Introduction
Cerebral ischemia is a condition in which there is
insufficient blood flow to the brain caused by cerebral vasospasm
or an embolism. Previous studies have demonstrated that cerebral
ischemia triggers a cascade of pathophysiological events, including
glutamate-dependent excitotoxicity, calcium overload, apoptosis,
inflammation, free radical formation, nitric oxide production and
mitochondrial damage, leading to neuronal cell death (1–5).
Through investigating the mechanism underlying cerebral ischemic
injury, a theoretical basis for clinical treatment can be proposed
and may have far-reaching significance for the prevention and
treatment of cerebral ischemia (6).
Iron is an essential trace element in the human
body. It is involved in synthesis of the myelin sheath and
neurotransmission. Iron is also a type of catalyst, which increases
the concentration of reactive oxygen species. The increased
production of reactive oxygen species and lipid peroxidation
damages neurons in cerebral ischemia (7,8).
Cerebral hypoxia leads to iron accumulation and lipid peroxidation
in oligodendrocytes in one-day old Wistar rats during development
(7). Whether iron accumulation and
overload in neurons following cerebral ischemic injury is a novel
mechanism requires further investigation.
Iron is composed of heme and non-heme iron in the
human body (9). Heme iron is
required for the synthesis of heme. Intracellular heme iron can
increase heme oxygenase 1 expression and lead to oxidative stress
and cell membrane damage (10).
Non-heme iron is involved in cell respiration and catalyzing
antibody production (11). Serum
ferritin is a marker of the body’s non-heme iron store (12). Hepcidin is secreted by the liver
and is important in the regulation of iron transport (13). Mutation of the gene BCS1L leads to
a mitochondrial disorder and elevated serum ferritin (14). Accumulation of iron is observed in
various neurodegenerative disorders, including Alzheimer’s and
Parkinson’s disease (15). Excess
iron increases ROS expression and activates the caspase protein
family, which contributes to apoptosis. The presence of excess iron
is therefore recognized as a major risk factor for
neurodegenerative diseases (16–17).
Previous studies have demonstrated that patients undergoing
transfusion therapy are at risk of iron overload with associated
tissue damage (15–17). Hyperferritinemia and increased iron
stores are associated with the severity of liver damage in
non-alcoholic fatty liver disease (18). Iron overload and oxidative stress
are involved in the endometriosis-associated inflammatory reaction
(19). Certain characteristics of
metabolic syndrome are mainly attributed to iron overload (20). As a type of non-heme iron export
protein (21–23), ferroportin (Fpn) is abundant in the
small intestine and macrophages (24). Previous studies demonstrated that
Fpn is also expressed in the hippocampus, cerebral cortex,
thalamus, brainstem and cerebellum (25,26).
Hepcidin binds to Fpn and promotes its internalization and
degradation. Fpn disease, the most common non-HFE hereditary
iron-loading disorder, is caused by a loss of iron export function
of Fpn resulting in early and preferential iron accumulation in
kupffer cells and macrophages (27). Schulz et al found that
astrocytes can secrete Fpn to promote remyelination following
axonal injury (28). Certain
studies have proposed that inflammatory cytokines alter the
expression of Fpn resulting in iron accumulation (29). Fpn can therefore respond to
intraneural non-heme iron metabolism in cerebral ischemia.
Traditional Chinese medicine has made certain
achievements for cerebral ischemia (30–32).
Naotaifang extract (NTE) is an extract of a traditional Chinese
medicine compound, which improves blood circulation. A previous
study demonstrated that NTE is clinically effective for the
treatment of cerebral ischemia and the therapeutic mechanism
includes anticoagulation and angiogenesis (33).
In order to reveal the dysregulation of
intracellular iron and examine the mechanisms underlying cerebral
brain, the present study investigated the expression of Fpn in the
hippocampal CA2 region cells following induction of cerebral
ischemia in rats treated with NTE.
Materials and methods
Animals
A total of 100 healthy adult male Sprague Dawley
rats weighing 220–250 g (SPF grade) were provided by the
Experimental Animal Center of Hunan University of Chinese Medicine
(Changsha, China). The study was approved by the ethics committee
of the College of Traditional Chinese Medicine (Changsha,
China).
Drugs
NTE consists of astragalus root, chuanxiong and
dilong. The extract was acquired by water decoction and alcohol
extraction. The active ingredients include astragaloside,
ligustrazine and ferulic acid (extracted by pharmaceutical
preparation at the Department of Hunan Traditional Chinese Medicine
University) mixed with physiological saline to achieve the required
concentration.
Experiment one
A total of 50 healthy male Sprague Dawley rats were
assigned to the 2 h group (n=10), 6 h group (n=10), 12 h group
(n=10), 24 h group (n=10) and 72 h group (n=10) using a random
digits table. The rats were used to establish the middle cerebral
artery occlusion (MCAO) model.
Experiment two
A total of 50 healthy male Sprague Dawley rats were
assigned to either the sham surgery group (n=10) or the surgery
group (n=40) using a random digits table. The rats in the surgery
group were used to establish the MCAO model. According to the
postoperative treatment, the surgery group was divided into the
model group (0.9% NaCL), low-dose group (3 g/g NTE), medium dose
group (9 g/kg NTE) and high-dose group (27 g/kg NTE). Each group
was treated with the corresponding dose through intragastric
administration for the following three days after surgery. The
animal specimens were collected 72 h after surgery.
Animal modeling
Focal cerebral ischemia was induced by
intra-arterial suture occlusion of the right middle cerebral artery
(MCA) (34). MCAO was induced by
using the intraluminal filament technique. Right common and
external carotid arteries were ligated and the internal carotid
artery was closed. A fish wire (d=0.28 mm) was advanced through the
right internal carotid artery to the origin of the MCA. The sham
group was treated identically, with the exception that no
intraluminal filament was insert into the MCA. Neurological
assessment was used to confirm successful MCAO. Following surgery
(35), the cerebral cortex and
hippocampus CA2 area exhibited marked neuronal damage on the right
side.
Neurobehavioral assessment
Neurobehavioral scores were assessed in each animal
following the final treatment at 72 h. A modification of a previous
method was used to evaluate the neurological deficit (36). The five categories of motor
neurological findings were scored: 0, no observable symptom; 1,
contralateral forelimb flexion; 2, contralateral circling; 3,
tumble contralateral side; 4, unable to walk, loss of
consciousness.
Preparation of tissue slices
All rats were perfused with paraformaldehyde (4%;
Sigma-Aldrich, St. Louis, MO, USA) under anesthesia and fixed at
different time points (2, 6, 12, 24 and 72 h) following brain
ischemia. The brains were then removed and fixed for 1 h, washed
with sodium chloride (Sigma-Aldrich), dehydrated with gradient
alcohol (Sigma-Aldrich), embedded in paraffin (Sigma-Aldrich) and
sectioned in 4 μm thick coronal sections.
Immunohistochemical staining and image
analysis
Paraffin sections were incubated at 60°C for 30 min,
then dewaxed in xylene (Sigma-Aldrich) and gradient alcohol. Slides
were soaked in a solution of 3% H2O2
(Sigma-Aldrich) for 10 min at room temperature to block endogenous
peroxidases. Rabbit-anti-rat Fpn monoclonal antibody (1:100;
Proteintech, Chicago, IL, USA) was added and incubated for 2 h at
37°C. The sections were washed three times in 0.01 mol/l
phosphate-buffered saline (PBS; Sigma-Aldrich) for 5 min. The
sections were placed in wet boxes and incubated for 40 min at 37°C
with polyclonal biotin-labeled goat anti-rabbit IgG (cat no.
PV-600; Proteintech). Subsequently, the sections were washed twice
in PBS for 5 min and stained with 3,3′-diaminobenzidine
(Sigma-Aldrich). The sections were then observed under an optical
microscope (Olympus, Tokyo, Japan). Five sections were randomly
selected from each slice. Image analysis was performed using Image
Pro Plus 5.0 software (Media Cybernetics, Inc., Rockville, MD, USA)
to determine the integral optical density (IOD) of Fpn positive
areas in each visual field (magnification, ×400) and an average was
calculated.
Expression of Fpn mRNA detected by
RT-PCR
At different time points following surgery or 72 h
after NTE treatment, 100 mg tissue was extracted from the
hippocampus using 1 ml TRIzol (Invitrogen Life Technologies,
Carlsbad, CA, USA). RNA was reverse transcribed in a final volume
of 10 μl containing 1 μg of total RNA, 1 μl
oligo (dT), 10 mM of each deoxyribonucleoside triphosphate (2
μl), 20 units RNasin, 200 units AMV Reverse Transcriptase
and 4 μl of 5X Reverse Transcriptase buffer with
diethylpyrocarbonate H2O (Reverse-transcription kit,
Invitrogen Life Technologies). PCR was performed using 3 μl
of synthesized cDNA with 10 μl 2X PCR mix, 1 μl of
each primer, 4 μl of PCR buffer and ddH2O to give
a total reaction volume of 20 μl. All common components were
added to a master mix (Takara, Dalian, China) and then aliquoted.
The cycling conditions were as follows: Initial denaturation at
94°C for 4 min followed by 28 cycles of 94°C for 15 sec, 56°C for
30 sec, 72°C for 30 sec and a final extension at 72°C for 5 min.
The primers of Fpn were designed (Table I) and Actin was used as a loading
control.
 | Table INucleotide sequence for
oligonucleotide primers of polymerase chain reaction products |
Table I
Nucleotide sequence for
oligonucleotide primers of polymerase chain reaction products
| Primer | Sequence |
|---|
| Fpn sense |
5′-TCCAGTACAGCAGCATCAGCA-3′ |
| Fpn antisense |
5′-ACCTCCTTGGGTCCAAACC-3′ |
| Actin sense |
5′-CCCATCTATGAGGGTTACGC-3′ |
| Actin
antisense |
5′-TTTAATGTCACGCACGATTTC-3′ |
Statistical analysis
All data are expressed as the mean ± standard
deviation and were statistically analyzed using SPSS 11.0 software
(SPSS, Inc., Chicago, IL, USA). The two-sample t-test was used for
comparison among groups. P<0.05 was considered to indicate a
statistically significant difference.
Results
Experiment one
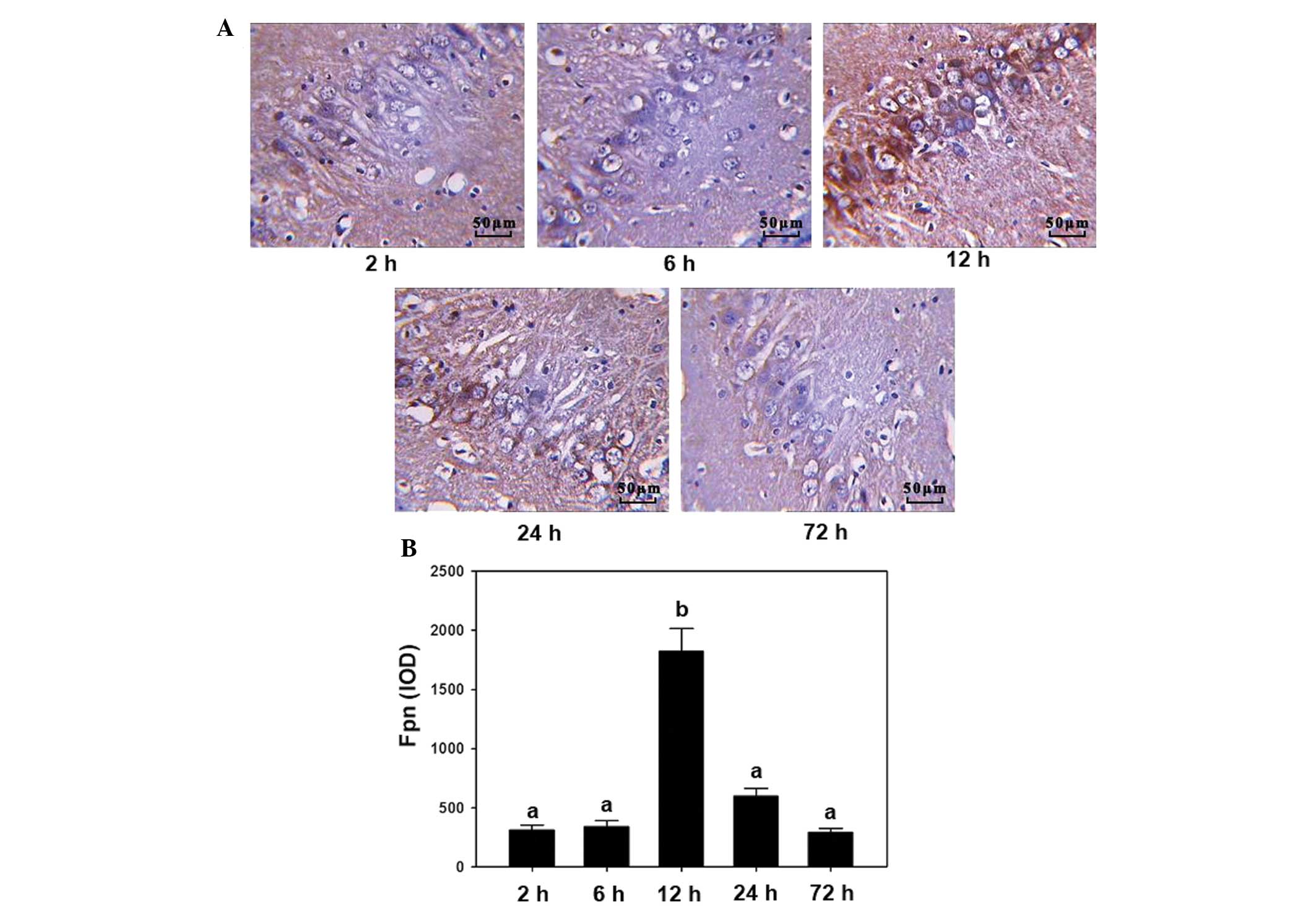
The expression of Fpn was detected using
immunohistochemistry and RT-PCR in the hippocampal CA2 region, at
different time points following induction of cerebral ischemia. The
results from the immunohistochemistry experiments showed that the
darkest staining was observed at 12 h while the changes were less
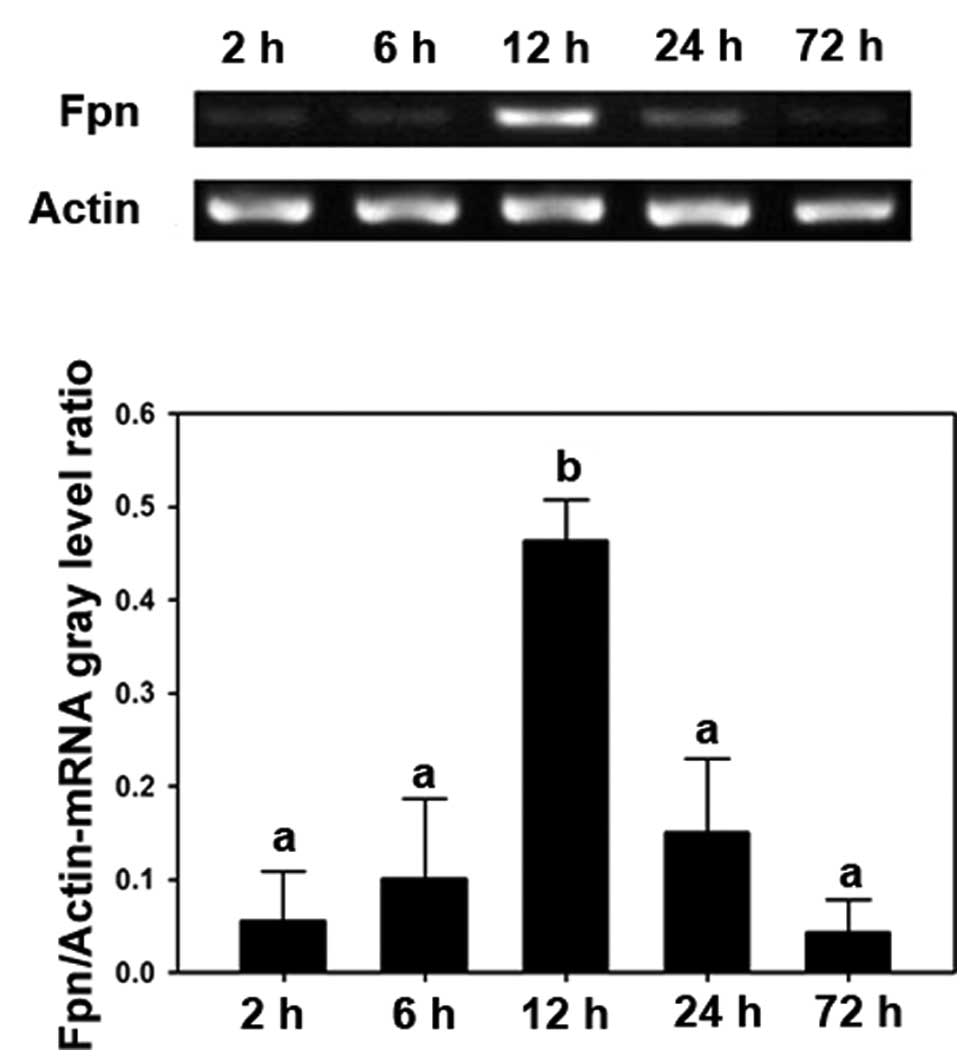
marked in the 2, 6, 24 and 72 h groups (Fig. 1A). RT-PCR demonstrated similar
results; after 12 h treatment, the expression of Fpn was
significantly increased compared with the time points (P<0.05;
Fig. 1B). The RT-PCR analysis also
supported the results that only the 12 h treatment group
demonstrated a significant increase among all the groups
(P<0.05; Fig. 2).
Experiment two
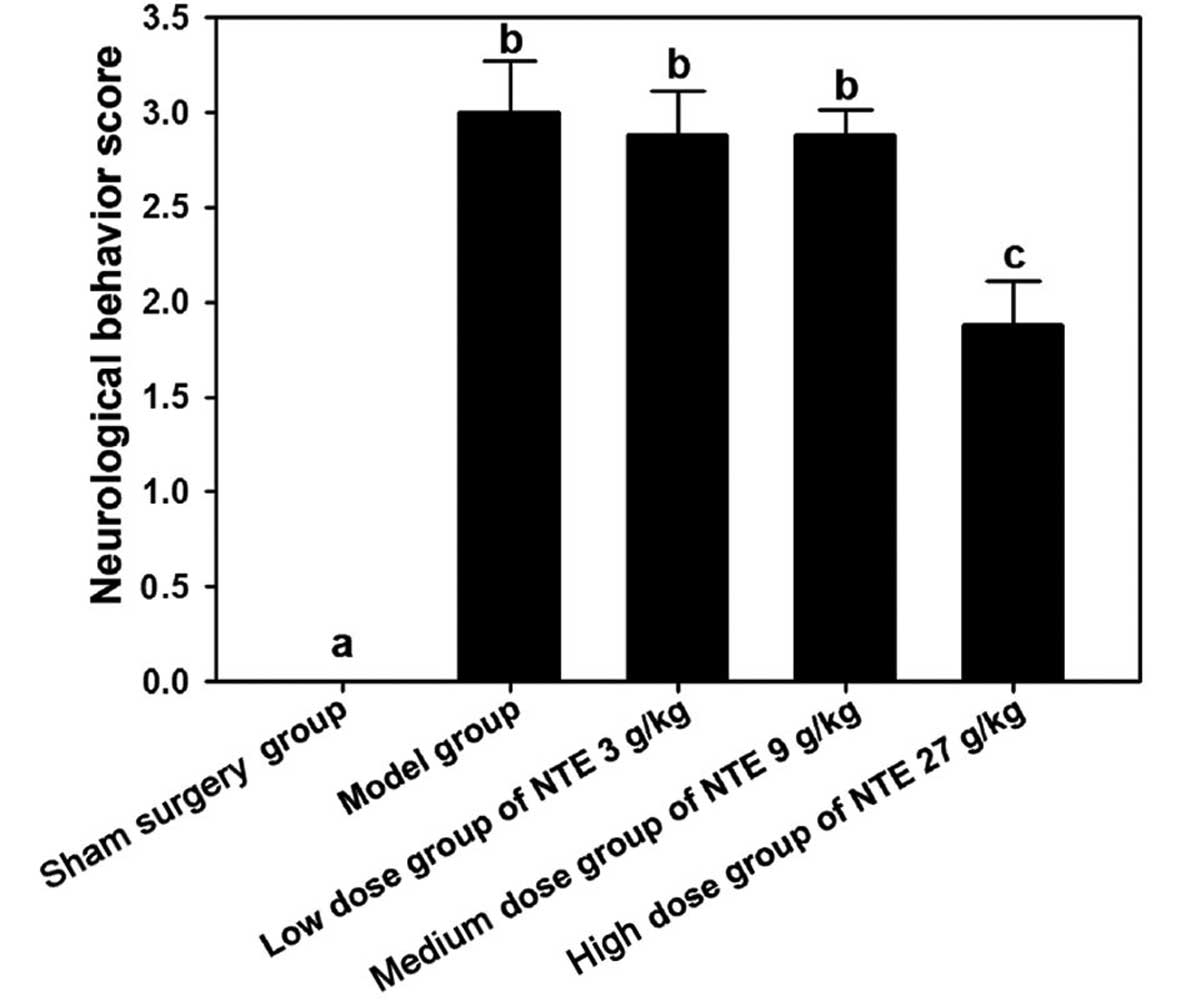
Compared with the sham surgery group, the surgery
group exhibited a higher neurological behavior score (P<0.05)
and compared with the model group, the high-dose group exhibited a
lower neurological behavior score (P<0.05; Fig. 3).
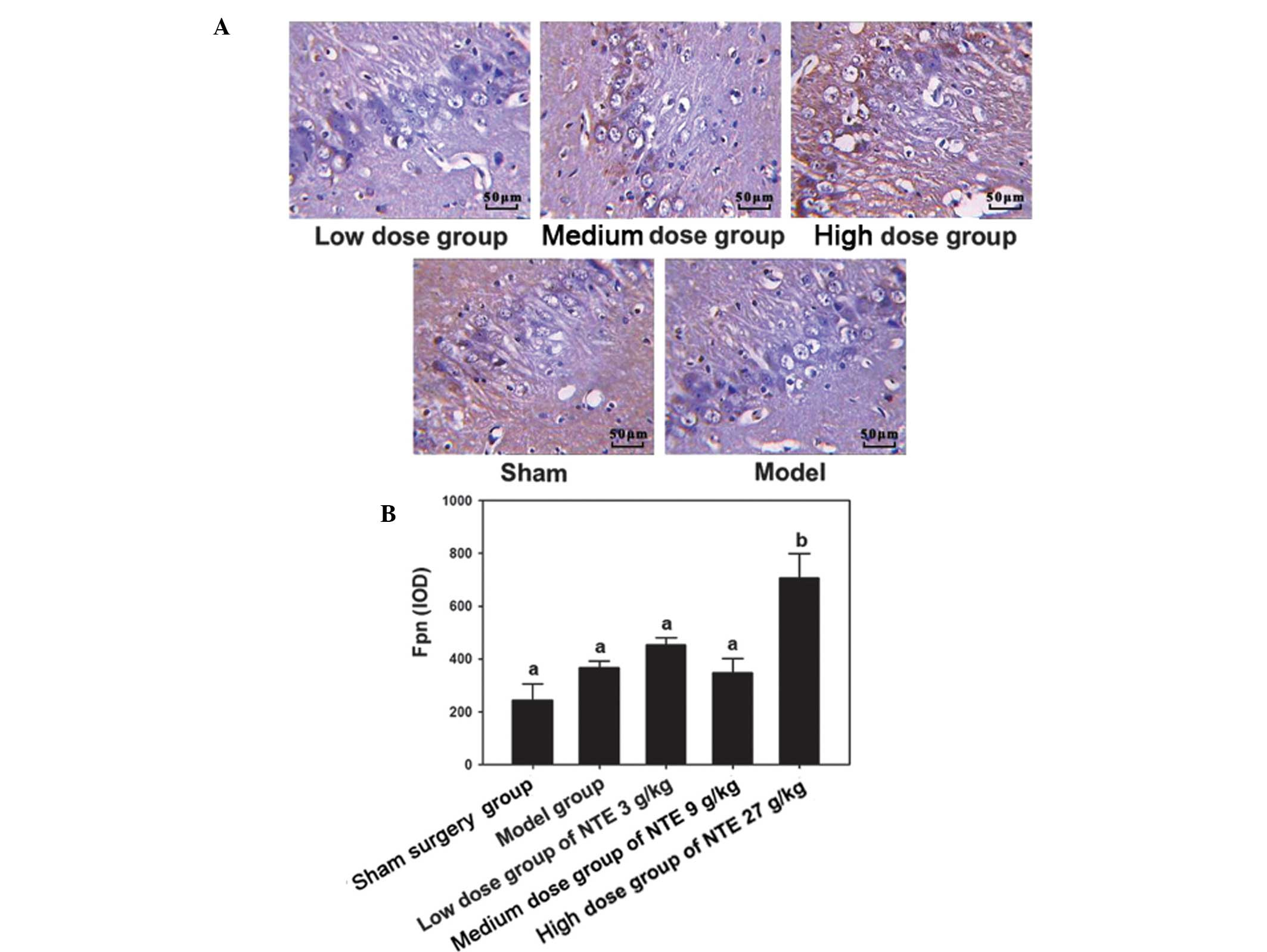
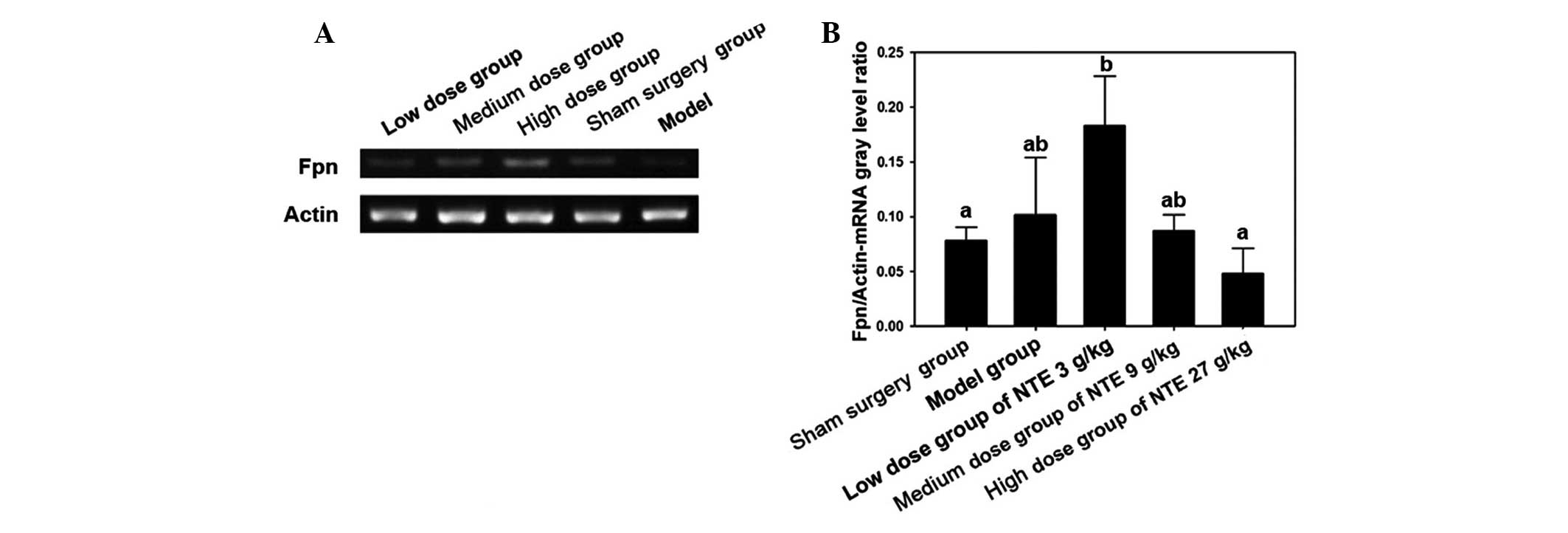
The immunohistochemical results suggested that
following treatment with 3 g/kg NTE, the expression of Fpn
increased significantly compared with the other treatment doses
(P<0.05). However, no significant changes were observed among
other groups (P>0.05; Fig. 4).
The RT-PCR analysis also supported the results that only the 12 h
treatment group demonstrated a significant increase among all the
groups (P<0.05; Fig. 5).
Discussion
Fpn is a type of non-heme iron exporter protein in
the cell membrane. It can be internalized and degraded by binding
to hepcidin. The present study demonstrated that expression of Fpn
in the hippocampal CA2 region was increased following occlusion of
the right MCA, reaching a peak at 12 h, and then decreasing to a
minimum at 72 h. The present study demonstrated that intraneural
iron metabolism is imbalanced in cerebral ischemia. In addition,
the results demonstrate that cellular iron accumulation promotes
the expression of Fpn. Iron metabolism imbalance can promote
cellular iron efflux. Intracellular iron accumulation may promote
the expression of Fpn by a variety of signaling pathways (37). In the present study, following 12
h, intracellular iron efflux increased due to iron overload,
however, compensatory adjustment of neurons was limited. After 12
h, the expression of Fpn reduced and iron outflow decreased. The
accumulation of iron leads to the increased production of reactive
oxygen species and lipid peroxidation, which may damage neurons
(38). Thus, it was proposed that
if a drug intervention can maintain high Fpn expression following
cerebral ischemia, then it may regulate iron metabolism to reduce
the damaging effect.
NTE is a traditional Chinese extract that promotes
the recovery of neurological function and improvement of blood
circulation (39). NTE consists of
astragalus root, chuanxiong and dilong. Previous studies have
demonstrated that Astragalus may reduce the expression of HIF-1a
(hypoxia inducible factor-1a), which protects hippocampal neurons
following ischemic brain damage (40). Astragalus may reduce apoptosis in
hippocampal neurons through reducing the cellular malondialdehyde
and nitric oxide content, thus increasing the activity of
superoxide dismutase (41). The
active ingredients of chuanxiong contain ligustrazine and ferulic
acid (42). Animal experiments
have demonstrated that ligustrazine may stimulate neurogenesis
following focal cerebral ischemia (43). Previous studies have indicated that
ferulic acid may enhance the expression of GABAB1 receptors at 3 h
of reperfusion and thereby provide neuroprotection (44). All components in NTE work together
in order to promote blood circulation and improve the recovery of
neurological function following cerebral ischemia.
Previous studies have demonstrated that Astragalus
polysaccharide can prevent neuronal apoptosis following cerebral
ischemia (32). Ferulic acid has a
neuroprotective effect by promoting the formation of nitric oxide
in rat cerebral ischemia (32).
Previous studies have verified that NTE is clinically effective for
the treatment of cerebral ischemia (45,46).
The therapeutic mechanism includes anticoagulation and
angiogenesis. Experiment one demonstrated that Fpn expression was
reduced to the lowest point 72 h after cerebral ischemia,
therefore, in experiment two the animal specimens were produced at
the same time point following NTE intervention. The present study
demonstrated that a high dose of NTE can increase the expression of
Fpn in the hippocampal CA2 region. The traditional Chinese medicine
NTE can enhance the expression of Fpn, increase iron excretion in
neurons and reduce neuronal oxidative damage caused by iron
accumulation. Therefore, the present study concluded that NTE can
protect the neurons in the hippocampal CA2 region by increasing the
expression of Fpn and promoting neuronal iron efflux in cerebral
ischemia.
Acknowledgments
This study was supported by the National Natural
Science Foundation of China (grant nos. 81303078 and 81202794), the
Natural Science Foundation of Hunan Province (grant no. 12JJ6076),
the Foundation of Hunan Provincial Administration of Traditional
Chinese Medicine (grant no. 201240), the Foundation of Education
Bureau of Hunan Province for Young Teachers (grant no. 11B090), the
Key Laboratory of Hunan Province for Integrated Traditional Chinese
and Western Medicine on Prevention and Treatment of Cardio-Cerebral
Diseases, the Key Laboratory of Colleges and Universities in Hunan
Province for Cytobiology and Molecular Biotechnology, Hunan
University of Chinese Medicine Aid program for Science and
Technology and the Innovative Research Team in Higher Educational
Institutions of Hunan Province.
References
|
1
|
Chu XP and Xiong ZG: Physiological and
pathological functions of acid-sensing ion channels in the central
nervous system. Curr Drug Targets. 13:263–271. 2012. View Article : Google Scholar :
|
|
2
|
Xu M and Zhang HL: Death and survival of
neuronal and astrocytic cells in ischemic brain injury: a role of
autophagy. Acta Pharmacol Sin. 32:1089–1099. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Xu F, Gu JH and Qin ZH: Neuronal autophagy
in cerebral ischemia. Neurosci Bull. 28:658–666. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Olmez I and Ozyurt H: Reactive oxygen
species and ischemic cerebrovascular disease. Neurochem Int.
60:208–212. 2012. View Article : Google Scholar
|
|
5
|
Belousov AB: Novel model for the
mechanisms of glutamate-dependent excitotoxicity: Role of neuronal
gap junctions. Brain Res. 1487:123–130. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Giuliani D, Minutoli L, Ottani A, et al:
Melanocortins as potential therapeutic agents in severe hypoxic
conditions. Front Neuroendocrinol. 33:179–193. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rathnasamy G, Ling EA and Kaur C: Iron and
iron regulatory proteins in amoeboid microglial cells are linked to
oligodendrocyte death in hypoxic neonatal rat periventricular white
matter through production of proinflammatory cytokines and reactive
oxygen/nitrogen species. J Neurosci. 31:17982–17995. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gebril OH, Simpson JE, Kirby J, Brayne C
and Ince PG: Brain iron dysregulation and the risk of ageing white
matter lesions. Neuromolecular Med. 13:289–299. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Muñoz P and Humeres A: Iron deficiency on
neuronal function. Biometals. 25:825–835. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Khan AA and Quigley JG: Heme and
FLVCR-related transporter families SLC48 and SLC49. Mol Aspects
Med. 34:669–682. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Garrick MD and Garrick LM: Cellular iron
transport. Biochim Biophys Acta. 1790:309–325. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ferraro S, Mozzi R and Panteghini M:
Revaluating serum ferritin as a marker of body iron stores in the
traceability era. Clin Chem Lab Med. 50:1911–1916. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Vermeulen E and Vermeersch P: Hepcidin as
a biomarker for the diagnosis of iron metabolism disorders: a
review. Acta Clin Belg. 67:190–197. 2012.PubMed/NCBI
|
|
14
|
Fellman V: GRACILE syndrome-a severe
neonatal mitochondrial disorder. Duodecim. 128:1560–1567. 2012.
|
|
15
|
Chu WC, Au WY and Lam WW: MRI of cardiac
iron overload. J Magn Reson Imaging. 36:1052–1059. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shander A, Berth U, Betta J and Javidroozi
M: Iron overload and toxicity: implications for anesthesiologists.
J Clin Anesth. 24:419–425. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Elborai Y, Uwumugambi A and Lehmann L:
Hematopoietic stem cell transplantation for thalassemia.
Immunotherapy. 4:947–956. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Valenti L, Dongiovanni P and Fargion S:
Diagnostic and therapeutic implications of the association between
ferritin level and severity of nonalcoholic fatty liver disease.
World J Gastroenterol. 18:3782–3786. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
González-Ramos R, Defrère S and Devoto L:
Nuclear factor-kappaB: a main regulator of inflammation and cell
survival in endometriosis pathophysiology. Fertil Steril.
98:520–528. 2012. View Article : Google Scholar
|
|
20
|
Annaloro C, Airaghi L, Saporiti G, Onida
F, Cortelezzi A and Deliliers GL: Metabolic syndrome in patients
with hematological diseases. Expert Rev Hematol. 5:439–458. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Theil EC: Iron homeostasis and nutritional
iron deficiency. J Nutr. 141:724S–728S. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen J and Enns CA: Hereditary
hemochromatosis and transferrin receptor 2. Biochim Biophys Acta.
1820:256–263. 2012. View Article : Google Scholar
|
|
23
|
Graham RM, Chua AC, Herbison CE, Olynyk JK
and Trinder D: Liver iron transport. World J Gastroenterol.
13:4725–4736. 2007.PubMed/NCBI
|
|
24
|
Kasvosve I: Effect of ferroportin
polymorphism on iron homeostasis and infection. Clin Chim Acta.
416:20–25. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zheng W and Monnot AD: Regulation of brain
iron and copper homeostasis by brain barrier systems: implication
in neurodegenerative diseases. Pharmacol Ther. 133:177–188. 2012.
View Article : Google Scholar :
|
|
26
|
Boserup MW, Lichota J, Haile D and Moos T:
Heterogenous distribution of ferroportin-containing neurons in
mouse brain. Biometals. 24:357–375. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Pietrangelo A, Caleffi A and Corradini E:
Non-HFE hepatic iron overload. Semin Liver Dis. 31:302–318. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Schulz K, Kroner A and David S: Iron
efflux from astrocytes plays a role in remyelination. J Neurosci.
32:4841–4847. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Urrutia P, Aguirre P, Esparza A, et al:
Inflammation alters the expression of DMT1, FPN1 and hepcidin, and
it causes iron accumulation in central nervous system cells. J
Neurochem. 126:541–549. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang HW, Liou KT, Wang YH, et al:
Deciphering the neuroprotective mechanisms of Bu-yang Huan-wu
decoction by an integrative neurofunctional and genomic approach in
ischemic stroke mice. J Ethnopharmacol. 138:22–33. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Loh KP, Qi J, Tan BK, Liu XH, Wei BG and
Zhu YZ: Leonurine protects middle cerebral artery occluded rats
through antioxidant effect and regulation of mitochondrial
function. Stroke. 41:2661–2668. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Koh PO: Ferulic acid modulates nitric
oxide synthase expression in focal cerebral ischemia. Lab Anim Res.
28:273–278. 2012. View Article : Google Scholar
|
|
33
|
Yunhe H, Jinwen G and Zhanying C: Effect
of Naotaifang on TXB 2, 6-Keto-PGF 1α in plasma and TNF-α in serum
of patients with cerebral infarction with deficiency of Qi and
blood stasis. Chinese Journal of Information on Traditional Chinese
Medicine. 4:16–17. 2002.
|
|
34
|
Garcia JH: A reliable method to occlude a
middle cerebral in wistar rats. Stroke. 24:14231993.
|
|
35
|
González-Delgado M and Bogousslavsky J:
Superficial middle cerebral artery territory infarction. Front
Neurol Neurosci. 30:111–114. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Longa EZ, Weinstein PR, Carlson S and
Cummins R: Reversible middle cerebral artery occlusion without
craniectomy in rats. Stroke. 20:84–91. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Eisenstein RS: Iron regulatory proteins
and the molecular control of mammalian iron metabolism. Annu Rev
Nutr. 20:627–662. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Bandyopadhyay U, Das D and Banerjee RK:
Reactive oxygen species: oxidative damage and pathogenesis. Curr
Sci. 77:658–666. 1999.
|
|
39
|
Jinwen G, Song C and Huibin Z: Effect of
naotaifang extract on functional changes of coagulation and
fibrinolysis of human umbilical veins endothelial cell induced by
recombined human tumor necrosis factor alpha. J Trad Chin Med U
Hun. 6:4–6. 2005.
|
|
40
|
Zhang Q, Gao WY, Zhang Y, et al:
Protective effects of astragalus extract against intermittent
hypoxia-induced hippocampal neurons impairment in rats. Chin Med J
(Engl). 126:1551–1554. 2013.
|
|
41
|
Yin YY, Zhu FF and Wu GC: Protective
effect of astragalosides on anoxia/reoxygenation injury of
hippocampal neuron. Zhongguo Zhong Xi Yi Jie He Za Zhi.
30:1173–1177. 2010.In Chinese.
|
|
42
|
Li SL, Chan SS, Lin G, et al: Simultaneous
analysis of seventeen chemical ingredients of Ligusticum chuanxiong
by online high performance liquid chromatography-diode array
detector-mass spectrometry. Planta Med. 69:445–451. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Qiu F, Liu Y, Zhang PB, et al: Effects of
ligustrazine on hippocampal dentate gyrus cell proliferation after
focal cerebral ischemia in adult rats. Nan Fang Yi Ke Da Xue Xue
Bao. 26:1400–1403. 2006.PubMed/NCBI
|
|
44
|
Cheng CY, Su SY, Tang NY, et al: Ferulic
acid inhibits nitric oxide-induced apoptosis by enhancing GABA(Bl)
receptor expression in transient focal cerebral ischemia in rats.
Acta Pharmacol Sin. 31:889–899. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
He YH, Hao XY, Ge JW, et al: Clinical
studies on naotaifang in treating patients with cerebral infarction
with deficiency of qi and blood stasis in TCM. J Emerg Trad Chin
Med. 10:319–320. 2001.
|
|
46
|
Zhu HB, Chen Y, Tan H and Ge JW: Effects
of Naotai recipe extracts on cerebral CD34 expression in rats with
focal cerebral ischemia. Trad Chin Drug Res Clin Pharma.
22:141–144. 2011.
|