infected cells for
presentation of cytoplasmic antigens to class II-restricted T
cells. J Exp Med. 172:947–954. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Schuhbauer DM, Mitchison NA and Mueller B:
Interaction within clusters of dendritic cells and helper T cells
during initial Th1/Th2 commitment. Eur J Immunol. 30:1255–1262.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
McClellan AD, Heiser A and Hart DN:
Induction of dendritic cell costimulator molecule expression is
suppressed by T cells in the absence of antigen-specific
signalling: role of cluster formation, CD40 and HLA-class II for
dendritic cell activation. Immunol. 98:171–180. 1999. View Article : Google Scholar
|
|
37
|
Stuhler G, Zobywalski A, Grünebach F, et
al: Immune regulatory loops determine productive interactions
within human T lymphocyte-dendritic cell clusters. Proc Natl Acad
Sci USA. 96:1532–1535. 1999. View Article : Google Scholar : PubMed/NCBI
|