Introduction
Pancreatic cancer (PC) is characterized by rapid
invasion, early metastasis and resistance to current standard
therapies (1). Understanding of
the molecular mechanisms in PC is urgently required in order to
identify novel treatment strategies. MicroRNAs (miRNAs, miRs) are
endogenous short non-coding RNA molecules consisting of 17–25
nucleotides, which have been frequently observed to be dysregulated
in diverse types of human cancer (2–4). The
functions of miRNAs as either oncogenes or tumor suppressors has
generated interest as to their possible use as novel targets or
tools for anticancer therapies (5,6).
miR-145 was identified as a tumor-suppressive miRNA,
which is downregulated in several types of cancer, including
prostate, bladder, breast, lung and ovarian cancer (7–12).
Microarray studies have revealed that miR-145 is also
down-regulated in PC (13,14). However, the biological function of
miR-145 in PC remains to be fully elucidated. In the present study,
it was identified that miR-145 is downregulated in PC tissues
compared with matched normal adjacent pancreatic tissues. Notably,
it was identified that neural precursor cell expressed,
developmentally downregulated 9 (NEDD9, also termed HEF1 or Cas-L),
a non-catalytic scaffolding protein implicated in the invasive
ability of several types of cancer (15,16),
is a novel target of miR-145 in PC. Restoration of miR-145
suppresses Panc-1 cell proliferation, invasion and migration
through reducing levels of NEDD9.
Materials and methods
Cell lines and human tissue samples
The Panc-1 human pancreatic ductal adenocarcinoma
(PDAC) cell line was obtained from the American Type Culture
Collection (Manassas, VA, USA). All cells were cultured in
Dulbecco’s modified Eagle’s medium (Invitrogen Life Technologies,
Carlsbad, CA, USA) supplemented with 10% fetal bovine serum
(Invitrogen Life Technologies) at 37°C in a humidified atmosphere
containing 5% CO2. A total of 20 PDAC tissues and paired
normal adjacent pancreatic tissues were obtained from the patients
who were confirmed to have PDAC by pathological analysis and
underwent the Whipple procedure at the Department of General
Surgery, Xiangya Hospital, Central South University (Changsha,
China). Specimens were flash-frozen in liquid nitrogen immediately
and stored at −80°C for future use. The protocol for the study was
approved by the Xiangya Hospital Clinical Research Ethics
Committee.
miRNA transfection
miR-145 mimics and negative control miRs (miR-NC)
were synthesized by Yingrun Biotechnology Inc. (Changsha, China). A
total of 1×104 Panc-1 cells/well were seeded into
six-well plates and then cells were transfected in a solution with
75 nM miR-145 mimics or miR-NC (Yingrun Biotechnology Inc.) using
Lipofectamine 2000 (Invitrogen Life Technologies) according to the
manufacturer’s instructions. Total RNA and protein were extracted
at 48 h post-transfection and used for reverse
transcription-quantitative polymerase chain reaction (RT-qPCR) and
western blot analysis.
RNA isolation and RT-qPCR
Total RNAs were isolated using TRIzol reagent
(Invitrogen Life Technologies) according to the manufacturer’s
instructions. The miR-145 levels were assayed using TaqMan MicroRNA
assays (Applied Biosystems, Carlsbad, CA, USA). The u6 small
nuclear B noncoding RNA (Applied Biosystems) level was used as an
internal normalization control. For NEDD9 mRNA analysis the primer
sequences were as follows: Forward: 5′-GAGCTGGATGGATGACTACGA-3′ and
reverse: 5′-AGCTCTTTCTGTTGCCTCTCA-3′. Total RNA was
reverse-transcribed with the SuperScript III first-strand synthesis
system for RT-PCR (Invitrogen Life Technologies) and amplified with
2X SYBR Green real-time PCR master mix (Toyobo, Osaka, Japan). PCR
was conducted using an ABI 7500 Real-Time PCR System (Applied
Biosystems) and the results were normalized. Relative fold changes
were calculated using the 2∆∆Ct method and standard
curves were produced. β-actin was used as an internal normalization
control.
Western blot analysis
At 48 h after transfection, Panc-1 cells were lysed
with radioimmunoprecipitation assay lysis buffer and proteins were
harvested. Proteins were resolved on an SDS denatured
polyacrylamide gel and then transferred onto a nitrocellulose
membrane (Millipore Corp., Billerica, MA, USA). A mouse anti-human
anti-NEDD9 polyclonal antibody (1:1,000; 4044S; Cell Signaling
Technology Inc., Beverly, MA, USA) and a mouse anti-human
anti-β-actin polyclonal antibody (1:4,000; sc-130301; Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA) were incubated with the
blot overnight at 4°C. Membranes were washed and incubated with
goat anti-mouse secondary antibodies (1:10,000) and were
visual-ized by enhanced chemiluminescence (Millipore, Billerica,
MA, USA) according to the manufacturer’s instructions.
Prediction of miRNA targets
In order to investigate the predicted target genes,
the TargetScan program (http://www.targetscan.org/), the miRanda program
(http://microrna.sanger.ac.uk), the
miRWalk program (http://www.umm.uni-heidelberg.de/apps/zmf/mirwalk/)
and the PicTar4 program (http://pictar.bio.nyu.edu) were used. The TargetScan
program (http://www.targetscan.org/) was used
to predict the seed region.
Plasmid construction and the
dual-luciferase assay
For the validation of NEDD9 as a direct target of
miR-145, an miRNA target luciferase reporter assay was performed
using a target reporter plasmid containing wild-type (WT) NEDD9
3′-untranslated region (UTR) and mutant NEDD9 3′UTR.
Co-transfection experiments were performed in 96-well plates. A
total of 1×104 HEK-293 cells (American Type Culture
Collection, Manassas, VA, USA) were seeded per well in 200
μl medium. A total of 100 ng WT or mutant (MT) reporter
constructs were co-transfected with Lipofectamine 2000 transfection
reagent into the PC cells with 50 nM miR-145 mimics or miR-NC
according to the manufacturer’s instructions. After 48 h, the
luciferase activity was measured with the dual luciferase reporter
assay system (Promega Corporation, Madison, WI, USA). The relative
luciferase activity was normalized to that of firefly
luciferase.
Cell proliferation
Cell proliferation was determined using an MTT
assay. Briefly, the Panc-1 cells were seeded in 96-well culture
plates at a density of 1×104 cells per well. After 24 h
of culturing, the cells were transfected with 75 nM miR-145 mimics
or miR-NC using Lipofectamine 2000. The cells were then cultured in
the medium and proliferation rates at 1, 2, 3, 4 and 5 days after
transfection were assessed by a colorimetric assay using 5 mg/ml
MTT solution at 490 nm. All the experiments were performed three
times with five replicates.
Cell migration and invasion analysis
Panc-1 cells were transfected with 75 nM miR-145
mimics or miR-145 NC for 48 h, trypsinized and plated for migration
and invasion assays. For the migration assay, 5×104
cells were plated in the top chamber of monocoated polyethylene
teraphthalate membrane (6-well insert, 8 μm pore size; BD
Biosciences, Bedford, MA, USA). For the invasion assay,
2×105 cells were plated in the top chamber of the
transwell with a Matrigel-coated polycarbonate membrane (24 wells
insert, 8 μm pore size; BD Biosciences). Respective medium
with 10% fetal bovine serum (Invitrogen Life Technologies) was
added to the lower chamber as a chemoattractant. After 24 h of
incubation, cells remaining on the upper surface of the insert
membrane were removed using a cotton swab. Cells, which had
migrated or invaded through the membrane/Matrigel to the bottom of
the insert were stained with 0.1% crystal violet for 30 min at 37°C
and washed with phosphate-buffered saline. Finally, migrated or
invaded cells were counted under a microscope (IX71; Olympus Corp.,
Tokyo, Japan) in five random visual fields and the relative number
was calculated.
Statistical analysis
Each experiment was conducted at least three times.
All values are expressed as the mean ± standard deviation.
Differences between experimental groups and controls were assessed
via Student’s t-test using Excel software (Microsoft Inc., Redmond,
WA, USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
miR-145 expression is downregulated in
PC
miR-145 has been observed to be underexpressed in
various types of cancer; however, its expression in PC has not been
previously investigated, to the best of our knowledge. The
expression of miR-145 was examined in different grades of PC using
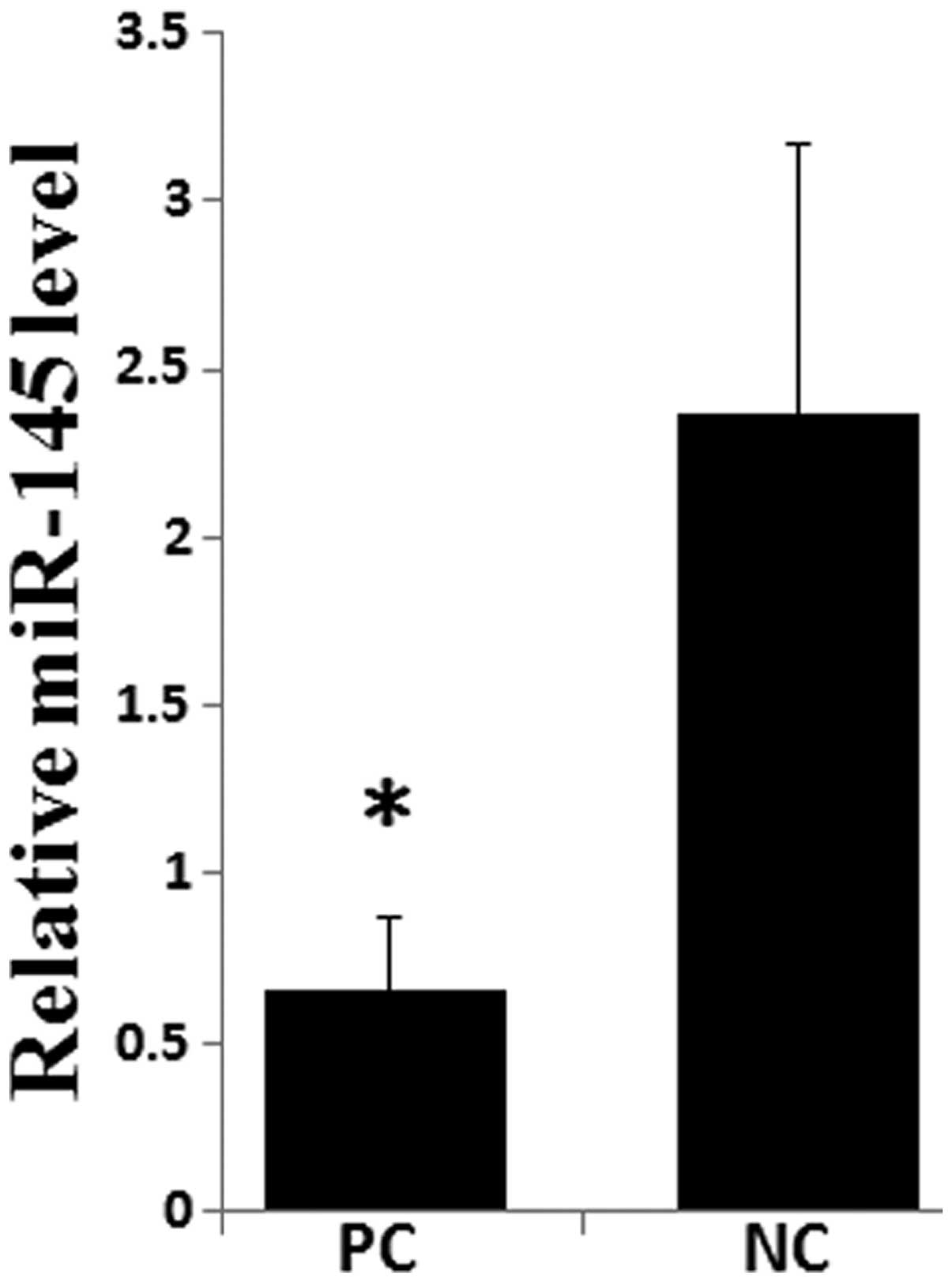
RT-qPCR. As shown in Fig. 1, the
expression of miR-145 was significantly lower in PC tissues
compared with paired adjacent normal pancreatic tissues
(P<0.05). These data support the hypothesis that miR-145 may act
as a tumor suppressor in PC.
Cell proliferation is suppressed by
re-expression of miR-145 in Panc-1 cells
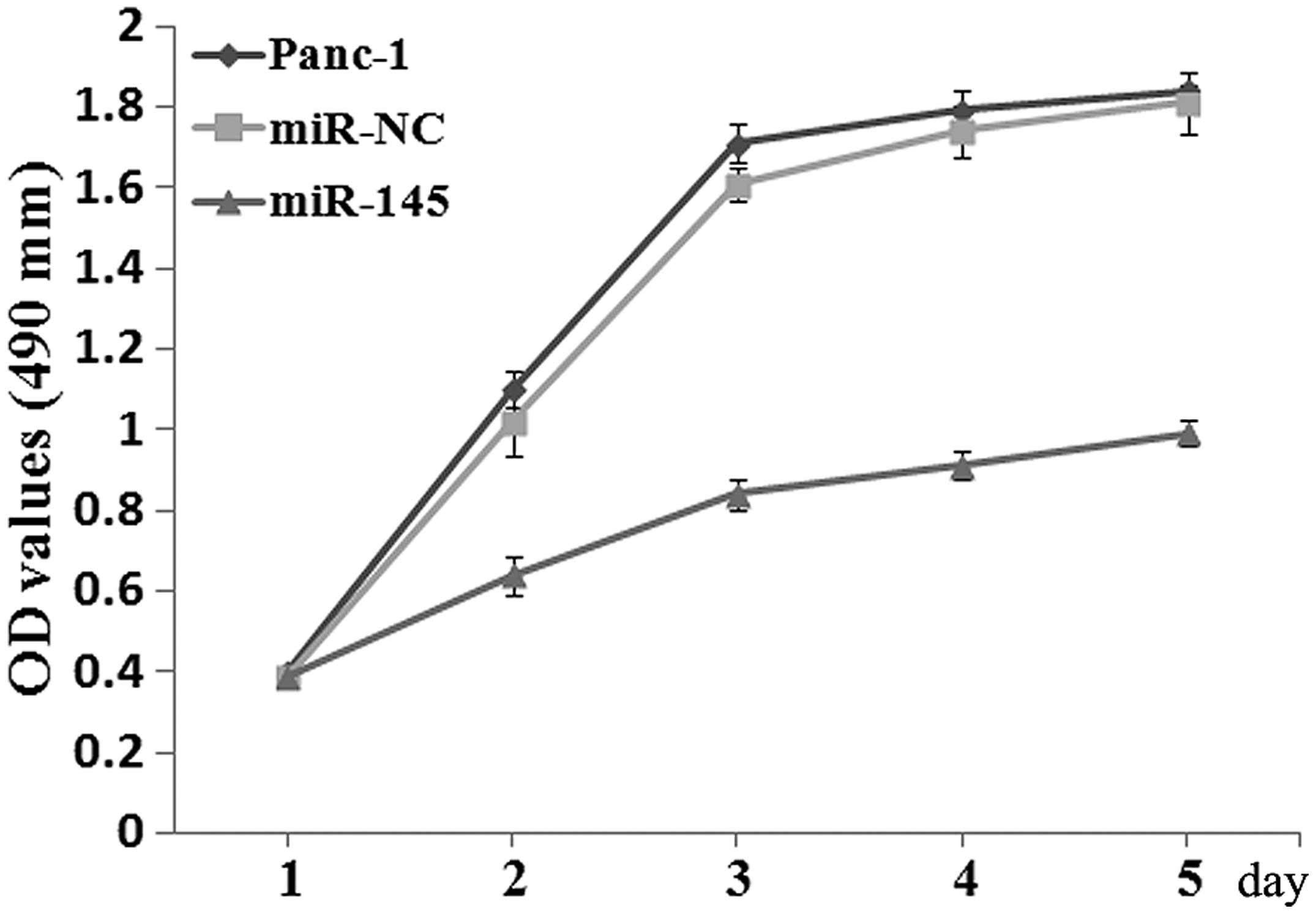
Initially, the effects of miR-145 on the
proliferation of PC cells were investigated using an MTT assay.
Panc-1 cells were transfected with 75 nM miR-145 mimics or miR-NC.
The miR-145 mimics caused a 40-fold increase of the miR-145
expression in Panc-1 cells (data not shown). The MTT value of cells
transfected with miR-145 mimics was significantly lower than that
of cells transfected with miR-NC at 48 h post-transfection
(P<0.05; Fig. 2).
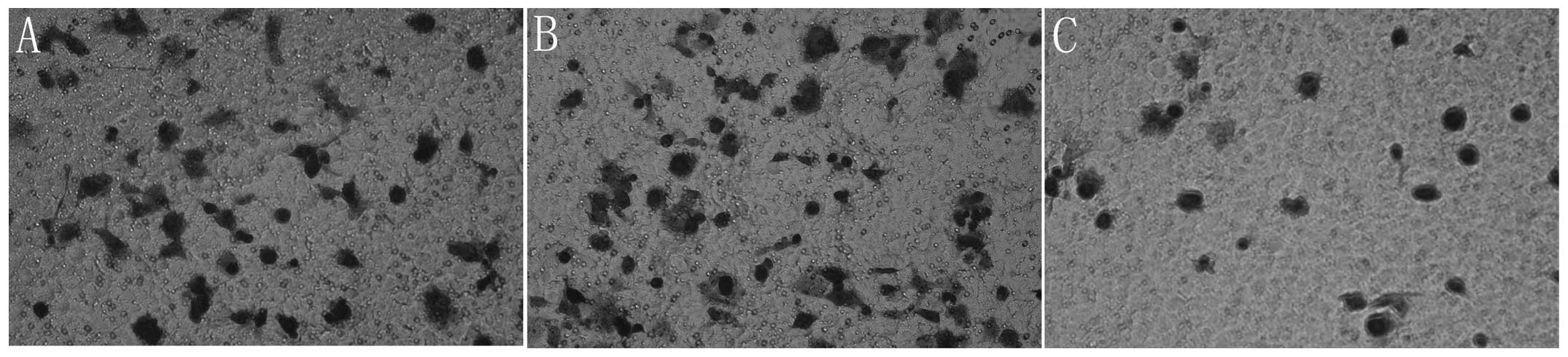
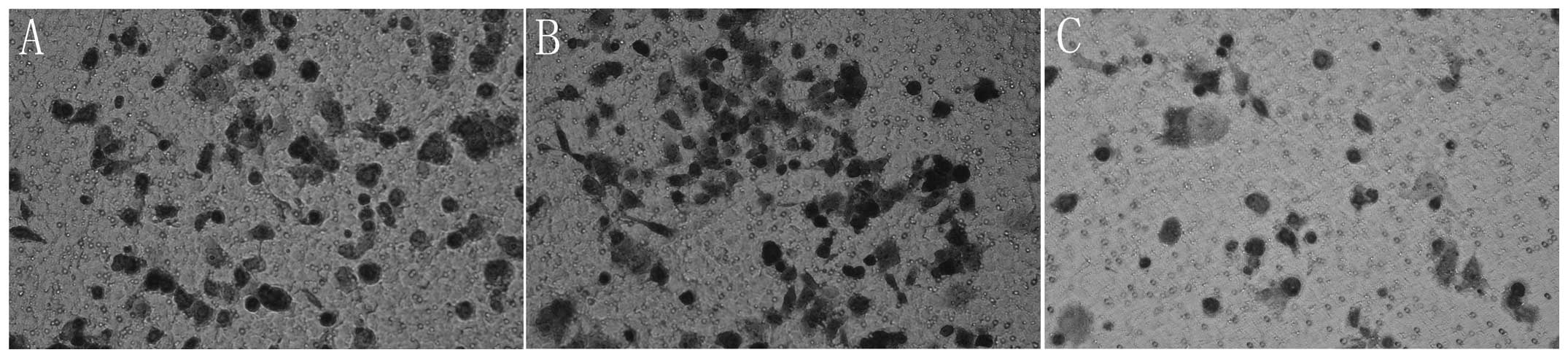
Cell invasion and migration are
significantly decreased by re-expression of miR-145
As PC is a malignant type of cancer with a potent
capacity to invade locally and cause distant metastases, the effect
of miR-145 restoration on Panc-1 cell invasion and migration was
subsequently examined. Panc-1 cells were transfected with miR-145
mimics and then levels of cell invasion and migration were examined
using a Transwell invasion and migration assay. The results
revealed that the re-expression of miR-145 significantly decreased
cell invasion and migration in Panc-1 cells (P<0.05; Figs. 3 and 4).
miR-145 directly targets 3′UTR of
NEDD9
To elucidate the molecular mechanism underlying
miR-145 mediated regulation of proliferation, invasion and
migration, in silico analysis was performed based on the
computer-aided algorithms: PicTar, Targetscan, miRWalk and miRanda
in conjunction with the miRGen Target program for predicted target
genes. The most promising candidate was NEDD9, which was predicted
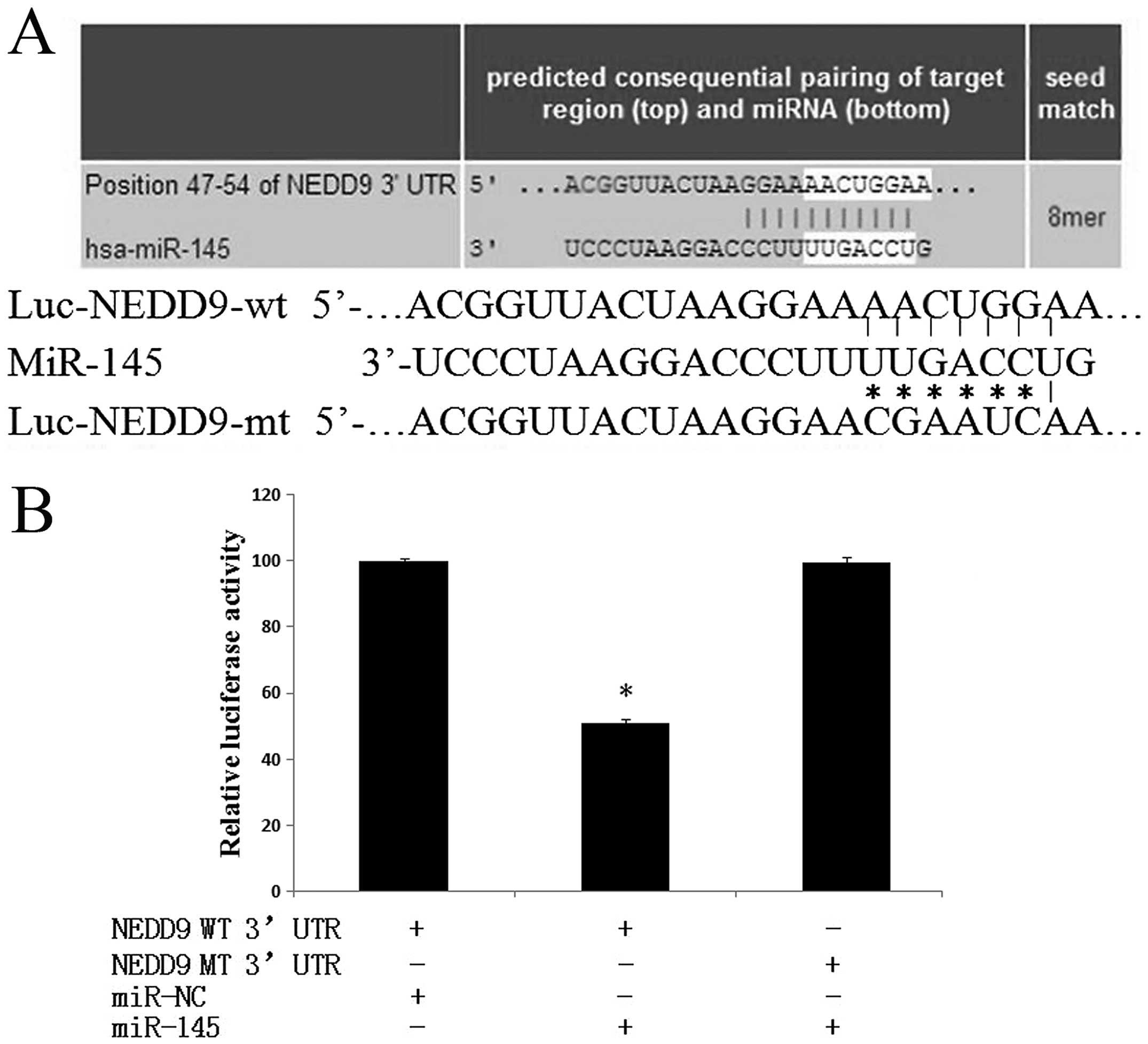
by all of the applied algorithms. As shown in Fig. 5A, there is a putative 8-mer-binding
site for miR-145 in the 3′UTR of the NEDD9 transcript [in addition
to 7-mer sites, TargetScan predicts 8-mer sites defined as: An
exact match to positions 2–8 of the mature miRNA (the seed+position
8) followed by an ‘A’]. In addition, it is a well-established
metastasis- and migration-promoting gene, which is upregulated in
several types of cancer, including PC. To identify whether miR-145
directly targets NEDD9, dual-luciferase reporter gene assays were
performed. Luciferase reporter plasmids containing the wild-type
3′UTR (Luc-NEDD9-wt) or mutant 3′-UTR (Luc-NEDD9-mt) of NEDD9 were
constructed to determine the targeted region (Fig. 5A). As shown in Fig. 5B, miR-145 significantly decreased
the firefly luciferase activity in the reporter with wild type
3′UTR (P<0.05); however the activity of the mutant 3′UTR vector
remained unaffected (P>0.05). These observations indicated that
miR-145 directly targeted NEDD9 through interacting with the
predicted binding site in its 3′UTR.
miR-145 expression is inversely
correlated with NEDD9 in PC
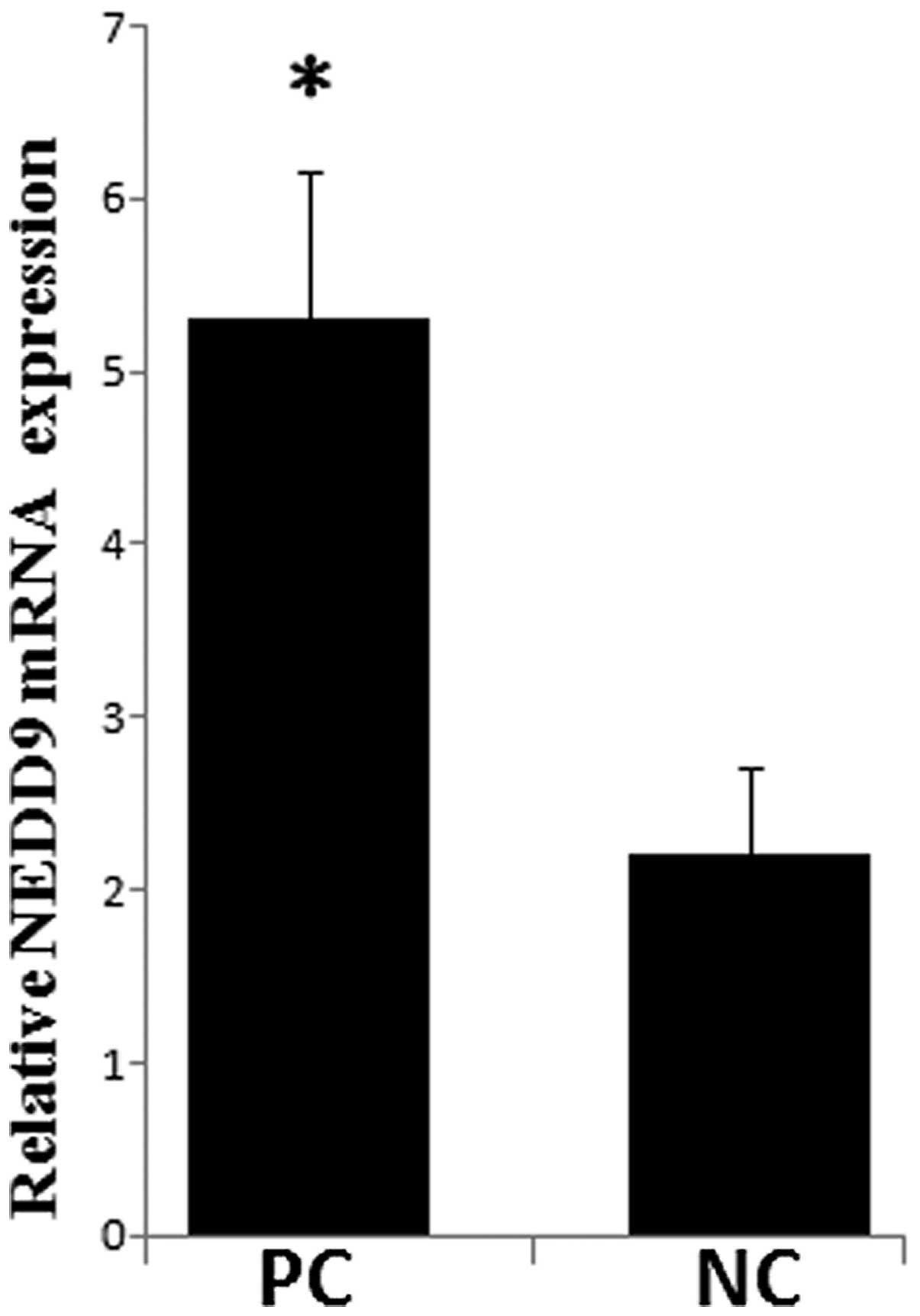
The NEDD9 mRNA levels were measured in PC tissues
and paired normal adjacent pancreatic tissues. As shown in Fig. 6, the average level of NEDD9
expression was significantly higher in PC tissues as compared with
that of the NP tissues. A significant inverse correlation was
observed between NEDD9 and miR-145 expression in PC tissues and
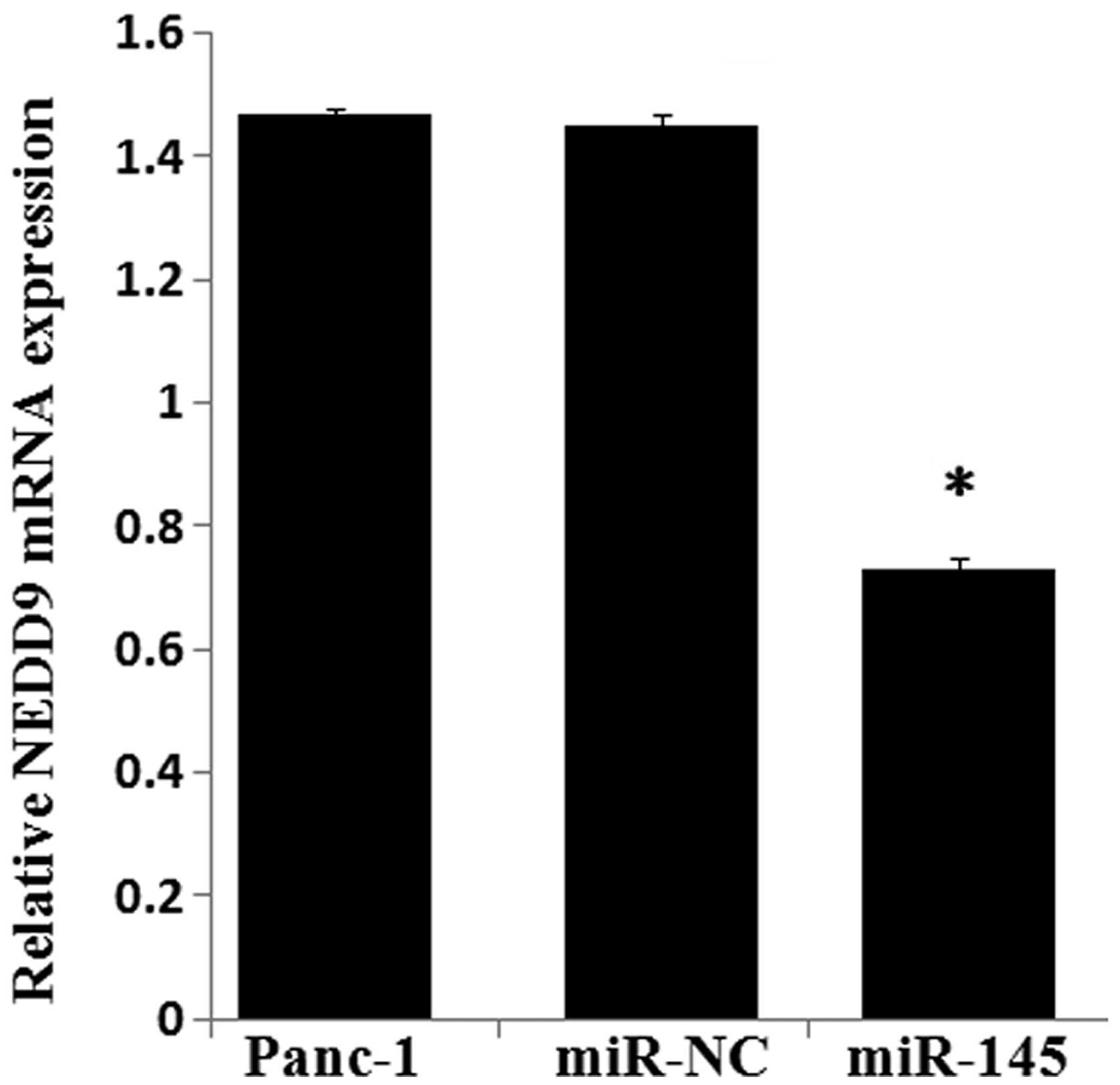
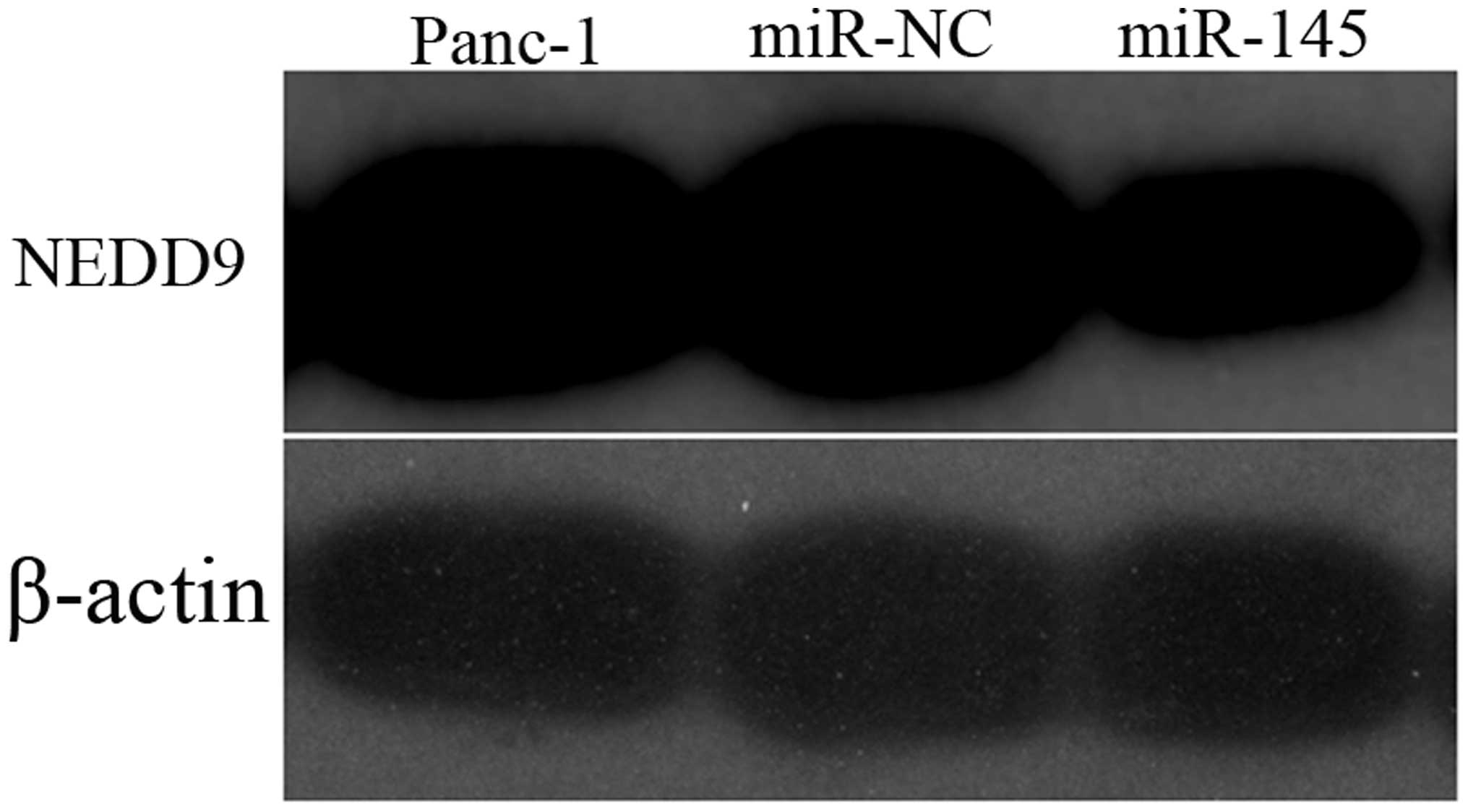
adjacent noncancerous tissues. Transfection of Panc-1 cells with
miR-145 mimics significantly decreased the expression of NEDD9 mRNA
(Fig. 7) by 51% and the NEDD9
protein by 52.21% (Fig. 8)
compared with levels in the control miR expressing cells
(P<0.05).
Discussion
miRNAs may negatively regulate gene expression by
interacting with the specific target mRNA 3′UTR, which can result
in gene silencing by either mRNA degradation or translation
inhibition (17). miRNAs have been
implicated in a broad range of biological processes, including cell
proliferation, apoptosis, differentiation, metabolism, migration
and invasion as each single miRNA may have more than one hundred
targets and >30% of protein coding may be under the control of
miRNAs (18). Mounting evidence
indicates that deregulation of miRNA expression is often associated
with a variety of disorders, including human cancer and numerous
miRNAs have been observed to be differentially expressed in PC as
compared with the normal pancreas (19,20).
Since invasion and metastasis are the major causes of a poor
prognosis in patients with PC, determining the complex role of
specific miRNAs and their targets in the process of PC invasion and
metastasis may provide novel insights for certain diagnoses and
therapeutic consequences.
Aberrant expression of miR-145 has been observed in
several types of cancer, including prostate, bladder, lung, breast
and ovarian cancer. In addition, a close association between
downregulated miR-145 and cancer invasion/metastasis has been
observed (7–12). Microarray studies have identified
that miR-145 was also downregulated in PC (13,14).
However, the role of miR-145 in PC remains to be fully elucidated.
In the present study, it was identified that miR-145 expression in
PC tissues is significantly downregulated compared with that in
adjacent normal pancreatic tissues, suggesting that miR-145 is a
candidate tumor suppressor in the pathogenesis of PC.
Downregulation or silencing of miR-145 may eliminate tumor
suppression so as to contribute to tumorigenesis. Subsequently, the
biological function of miR-145 in PC cells was examined and the
results demonstrated that restoration of miR-145 in Panc-1 cells
can significantly inhibit cell proliferation, invasion and
migration, confirming previous studies suggesting a
tumor-suppressive role for this miRNA. These results provided
substantial evidence that miR-145 may be involved in the invasive
and metastatic progression of PC. Therefore, approaches to
introduce miR-145 into cancer cells may potentially be feasible in
the clinical treatment of PC, particularly for patients with lower
levels of miR-145 expression in their tumor tissues. However, the
exact mechanism by which this treatment may aid therapy remains to
be elucidated, largely due to the limited knowledge of miR-145
targets.
NEDD9, also termed HEF1 or Cas-L was initially
identified by its developmentally regulated expression pattern in
the early embryonic mouse brain. NEDD9 is a non-catalytic
scaffolding protein, which is a member of the Crk-associated
substrate (CAS) family (21). A
series of studies have implicated the NEDD9 protein as a biomarker
of invasive ability and an essential switch for pro-metastatic
behavior in numerous types of cancer, including lung cancer, breast
cancer and melanoma (22–24). The interaction of NEDD9 with FAK
and Src leads to the tyrosine phosphorylation of NEDD9 to generate
binding sites for effector proteins, including the Rac and the
Cas-Crk complex, which then regulate and activate transcription
pathways involved in metastasis and cancer progression (15). Recently, Speranza et al
(25) identified that miR-145 is
markedly downregulated whereas NEDD9 is significantly upregulated
in glioblastoma specimens and corresponding
glioblastoma-neurospheres (GB-NS) compared with normal brain and
low-grade gliomas. The results suggested that miR-145 downregulates
NEDD9, while NEDD9 down-regulates miR-145, forming a
double-negative feedback loop in GB-NS (25). However, little is known about the
function of NEDD9 in PC, and to the best of our knowledge, there
are no previous studies investigating whether NEDD9 expression is
regulated by specific miRNAs, such as miR-145, in PC.
In the present study, an important molecular
association between miR-145 and NEDD9 was demonstrated. Firstly,
the bioinformatics analysis indicated that NEDD9 may be one of the
potential targets for miR-145. Subsequently, it was identified that
NEDD9 is markedly upregulated in PC tissues, which is consistent
with a previous study (26). More
importantly, a significant negative correlation was observed
between miR-145 and NEDD9 expression in PC tissues, and the
expression of NEDD9 was decreased in Panc-1 cells accompanied by
suppressed cell proliferation, invasion and migration when
overexpressing miR-145, suggesting that miR-145 may be involved in
the regulation of NEDD9 in PC. This hypothesis was further
supported by the luciferase activity assay, in which the data
demonstrated that miR-145 was able to directly target the 3′UTR of
NEDD9 and the binding site was consistent with the seed region
predicted by Targetscan. These data demonstrated that miR-145 may
inhibit cell growth, invasion and migration of PC by targeting
NEDD9.
In conclusion, data from the present study has
demonstrated that miR-145 is downregulated in PC, restoration of
miR-145 may inhibit the proliferation, migration and invasion
capacity of PC cells by negatively regulating NEDD9 at the
post-transcriptional level via directly binding to non-coding
regions of NEDD9. The present data suggested that miR-145 may be
useful as a novel potential therapeutic approach for the treatment
of PC.
References
|
1
|
Muniraj T, Jamidar PA and Aslanian HR:
Pancreatic cancer: a comprehensive review and update. Dis Mon.
59:368–402. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bartel DP: MicroRNAs: genomics,
biogenesis, mechanism and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Xi JJ: MicroRNAs in Cancer. Cancer Treat
Res. 158:119–137. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Calin GA and Croce CM: MicroRNA signatures
in human cancers. Nat Rev Cancer. 6:857–866. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang B, Pan X, Cobb GP and Anderson TA:
microRNAs as oncogenes and tumor suppressors. Dev Biol. 302:1–12.
2007. View Article : Google Scholar
|
|
6
|
Wang Z: Advances with microRNAs in
tumorigenesis and cancer therapy. Curr Pharm Des. 20:52452014.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Xing AY, Wang B, Shi DB, et al:
Deregulated expression of miR-145 in manifold human cancer cells.
Exp Mol Pathol. 95:91–97. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wu H, Xiao Z, Wang K, Liu W and Hao Q:
MiR-145 is down-regulated in human ovarian cancer and modulates
cell growth and invasion by targeting p70S6K1 and MUC1. Biochem
Biophys Res Commun. 441:693–700. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fuse M, Nohata N, Kojima S, et al:
Restoration of miR-145 expression suppresses cell proliferation,
migration and invasion in prostate cancer by targeting FSCN1. Int J
Oncol. 38:1093–1101. 2011.PubMed/NCBI
|
|
10
|
Chiyomaru T, Enokida H, Tatarano S, et al:
miR-145 and miR-133a function as tumour suppressors and directly
regulate FSCN1 expression in bladder cancer. Br J Cancer.
102:883–891. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cho WC, Chow AS and Au JS: Restoration of
tumour suppressor hsa-miR-145 inhibits cancer cell growth in lung
adenocarcinoma patients with epidermal growth factor receptor
mutation. Eur J Cancer. 45:2197–2206. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang S, Bian C, Yang Z, et al: miR-145
inhibits breast cancer cell growth through RTKN. Int J Oncol.
34:1461–1466. 2009.PubMed/NCBI
|
|
13
|
Mees ST, Schleicher C, Mardin WA,
Senninger N, Colombo-Benkmann M and Haier J: Analyzing miRNAs in
ductal adenocarcinomas of the pancreas. J Surg Res. 169:241–246.
2011. View Article : Google Scholar
|
|
14
|
Papaconstantinou IG, Manta A, Gazouli M,
et al: Expression of microRNAs in patients with pancreatic cancer
and its prognostic significance. Pancreas. 42:67–71. 2013.
View Article : Google Scholar
|
|
15
|
O’Neill GM, Seo S, Serebriiskii IG, Lessin
SR and Golemis EA: A new central scaffold for metastasis: parsing
HEF1/Cas-L/NEDD9. Cancer Res. 67:8975–8979. 2007. View Article : Google Scholar
|
|
16
|
Izumchenko E, Singh MK, Plotnikova OV, et
al: NEDD9 promotes oncogenic signaling in mammary tumor
development. Cancer Res. 69:7198–7206. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lai EC: Micro RNAs are complementary to 3′
UTR sequence motifs that mediate negative post-transcriptional
regulation. Nat Genet. 30:363–364. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Iorio MV and Croce CM: MicroRNAs in
cancer: small molecules with a huge impact. J Clin Oncol.
27:5848–5856. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Szafranska AE, Davison TS, John J, et al:
MicroRNA expression alterations are linked to tumorigenesis and
non-neoplastic processes in pancreatic ductal adenocarcinoma.
Oncogene. 26:4442–4452. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lee EJ, Gusev Y, Jiang J, et al:
Expression profiling identifies microRNA signature in pancreatic
cancer. Int J Cancer. 120:1046–1054. 2007. View Article : Google Scholar
|
|
21
|
Seo S, Ichikawa M and Kurokawa M:
Structure and function of cas-L and integrin-mediated signaling.
Crit Rev Immunol. 26:391–406. 2006. View Article : Google Scholar
|
|
22
|
Singh MK, Izumchenko E, Klein-Szanto AJ,
Egleston BL, Wolfson M and Golemis EA: Enhanced genetic instability
and dasatinib sensitivity in mammary tumor cells lacking NEDD9.
Cancer Res. 70:8907–8916. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chang JX, Gao F, Zhao GQ and Zhang GJ:
Expression and clinical significance of NEDD9 in lung tissues. Med
Oncol. 29:2654–2660. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kim M, Gans JD, Nogueira C, et al:
Comparative oncogenomics identifies NEDD9 as a melanoma metastasis
gene. Cell. 125:1269–1281. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Speranza MC, Frattini V, Pisati F, et al:
NEDD9, a novel target of miR-145, increases the invasiveness of
glioblastoma. Oncotarget. 3:723–734. 2012.PubMed/NCBI
|
|
26
|
Xue YZ, Sheng YY, Liu ZL, et al:
Expression of NEDD9 in pancreatic ductal adenocarcinoma and its
clinical significance. Tumour Biol. 34:895–899. 2013. View Article : Google Scholar
|