Introduction
Multiorgan dysfunction syndrome (MODS) commonly
occurs subsequent to severe burns, trauma or major surgical stress.
It is currently a leading cause of mortality in critically ill
patients and has become a complicated problem in surgery (1,2).
MODS refers to the dysfunction or failure of two or more systems or
organs and occurs 24 h after the body suffers severe trauma, shock,
infection or acute injury, which results in multiple organs being
unable to maintain a stable internal environment (3). In 1991, the joint conference
committee of the American College of Chest Physicians and the
Society of Critical Care Medicine established the concept of
systemic inflammatory response syndrome and the corresponding
compensatory anti-inflammatory response (4). It is thought that a series of chain
reactions or cascade effects arising from an imbalance between
pro-inflammatory and anti-inflammatory mediators leads to the
development of MODS; thus, the severity and fate of MODS depend on
the balance between the two mediators (5). Currently, neither effective
treatments nor preventive measures against MODS are available.
Studies regarding the pathogenesis of MODS have focused on
immunological function disorder, which is important in the
occurrence and development of MODS (6). During severe injury and infection,
large quantities of inflammatory mediators are released, resulting
in tissue deterioration and exudation, whereas continuously
increasing levels of mediators, such as cytokines, place the immune
system in a bipolar state of activation and paralysis. Therefore,
the high expression levels of immunological mediators jointly
result in tissue injury and the occurrence and development of MODS.
Immunological studies have demonstrated that the main
manifestations of ̔immunological paralysis’ in MODS are caused by
the human leukocyte antigen DR, which continuously reduces
intracellular defects, including the proliferation of
antigen-specific T lymphocytes. T lymphocytes have been shown to be
inhibited by the reduced expression of major histocompatibility
complex II (I-Ab, also termed MHC-II) antigen (6–8).
These defects include the loss of important cell surface antigen
expression, cytokine dysregulation, changes in antigen expression
levels and acceleration of cytokine apoptosis (9–14).
A previous study revealed that in advanced MODS, the
reduction in dendritic cell activity and the resulting massive
lymphocyte apoptosis are important in immunosuppression and MODS
formation (15). Relieving the
immune imbalance and immunosuppression at this stage may prevent
the occurrence of MODS.
Fms-related tyrosine kinase 3 ligand (Flt3L) is a
cytokine that promotes the cytopoiesis and differentiation of
various stem cells, blood cells and precursor blood cells, and it
promotes the proliferation, differentiation and maturation of
prolymphocytes, dendritic cells, natural killer cells and B
lymphocytes (16–18). One study found that Flt3L reversed
endotoxin-induced septic immunological paralysis in mice (19). In the present study, the
zymosan-induced MODS model was used, which is currently employed to
simulate MODS in clinical patients. Flt3L was injected into mice
with advanced MODS to evaluate whether it exhibited a protective
effect on the mice.
Materials and methods
Model preparation
Specific pathogen-free-grade male C57BL/6 mice, aged
six to eight weeks with body weights ranging from 20–25 g, were
purchased from the Experimental Animal Center of the Academy of
Military Medical Sciences (Beijing, China). As determined by a
previously published procedure (3), 1 g zymosan powder (Sigma-Aldrich, St.
Louis, MO, USA) and 40 ml medicinal liquid paraffin were mixed
using a high frequency magnetic stirrer for 1 h to prepare a 25 g/l
zymosan suspension. Subsequently, the suspension was sterilized in
100°C water for 80 min and then cooled to room temperature. The
abdominal skin areas of the mice were locally sterilized and 1 mg/g
zymosan suspension was administered intraperitoneally. The present
study was conducted in strict accordance with the recommendations
of the Guide for the Care and Use of Laboratory Animals of the
National Institutes of Health. The animal use protocol has been
reviewed and approved by the Institutional Animal Care and Use
Committee of the First Affiliated Hospital of General Hospital of
PLA (Beijing, China).
Animal treatment
The experimental animals were randomly divided into
a normal control group (10 mice), an MODS group (30 mice) and an
Flt3L treatment group (30 mice). In the MODS group, normal saline
was intraperitoneally injected once a day for seven days beginning
from day five after the zymosan injection. For the Flt3L treatment
group, recombinant mouse Flt3L (5 μg/kg; CytoLab Ltd.,
Rehovot, Israel) was intraperitoneally injected once a day for
seven days beginning on day five after zymosan injection. For the
normal control group, normal saline was intraperitoneally injected
once a day for seven days beginning on day five without zymosan
intraperitoneal injection. Ten mice from each group were sacrificed
and samples were collected on day 12 after zymosan injection.
Detection of splenic dendritic cells
(DCs)
The mouse spleen was held in a dry dish with forceps
and 1 ml collagenase D solution (500 μl; Sigma-Aldrich) was
slowly injected by a 22-G needle. The spleen was then torn open
with the needle and transferred to another dish. Subsequently, the
spleen tissues were excised and incubated for 25 min at 37°C. A
volume of 10 mM EDTA was added and the tissues were incubated
continuously for 5 min. The tissues were ground on a 400-mesh metal
screen and the cell liquid was collected and centrifuged for 10 min
at 111 x g. A total of 2 ml red cell lysate (BD Biosciences,
Franklin Lakes, NJ, USA) was added and the sample was inverted four
times, incubated for 8 min in the dark and centrifuged for 4 min at
111 x g to remove the supernatant liquid. Subsequently, the cells
were suspended in 2 ml phosphate-buffered saline (PBS). Following
Trypan blue staining, the cells were smeared onto a blood cell
counting board and the number of living cells was determined under
light microscopy (ideally >95%). The cell concentration was
adjusted to 1×106 cells/ml. A total of 0.5 μl
mouse monoclonal anti-phycoerythrin (PE)-CD11c (N418; Biolegend,
Inc., San Diego, CA, USA) and 0.5 μl mouse monoclonal
anti-I-Ab-fluorescein isothiocyanate (FITC) (AF6-120.1;
Biolegend, Inc.) antibodies were added to the cell mixture, which
was mixed uniformly, allowed to stand for 30 min at 4°C, washed
twice with 0.1 M PBS and fixed with 1% paraformaldehyde. Flow
cytometry was used for detection.
Phenotypic analysis of peripheral blood T
lymphocytes
The animal was placed in a restraining device thus
only the tail was exposed for drawing blood. The tail was warmed by
a heat lamp in order to dilate the vessels, sterilized with 70%
ethanol and 1–2 ml blood was drawn from the caudal vein with a 23G
needle, prior to sacrifice of the animals.
Mouse anticoagulant (100 μl, 10.0–12.5 IU/ml)
was placed into three test tubes containing anti-CD3-FITC,
anti-CD4-phycoerythrin (PE), anti-CD8-PE-Cy5 and
anti-I-Ab-FITC, respectively (0.5
μl/106 cells; all from Becton Dickinson
Biosciences, San Jose, CA, USA), mixed uniformly and allowed to
stand for 30 min at room temperature. Whole blood cells were
reacted with antibody and the blood cells were then lysed using a
double volume of red cell lysate, following which the solution was
immediately mixed evenly. After 15 min at room temperature, the
mixture was centrifuged for 4 min at 187 x g to remove the
supernatant liquid. The cells were resuspended in 1%
paraformaldehyde. All data were acquired using FACScalibur (BD
Biosciences) and analyzed using CellQuest Pro software version 4.0
(BD Biosciences).
I-Ab labeling detection
Mouse anticoagulant (100 μl, 10.0–12.5 IU/ml)
was placed into a test tube containing 1 μl
anti-FITC-I-Ab antibody, mixed uniformly and left to
stand at room temperature for 30 min. Subsequently, a double volume
of red cell lysate was added and the solution was immediately mixed
evenly. After 15 min at room temperature, the mixture was
centrifuged for 4 min at 200 x g to remove the supernatant liquid.
PBS was added, and flow cytometry was used to observe the quality
and percentage of I-Ab-labeled mononuclear cells.
Pathological examination under light
microscopy
The liver, lung, kidney and heart tissues of mice
from the different groups were collected and fixed with 10% neutral
formaldehyde solution for 24 h. The samples were dehydrated by a
tissue-dehydrating machine, embedded in paraffin and sectioned into
4-μm thick slices. Following roasting, slicing and dewaxing,
routine hematoxylin-eosin staining was conducted and the sections
were observed under light microscopy using an Olympus BX40F
microscope (Olympus, Melville, NY, USA). Images were captured with
a Sony 3CCD color video camera (Sony, Tokyo, Japan).
Transmission electron microscopy
Fresh spleen tissues from each of the groups were
sectioned into 1-mm tissue blocks. The tissue blocks were
immediately placed in 3% glutaric dialdehyde, premixed for 24 h,
fixed with OsO4 for 2 h, dehydrated with a gradient
ethanol and acetone, embedded in Epson 812 and sectioned with an
LKB-V ultramicrotome. Following double staining with uranyl acetate
and lead citrate, the sections were viewed under a JEM-1200EX
transmission electron microscope (JEOL, Tokyo, Japan).
Statistical analysis
SPSS statistical analysis software (version 10;
SPSS, Inc., Chicago, IL, USA) was used for data processing and data
are expressed as the mean ± standard deviation. Student’s t-test
was used for statistical analysis of the data. P<0.05 was
considered to indicate a statistically significant difference.
Results
Comparison of animal mortality rate
At day 12 following intraperitoneal zymosan
injection, the mice with MODS presented with somnolence, dyspnea,
depression, eye closing, dirty fur, chills and anorexia. The
mortality rate in this group was 18%, whereas the mortality rate in
mice in the Flt3L treatment group was only 7%.
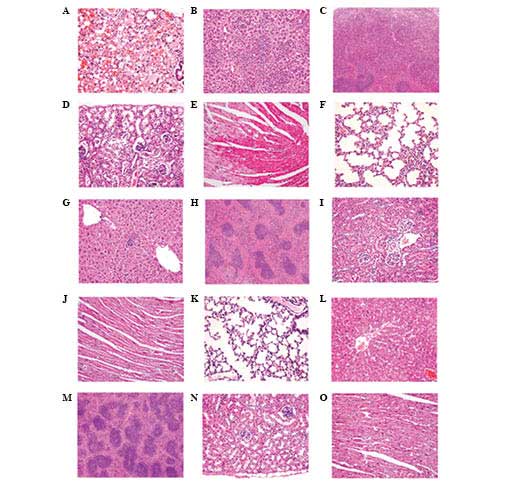
Pathological changes in main organs
The liver, lungs, kidneys, heart and spleen of the
mice with MODS exhibited marked pathological changes, whereas the
pathological changes in the Flt3L treatment mice appeared less
severe (Fig. 1).
The lungs of mice with MODS exhibited alveolar edema
complicated with hemorrhage as well as pulmonary interstitial edema
complicated with focal inflammatory cell infiltration. Alveolar
septum thickening and interstitial pulmonary perivascular
lymphocytic infiltration were also observed (Fig. 1A). In the Flt3L treatment group,
the lesions were markedly less pronounced; pulmonary interstitial
infiltration and inflammatory cell infiltration of the alveolar
septum were occasionally visible (Fig.
1F).
The liver cells of mice with MODS presented with
albuminoid degeneration and vacuolar degeneration. Locally,
eosinophilic variants and spotty liver necrosis were observed;
proliferating hepatic sinus Kupffer cells and further inflammatory
cell infiltrations were also identified (Fig. 1B). In the Flt3L treatment group,
the liver only presented with focal eosinophilic variants of liver
cells (Fig. 1G).
In the kidneys, the glomeruli in the MODS group
exhibited hyperemic and ischemic changes, and the renal tubular
epithelial cells exhibited albuminoid degeneration, granular
degeneration and partial epithelial vacuolar degeneration. Local
interstitial perivascular edema and lymphocyte infiltrations were
also observed (Fig. 1D). In the
Flt3L treatment group, only partial glomerular hyperemia and local
renal tubular epithelial albuminoid degeneration were visible
(Fig. 1I).
In the heart, the MODS group presented with
myocardial interstitial edema with vasodilatation and hyperemia.
Partial myocardial fibers shrank and appeared red, with a condensed
sarcoplasm, increased acidophilia and a flaky distribution
(Fig. 1E). In the Flt3L treatment
group, these lesions were clearly less severe and only a few
myocardial fibers exhibited focal degeneration (Fig. 1J).
In the spleen, the white pulp in the MODS group
appeared shrunken, the periarterial lymphatic sheath had
disappeared and lymphocytic maturation was greatly reduced. In
addition, acini were rare, the follicular germinal centers were not
clearly apparent, and the boundary between the white pulp and the
red pulp was unclear. In the red pulp, neutrophilic infiltration
was observed (Fig. 1C). In the
Flt3L treatment group, the number of white pulp lymphocytes was
clearly increased and the number of acini was close to that of the
normal group (Fig. 1H). The
tissues of the normal groups, including the lung, liver, spleen,
kidney and heart, exhibited no histopathological lesions (Fig. 1K–O).
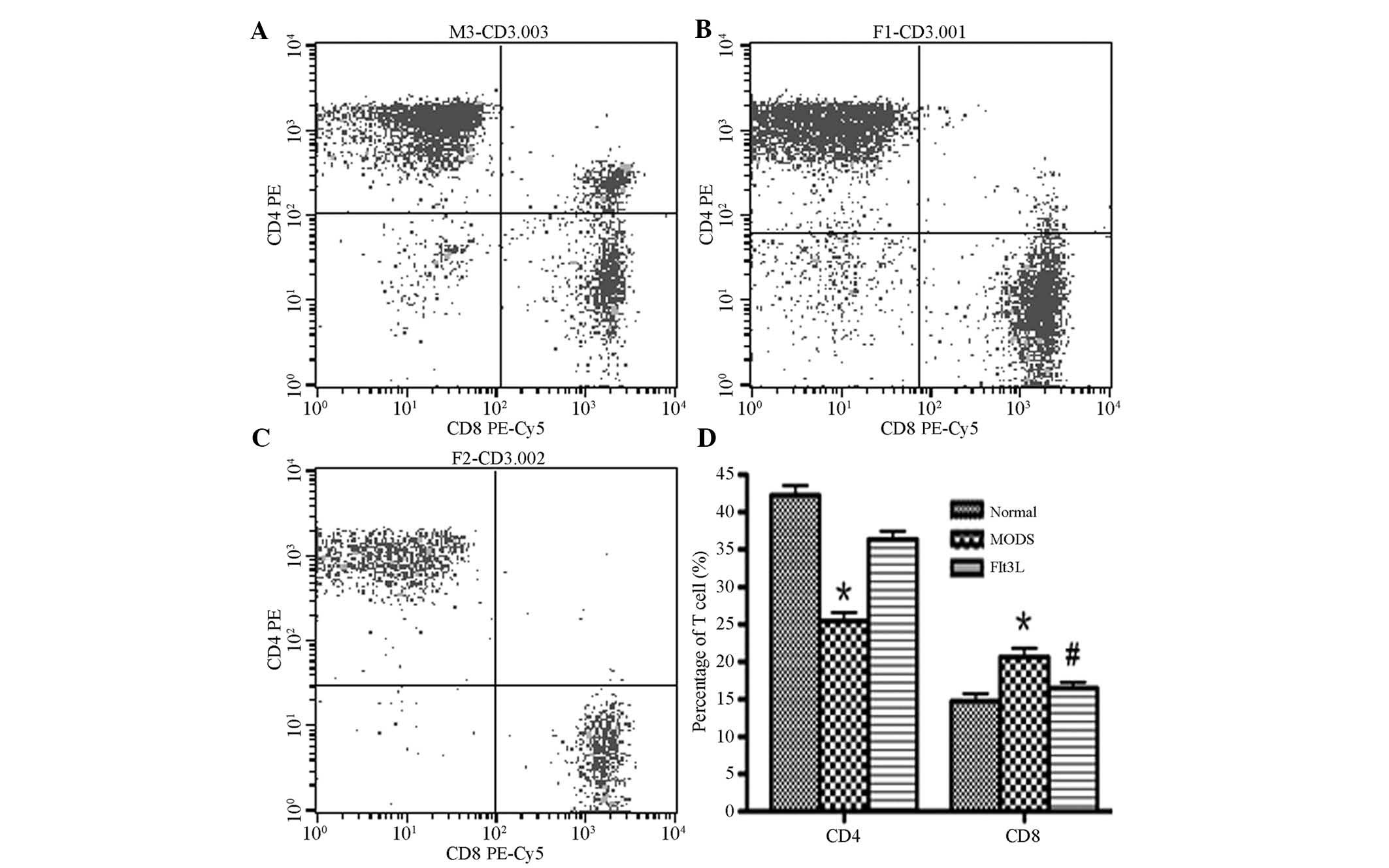
Phenotype analysis of peripheral blood T
lymphocytes
In the MODS group, the number of CD4+ T
cells was significantly reduced, whereas the number of
CD8+ T cells was significantly increased in comparison
with the normal control group (P<0.05). In the Flt3L treatment
group, the number of CD4+ T cells was increased in
comparison with the MODS group and approached normal levels,
whereas the number of CD8+ T cells was significantly
reduced in comparison with the MODS group (P<0.05; Fig. 2).
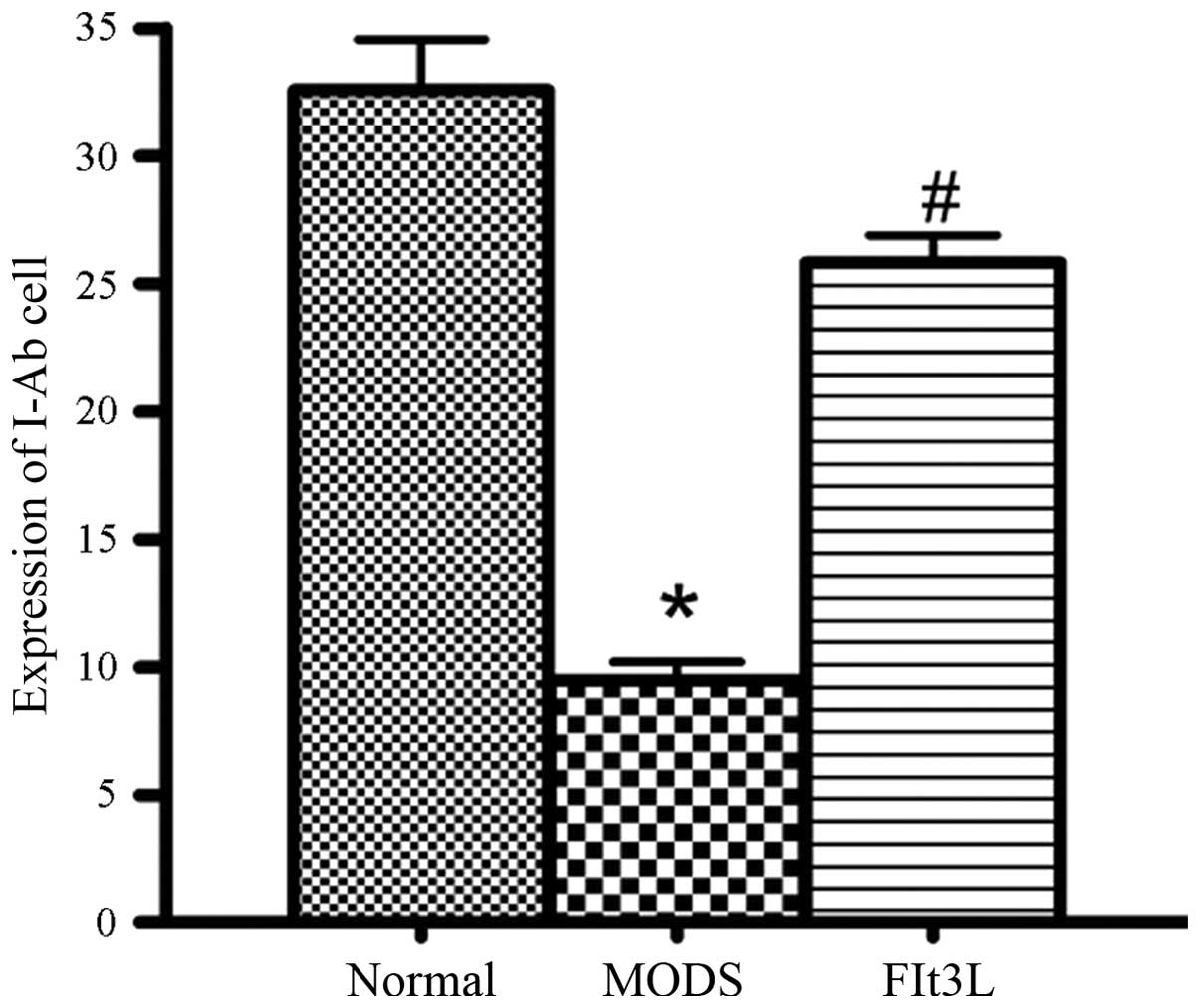
I-Ab labeling
In the MODS group, the I-Ab expression
levels in the peripheral blood were significantly lower than those
in the normal control group (P<0.01). In the Flt3L treatment
group, the I-Ab expression levels in the peripheral
blood were significantly higher than those in the MODS group
(P<0.01) and close to normal levels. This result indicated that
the zymosan-induced reduction in the immune activity of antigen
presenting cells and DCs was restored following Flt3L treatment
(Fig. 3).
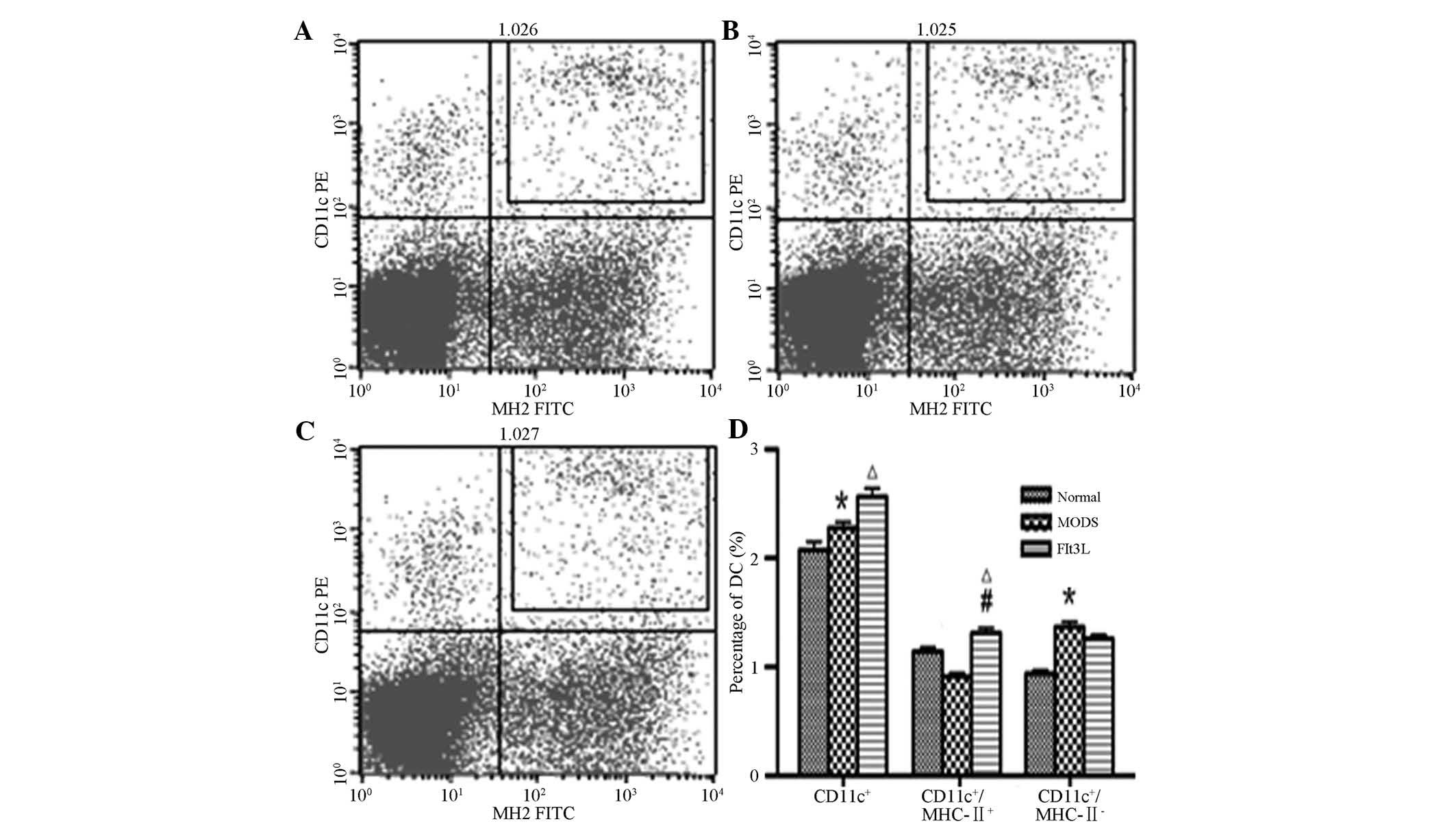
Immunophenotype analysis of splenic
DCs
Compared with the normal control group, the total
number of CD11c+ DCs and the number of immature DCs
(CD11c+/I-Ab−) in the MODS group were
significantly increased (P<0.05). In the Flt3L treatment group,
the total number of CD11c+ DCs along with the number of
mature DCs (CD11c+/I-Ab+) were significantly
increased compared with those in the normal control group
(P<0.05). The number of mature DCs
(CD11c+/I-Ab+) in the Flt3L treatment group
was also significantly increased when compared with that in the
MODS group (P<0.05). No significant differences were identified
(P>0.05) between the numbers of immature DCs
(CD11c+/I-Ab−) in the Flt3L group and those
in the MODS or the normal control group (Fig. 4).
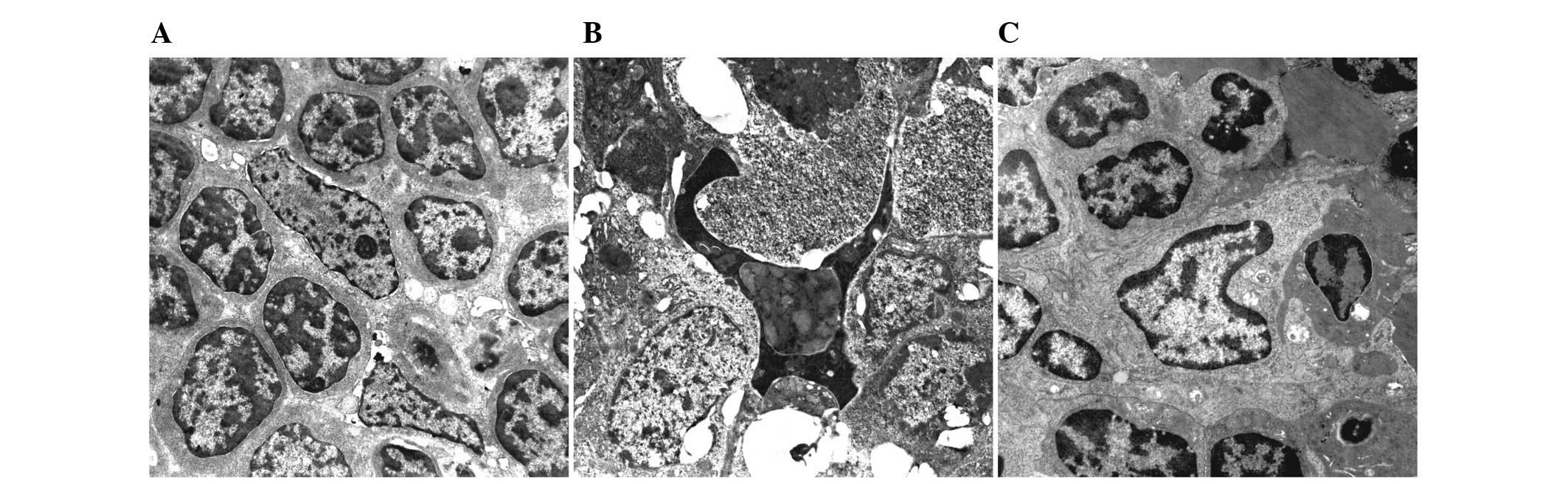
Ultrastructural observation of splenic
DCs
In the normal control group, large volumes of
irregularly or spindle shaped splenic DCs were observed. Other
observations included the formation of several projections on the
cell surface (extending towards gaps in the surrounding
lymphocytes), reduced cytoplasmic electron density, underdeveloped
organelles and lymphocytes centered on DCs (Fig. 5A). On day 12, the majority of
splenic DCs in the MODS group presented with regressive and
apoptotic changes, and the surrounding lymphocytes exhibited
cytolytic and apoptotic degeneration (Fig. 5B). In the Flt3L treatment group,
the splenic DCs were activated, with increased cell bodies,
lengthened projections, increased organelles and lymphocytes
centered on DCs (Fig. 5C).
Discussion
Researchers have conducted a number of studies and
developed clinical applications to prevent the progression of
advanced MODS, however with unsatisfactory results. These
strategies failed as they did not specifically target the
underlying mechanism (1).
Flt3L is a cytokine that promotes the cytopoiesis
and differentiation of various stem cells, blood cells and blood
precursor cells. Flt3L synergistically functions with other
cytokines and is a good amplification agent, although Flt3L does
not influence cell morphology or the cell phenotype. In
vitro studies have demonstrated that Flt3L promoted the
differentiation of CD34+ hemopoietic stem cells from
bone marrow and umbilical blood into DCs, and enhanced DC
amplification resulting from GM-CSF and TNF-α induction, thereby
increasing the number of DCs (15). In vivo studies have revealed
that Flt3L increased the number of DCs in the spleen, bone marrow,
lymph nodes and liver of mice (8,11–17).
The spleen is the largest peripheral immune organ
and is an important site for lymphocytic activation and immune
responses. The functions of peripheral immune organs are crucial in
the maintenance of the immune response and inflammatory reaction
balance.
I-Ab is a histocompatibility type-II
antigen in mice, whose receptors are only present on the surface of
activated antigen-presenting cells. Therefore, I-Ab
expression directly reflects the activation of mouse
antigen-presenting cells. CD11c, a sensitive DC marker, is
expressed in mature and immature DCs. In the present study, the
combined analysis of CD11c and I-Ab markers effectively
differentiated mature DCs from immature DCs.
The surfaces of mononuclear cells in the peripheral
blood of mice with advanced MODS exhibited significantly reduced
I-Ab expression levels (P<0.01) and a considerably
reduced CD4+/CD8+ T lymphocytic ratio
compared with those in the normal control group, which indicated a
severely reduced immune response. In the present study, splenic DCs
still proliferated, but were predominantly immature DCs
(CD11c+/I-Ab−). The splenic DCs were shrunken
and regressed with apoptotic and cytolytic changes. In addition,
white pulp lymphocytes were absent. For the mice in the Flt3L
treatment group, the splenic DC volume and the number of DC cells,
which were predominantly mature DCs
(CD11c+/I-Ab+), were increased in comparison
with those in the normal control group. The I-Ab
expression levels in the peripheral blood mononuclear cells of the
Flt3L treatment group were significantly higher than those in cells
of the MODS group (P<0.01). In addition, the number of white
pulp lymphocytes was evidently increased, along with the number of
CD4+ T cells in the peripheral blood. The
CD4+/CD8+ ratio was normal. Furthermore, the
mortality rate of experimental mice was reduced from 18% in the
MODS group to 7% in the treatment group, which suggests that the
immunological functions in the mice were restored and improved
following Flt3L treatment.
In the early stages of severe infections and MODS,
immune organs and DCs in the peripheral blood exhibit high
proliferation and activation, causing the body to produce an
excessive immune response, thereby initiating MODS. Following a
disease remission period of five to seven days, the number of DCs
in the MODS stage remained increased, but with a markedly reduced
immunological competence. Previous studies (3–5)
considered that immunosuppression during MODS is possibly
associated with massive consumption and apoptosis of DCs and
lymphocytes during the early stages of the disease. Other studies
(20–24) demonstrated that DCs are
dual-directional immune regulators, inducing the immune response
but negatively regulating the response to maintain homeostasis. DCs
are divided into static, active and tolerant DCs as determined by
function. The negative regulatory function of DCs is mainly
performed by tolerant DCs. These induce an immune tolerance under
physiological conditions, and immunosuppression and immunological
paralysis under pathological conditions (25). Tolerant DCs perform negative
regulation by inducing T-cell deactivation, immune response
deflection, regulatory T-cell formation, and promotion of apoptosis
of activated T cells and other routes (15). DCs between the remission stage and
the end stage of MODS (12 days) are inactive or tolerant, and
exhibit a negative regulatory effect, which results in the
deactivation or suppression of T cells. In advanced MODS, DCs are
deactivated and the direction of immune regulation changes.
In the remission stage of MODS, injected Flt3L
reversed the activity of ‘tolerant DCs’ by amplifying the number of
DC precursor cells and activating DC activity. DCs have been
demonstrated to greatly enhance the cytotoxic efficacy towards
tumor cells (26). Flt3L enhanced
the ability of DCs to stimulate T cell activation to restore and
strengthen cellular immunological function. In conclusion, the the
present study suggested that Flt3L injections restore the
immunological functions disrupted by MODS, thereby preventing the
progression of MODS. Therefore, Flt3L may be suitable for further
studies and clinical applications of immune prevention in MODS and
sepsis.
References
|
1
|
Angus DC, Linde-Zwirble WT, Lidicker J,
Clermont G, Carcillo J and Pinsky MR: Epidemiology of severe sepsis
in the United States: analysis of incidence, outcome, and
associated costs of care. Crit Care Med. 29:1303–1310. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lawrence K: Pediatric sepsis and
multiorgan dysfunction syndrome: progress and continued challenges.
Crit Care Nurs Clin North Am. 23:323–337. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bone RC, Sibbald WJ and Sprung CL: The
ACCP/SCCM consensus conference on sepsis and organ failure. Chest.
101:1481–1483. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Davies MG and Hagen PO: Systemic
inflammatory response syndrome. Br J Surg. 84:920–935. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shimada H, Moriwaki Y, Kurosawa H, et al:
Inflammatory mediator and organ dysfunction syndrome. Nippon Geka
Gakkai Zasshi. 99:490–496. 1998.In Japanese.
|
|
6
|
Bone RC: Immunologic dissonance: a
continuing evolution in our understanding of the systemic
inflammatory response syndrome (SIRS) and the multiple organ
dysfunction syndrome (MODS). Ann Intern Med. 125:680–687. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Volk HD, Reinke P, Krausch D, et al:
Monocyte deactivation - rationale for a new therapeutic strategy in
sepsis. Intensive Care Med. 22(Suppl 4): S474–S481. 1996.
View Article : Google Scholar
|
|
8
|
Mentula P, Kylänpää-Bäck ML, Kemppainen E,
et al: Decreased HLA (human leucocyte antigen)-DR expression on
peripheral blood monocytes predicts the development of organ
failure in patients with acute pancreatitis. Clin Sci (Lond).
105:409–417. 2003. View Article : Google Scholar
|
|
9
|
Schefold JC: Measurement of monocytic
HLA-DR (mHLA-DR) expression in patients with severe sepsis and
septic shock: assessment of immune organ failure. Intensive Care
Med. 36:1810–1812. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hotchkiss RS, Swanson PE, Freeman BD, et
al: Apoptotic cell death in patients with sepsis, shock, and
multiple organ dysfunction. Crit Care Med. 27:1230–1251. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hotchkiss RS, Tinsley KW, Swanson PE, et
al: Depletion of dendritic cells, but not macrophages, in patients
with sepsis. J Immunol. 168:2493–2500. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Boomer JS, To K, Chang KC, et al:
Immunosuppression in patients who die of sepsis and multiple organ
failure. JAMA. 306:2594–2605. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Reddy RC, Chen GH, Tekchandani PK and
Standiford TJ: Sepsis-induced immunosuppression: from bad to worse.
Immunol Res. 24:273–287. 2001. View Article : Google Scholar
|
|
14
|
Monneret G, Debard AL, Venet F, et al:
Marked elevation of human circulating CD4+CD25+ regulatory T cells
in sepsis-induced immunoparalysis. Crit Care Med. 31:2068–2071.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jiangyang L, Qian L, Xiaohong W, et al:
Changes of spleen dendritic cells in the terminal stage of multiple
organ dysfunction syndrome. Acta Biomed. 82:146–153. 2011.
|
|
16
|
Shurin MR, Pandharipande PP, Zorina TD, et
al: FLT3 ligand induces the generation of functionally active
dendritic cells in mice. Cell Immunol. 179:174–184. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sakabe H, Kimura T, Zeng Z, et al:
Haematopoietic action of flt3 ligand on cord blood-derived
CD34-positive cells expressing different levels of flt3 or c-kit
tyrosine kinase receptor: comparison with stem cell factor. Eur J
Haematol. 60:297–306. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shaw SG, Maung AA, Steptoe RJ, Thomson AW
and Vujanovic NL: Expansion of functional NK cells in multiple
tissue compartments of mice treated with Flt3-ligand: implications
for anti-cancer and anti-viral therapy. J Immunol. 161:2817–2824.
1998.PubMed/NCBI
|
|
19
|
Wysocka M, Montaner LJ and Karp CL: Flt3
ligand treatment reverses endotoxin tolerance-related
immunoparalysis. J Immunol. 174:7398–7402. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Flohé SB, Agrawal H, Schmitz D, Gertz M,
Flohé S and Schade FU: Dendritic cells during polymicrobial sepsis
rapidly mature but fail to initiate a protective Th1-type immune
response. J Leukoc Biol. 79:473–481. 2006. View Article : Google Scholar
|
|
21
|
Fujita S, Seino K, Sato K, et al:
Regulatory dendritic cells act as regulators of acute lethal
systemic inflammatory response. Blood. 107:3656–3664. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wallet MA, Sen P and Tisch R:
Immunoregulation of Dendritic Cells. Clin Med Res. 3:166–175. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Volman TJ, Hendriks T and Goris RJ:
Zymosan induced generalized inflammation: experimental studies into
mechanisms leading to multiple organ dysfunction syndrome. Shock.
23:291–297. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang M, Tang H, Guo Z, et al: Splenic
stroma drives mature dendritic cells to differentiate into
regulatory dendritic cells. Nat Immunol. 5:1124–1133. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tan C, Reddy V, Dannull J, et al: Impact
of anti-CD25 monoclonal antibody on dendritic cell-tumor fusion
vaccine efficacy in a murine melanoma model. J Transl Med.
11:1482013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fan X, Ye M, Xue B, Ke Y, Wong CK and Xie
Y: Human dendritic cells engineered to secrete interleukin-18
activate MAGE-A3-specific cytotoxic T lymphocytes in vitro. Immunol
Invest. 41:469–483. 2012. View Article : Google Scholar : PubMed/NCBI
|