Introduction
Idiopathic pulmonary fibrosis (IPF) is the most
common fibrotic lung disorder with a poor prognosis and high
mortality rate (1–2). Its pathogenesis is hypothesized to
involve excessive fibroblast proliferation, abnormal
reepithelialization and dysregulated extracellular matrix
accumulation following alveolar injury, leading to progressive
respiratory failure (3). Although
the precise etiology of IPF remains to be elucidated, numerous
previous studies have indicated that IPF may result from an
interstitial inflammatory response to an unknown infection or
injury (4). In addition, markers
of oxidative stress have been identified in patient lungs with IPF
and aberrant antioxidant activity has been demonstrated to
exacerbate pulmonary fibrosis in several animal models (5). Additionally, nitric oxide (NO) has
been observed to lead to acute inflammation in the lung (6). These earlier results suggest that
reactive oxygen species (ROS) and reactive nitrogen species also
have important roles in this disease. Although an antifibrotic
drug, pirfenidone, has been approved for the treatment of IPF in
Japan (7) and Europe (8), searching for novel effective
therapeutic drugs for IPF is still required.
Tanshinone IIA (Tan IIA) is an important lipophilic
diterpene extracted from the root of a Chinese herb termed
Salvia miltiorrhiza Bunge (9). Ample evidence has demonstrated that
Tan IIA exerts antitumoral effects during tumorigenesis of numerous
types of cancer, including prostate cancer (10), colon carcinoma (11) and breast cancer (12). Tan IIA is also used as an effective
remedy for the treatment of cardiovascular (13,14)
and cerebrovascular (15)
diseases. Furthermore, a study by Chen et al (15) has revealed that Tan IIA effectively
inhibits the release of proinflammatory cytokines, including tumor
necrosis factor-α (TNF-α) and interleukin (IL)-6, in a rat model of
cerebral ischemia/reperfusion injury (15), suggesting that Tan IIA possesses
anti-inflammatory potential. Notably, several previous lines of
evidence have demonstrated a therapeutic effect of Tan IIA on
experimental liver fibrosis in rodent models (16,17).
In addition, similar inhibitory effects of Tan IIA on peritoneal
fibrosis have also been reported in a peritoneal dialysis rat model
(18). These previous findings
suggest a protective role of Tan IIA in fibrotic diseases. However,
there is a lack of data regarding the effects of Tan IIA on
fibrotic lung disease.
Therefore, the present study was conducted to
investigate the effects of Tan IIA on IPF in a bleomycin
(BLM)-induced pulmonary fibrosis rat model. The underlying
regulatory mechanisms associated with the potential
anti-inflammatory and antifibrotic effects of Tan IIA were also
investigated.
Materials and methods
Animals and treatment
Sprague-Dawley rats (age, 8 weeks; weight, ~250 g)
were obtained from Dalian Medical University (Dalian, China), and
maintained at a constant room temperature (20–22˚C) and humidity
(50–60%) with a 12 h light/dark cycle, and with access to a
standard diet and tap water ad libitum. All animal
procedures were approved by the institutional animal care and use
committee of Dalian Medical University, which conforms to the
provisions of the Declaration of Helsinki in 1995 (as revised in
Edinburgh 2000).
According to previously described methods (19,20),
a pulmonary fibrosis rat model was reproduced in the present study.
Briefly, rats were anesthetized with an intraperitoneal injection
of 10% chloride hydrate (3.5 ml/kg body weight; Sinopharm Chemical
Reagent Beijing Co., Ltd, Beijing, China) and then administered a
single intratracheal instillation of BLM dissolved in saline (5
mg/kg body weight; Melone Pharmaceutical Co., Ltd, Dalian, China).
Control rats received an equal volume of sterile saline using the
same procedure. To examine the role of Tan IIA in BLM-induced
pulmonary fibrosis pathogenesis, control and BLM-treated rats were
administered daily intraperitoneal injections of Tan IIA(15 mg/kg
body weight; Melone Pharmaceutical Co., Ltd) following BLM
infusion. Accordingly, the rats were divided into four groups (n=25
per group): i) Control group; ii) Tan IIA group, control rats
receiving a daily Tan IIA injection; iii) BLM; iv) BLM+Tan IIA,
BLM-treated rats receiving a daily Tan IIA injection. On day 28
after the initial instillation rats were sacrificed by
intraperintoneal injection of 10% chloral hydrate (5 ml/kg body
weight).
Lung index assay
Rats were anesthetized and sacrificed on day 28, and
their lungs were removed and weighed prior to and following drying
in an incubator at 60˚C for 72 h. The pulmonary edema was
calculated as a wet/dry (W/D) weight ratio.
Bronchoalveolar lavage (BAL)
BAL was performed three times by intratracheal
instillation of 1.5 ml sterile saline in the trachea. The BAL
fluids (BALF) were immediately centrifuged at 500 × g at 4˚C for 15
min. Cell-free supernatant of the first BAL sample was used to
measure the secretion levels of cytokines (TNF-α, IL-1β and IL-6)
using an Enzyme-Linked Immunosorbent Assay (ELISA) kit (USCN Life
Science, Wuhan, China) according to the manufacturer’s
instructions. The cytokine contents were expressed as pg/ml of
BALF. The cell pellet was resuspended in cold phosphate-buffered
saline (PBS; HyClone, Logan, UT, USA) and then subjected to
assessment for total cell counts. To determine the differential
cell counts, 200 cells from each BALF sample were stained with a
Wright-Giemsa Stain kit (Jiancheng Bioengineering Institute,
Nanjing, China), counted under a microscope (DP73; Olympus, Tokyo,
Japan) and expressed as a percentage of total cells.
Histological examination
Following sacrifice, the right lungs were removed
and fixed in 4% paraformaldehyde, dehydrated, and embedded in
paraffin. The paraffin-embedded tissue samples were sectioned into
5-μm slices and then stained with hematoxylin & eosin
(H&E) and Masson’s trichrome, and examined under a light
microscope (DP73; Olympus). The Ashcroft score was used to
determine the degree of fibrosis in lung specimens (21,22).
Immunohistochemical staining
For immunohistochemical staining analysis, the
5-μm slices were deparaffinized in xylene, hydrated with
graded alcohol and then washed with 1% PBS. Subsequently, these
slices were first incubated in 10 mmol/l citrate buffer (pH 6.0) at
100˚C for 10 min to retrieve the antigen and then placed in 3%
hydrogen peroxide for 15 min at room temperature to block the
endogenous peroxidase activity. Following rinsing with 1% PBS three
times, the sections were blocked with goat serum for 30 min,
incubated with rabbit polyclonal primary antibodies against the
cyclooxygenase-2 (COX-2; 1:100 diluted; Wanlei Life Sciences,
Shenyang, China) and inducible nitric oxide synthase (iNOS; 1:100
diluted; Wanlei Life Sciences) at 4˚C overnight. Subsequently, the
sections were washed with 1% PBS, incubated with the corresponding
goat anti-rabbit biotin-labeled secondary antibody (1:200;
Beyotime, Shanghai, China) at 37˚C for 30 min and subsequently
incubated in avidin-horseradish peroxidase complex (Beyotime).
Thereafter, the sections were visualized using
3,3′-diaminobenzidine (Solarbio, Beijing, China) and counterstained
with hematoxylin (Solarbio).
Western blot analysis
Protein extracts from rat lung tissues were prepared
using ice-cold NP-40 lysis buffer (Beyotime). Following
determination of the protein concentration with bicinchoninic acid
protein assay kit (Beyotime), equal quantities of protein samples
were fractionated on SDS-PAGE, transferred onto polyvinylidene
difluoride membranes (Millipore, Bedford, MA, USA), and blocked
with 5% (w/v) skimmed milk (Yili Industrial Group Company, Hohhot,
China). The membranes were blotted with mouse polyclonal antibodies
against COX-2 (1:500 diluted; Wanlei Life Sciences) and iNOS (1:500
diluted; Wanlei Life Sciences) at 4˚C overnight and then incubated
with the corresponding goat anti-rabbit horseradish
peroxidase-conjugated secondary antibodies (1: 5,000 diluted;
Beyotime) at room temperature for 45 min. The protein blots on the
membranes were visualized using an ECL kit (Millipore), and the
band density values were calculated as a ratio to β-actin.
Measurements of NO, prostaglandin E2
(PGE2) and malondialdehyde (MDA) levels in lung tissue samples
NO is usually oxidized to nitrate and nitrite in
vivo (23), therefore the
total NO levels were determined by assessing the sum of nitrate and
nitrite based on the Griess reaction using a total NO assay kit
(Beyotime). A standard curve was established with a set of serial
dilutions of sodium nitrite. NO production levels were expressed as
μmol/mg protein. In addition, the pulmonary protein expression of
prostaglandin E2 (PGE2) was determined using an ELISA, and
expressed as pg/mg protein. MDA content in rat lung tissue samples
was detected using an MDA assay kit (Jiancheng Bioengineering
Institute, Nanjing, China), and expressed in nmol/mg protein.
Statistical analysis
The present data were expressed as the mean ±
standard deviation. Statistical differences among several groups
were determined using a one-way analysis of variance followed by
the Bonferroni post hoc test using SPSS version 17.0 (SPSS,
Chicago, IL, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
Tan IIA attenuates the inflammatory
responses in BLM-induced pulmonary fibrosis
To determine the effect of Tan IIA on BLM-induced
pulmonary inflammation responses in rats, the inflammatory cell
counts in BALF were initially assayed. As indicated in Table I, a significant influx of
inflammatory cells was observed in BALF of the rats with pulmonary
fibrosis, and the percentages of neutrophils and lymphocytes were
also markedly increased. Following the Tan IIA injection,
BLM-induced increases of inflammatory cell counts were markedly
inhibited.
 | Table IEffect of Tan IIA on BLM-induced
changes in total and differential cell counts in the
bronchoalveolar lavage fluid of rats. |
Table I
Effect of Tan IIA on BLM-induced
changes in total and differential cell counts in the
bronchoalveolar lavage fluid of rats.
| Group | Total cells
(×105/ml) | Neutrophils
(%) | Lymphocytes
(%) | Macrophages
(%) |
|---|
| Control | 1.62±0.30 | 8.42±2.15 | 13.00±3.65 | 78.58±5.53 |
| Tan IIA | 1.76±0.29 | 8.75±2.70 | 11.67±4.07 | 79.58±6.70 |
| BLM | 12.10±1.62cd | 24.17±2.88cd | 25.58±3.77cd | 50.25±5.99cd |
| BLM+Tan IIA | 7.41±0.83b | 18.92±3.06a | 19.33±2.71a | 61.75±5.35a |
H&E staining results revealed that, compared
with lung tissues obtained from control or Tan IIA groups, the lung
structure of rats from the BLM-treated group was severely damaged
and the lung interstitium was evidently thickened (Fig. 1A). These pathological changes were
attenuated by the intraperitoneal injection of Tan IIA. Since the
thickening of the lung interstitium may due to extra fluid (edema)
or inflammation (24), the lung
W/D ratio and the secretion levels of the pro-inflammatory factors
in the experimental rats were subsequently detected. It was noted
that BLM-induced pulmonary edema and elevation of TNF-α, IL-1β and
IL-6 in BALF were alleviated by Tan IIA injection (Fig. 1B and C). Collectively, these
results suggested that Tan IIA may protect the lung against the
deleterious inflammation induced by BLM.
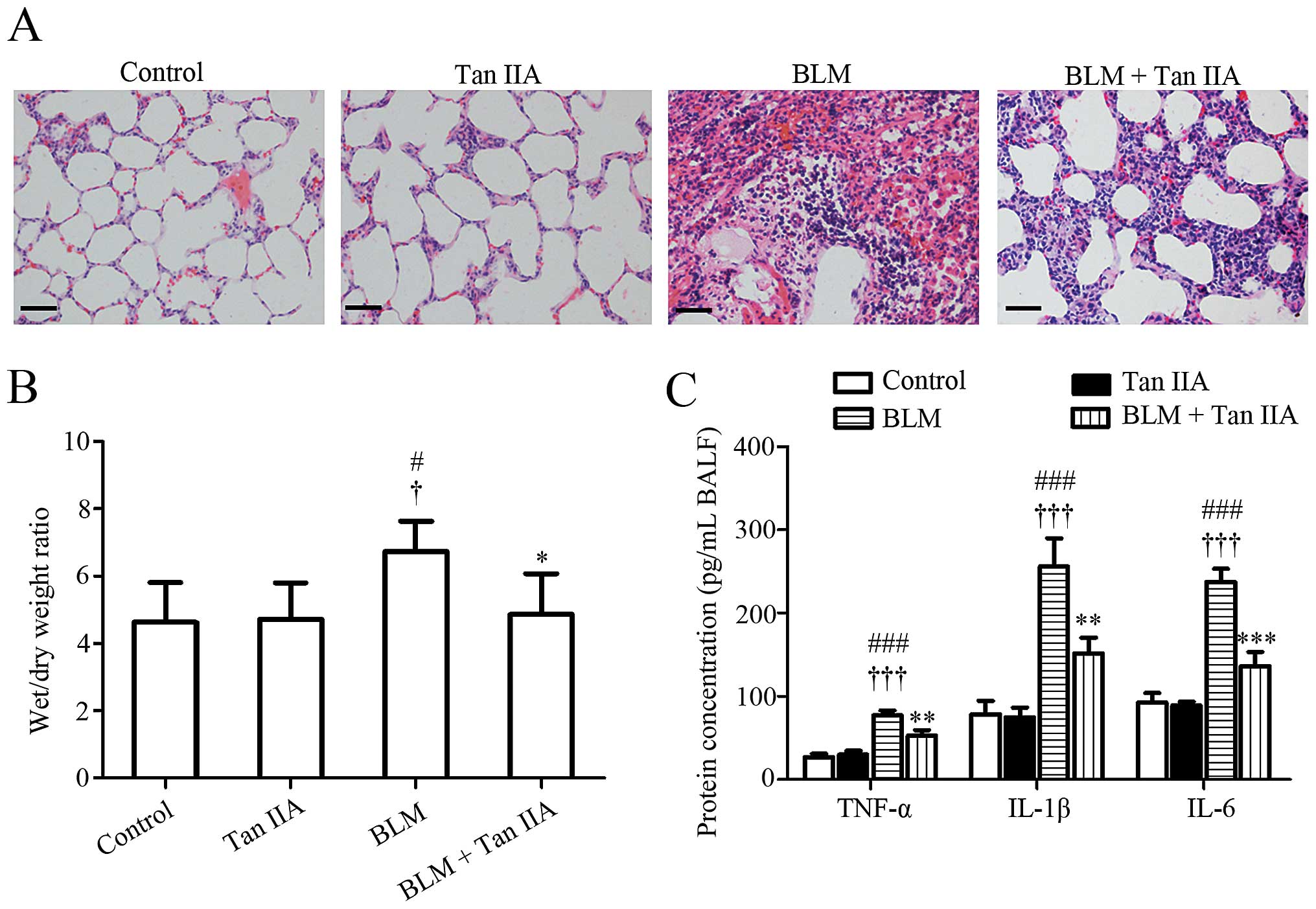 | Figure 1Effects of Tan IIA on histological
changes of rat lung tissues. (A) Representative images of
hematoxylin & eosin staining, scale bars=50 μm. (B)
Wet/dry weight ratio of rat lung (n=6 per group). (C) Secretion
levels of TNF-α, IL-1β and IL-6 in BALF quantified by ELISA (n=3
per group). Results are expressed as the mean ± standard deviation.
#P<0.05 and ###P<0.001, versus the
control group; †P<0.05 and †††P<0.001,
versus the Tan IIA group; *P<0.05, **P<0.01 and
***P<0.001, versus the BLM group. TNF-α, tumor
necrosis factor-α; IL-1β, interleukin 1β; IL-6, interleukin-6;
BALF, bronchoalveolar lavage fluids; Tan IIA, Tanshinone IIA; BLM,
bleomycin. |
Tan IIA mitigates BLM-induced pulmonary
fibrosis in rats
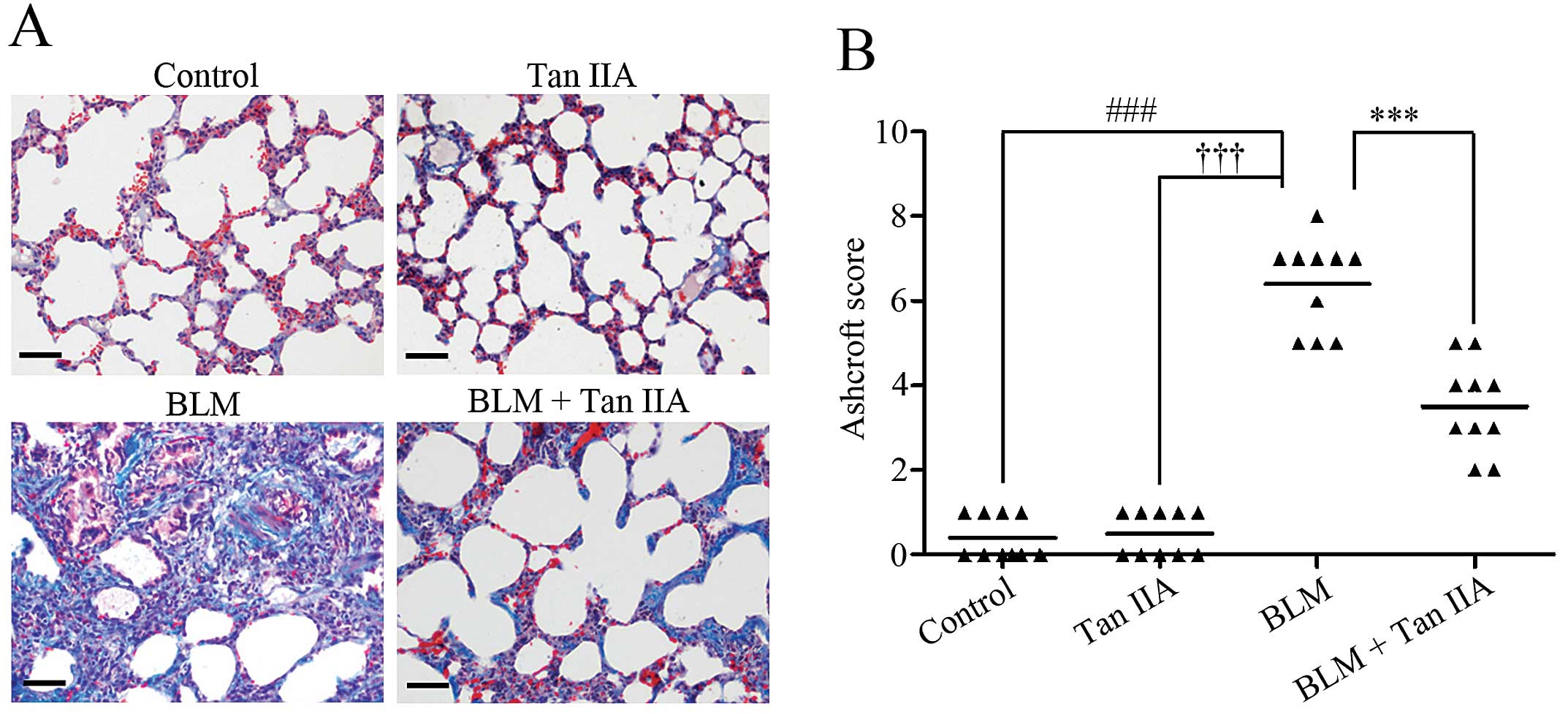
Masson’s trichrome staining results revealed severe
pulmonary fibrosis in BLM-treated rats (Fig. 2A). Following injection of Tan IIA,
the area of collagen deposition was markedly reduced in rats
treated with BLM (Fig. 2A). The
Ashcroft score was then used to determine the extent of lung
fibrosis. The data indicated that, following Tan IIA
administration, the score of rats with pulmonary fibrosis was
significantly decreased (Fig. 2B).
These findings indicated an antifibrotic effect of Tan IIA on
pulmonary fibrosis in rats.
Tan IIA attenuates oxidative stress in
BLM-induced pulmonary fibrosis in rats
Numerous studies have observed that oxidative stress
is involved in the fibrotic processes of various organs, including
the lung (25). Since
reduction-oxidation products are difficult to measure directly in
tissues, the effect of Tan IIA on oxidative stress was evaluated by
detecting the expression of several key factors implicated in this
reaction, including COX-2, PGE2 and MDA. Data from
immunohistochemistry (Fig. 3A) and
western blot analyses (Fig. 3B)
indicated that pulmonary COX-2 protein expression increased
following BLM treatment, but decreased by the following Tan -A
injection. In addition, the ELISA results revealed that the
expression pattern of COX-2 enzymatic product, PGE2 (26) paralleled that of COX-2 in rat lungs
(Fig. 3C). Additionally,
BLM-induced elevated expression of the final product of
polyunsaturated fatty acid peroxidation, MDA (27), was also inhibited by Tan -A
administration (Fig. 3D). These
results revealed that Tan IIA may suppress the abnormal oxidative
reaction caused by BLM in rat lungs.
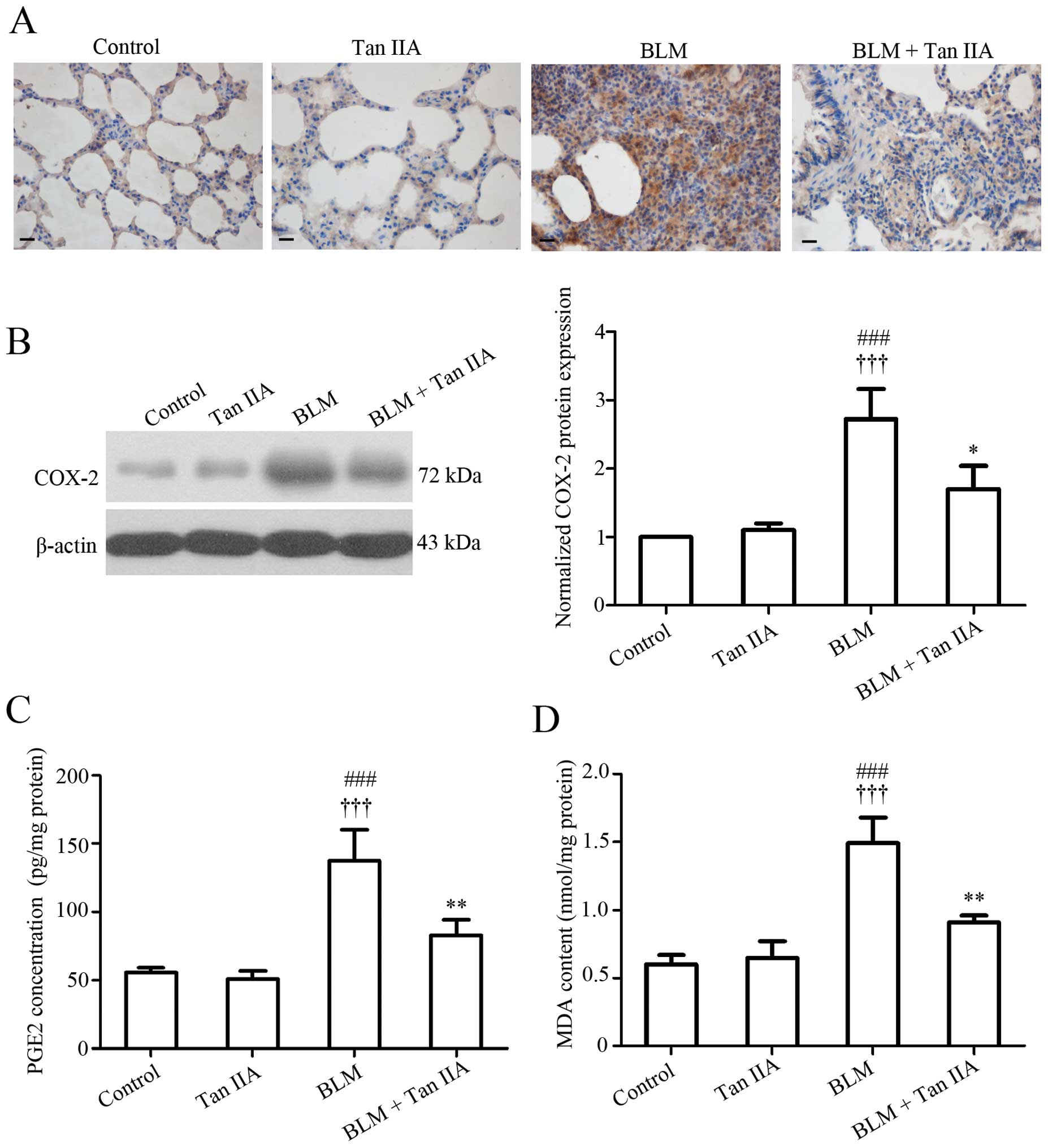 | Figure 3Effects of Tan IIA on oxidative
stress-associated factors in lung tissue. (A) Representative images
of immunohistochemical staining for COX-2 protein in lung tissue
samples, scale bars=20 μm. (B) COX-2 protein expression in
lung tissue specimens determined by western blot analysis. (C) PGE2
expression and (D) MDA concentration in lung tissues. Results are
expressed as the mean ± standard deviation, n=3 per group.
###P<0.001, versus the control group;
†††P<0.001, versus the Tan IIA group; *P<0.05 and
***P<0.001 versus the BLM group. COX-2, cyclooxygenase-2, PGE2,
prostaglandin E2; MDA, malondialdehyde; Tan IIA, Tanshinone IIA;
BLM, bleomycin. |
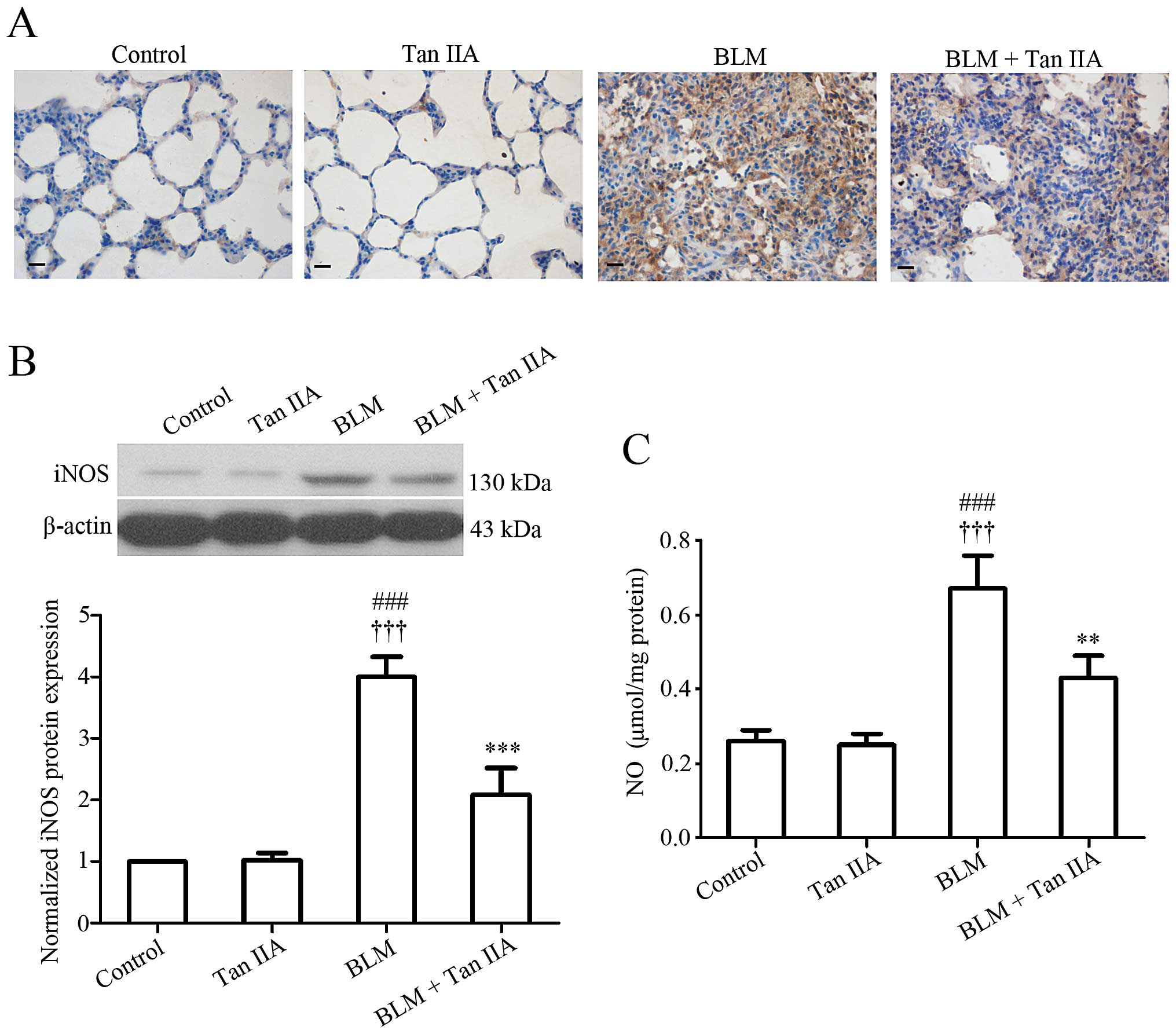
Tan IIA inhibits iNOS expression and NO
production in BLM-induced pulmonary fibrosis
Regarding the potent implication of iNOS-derived NO
in the pathogenesis of human pulmonary fibrosis (28), iNOS protein expression and NO
production level were measured in rat lung tissue samples.
Immunodetection (Fig. 4A) and
western blot analysis (Fig. 4B)
indicated that Tan IIA injection induced a significant reduction of
iNOS expression in rats treated with BLM. In addition, the ELISA
results revealed that the pulmonary NO level was increased
following BLM instillation, but decreased after Tan IIA treatment
(Fig. 4C). The present data
suggested that Tan IIA injection may reduce BLM-induced excessive
NO production in rat lungs.
Discussion
BLM-induced pulmonary fibrosis is a well-established
disease model for IPF and widely used in the investigation of the
efficacy and mechanism of therapeutic candidates (29,30).
In the present study, pulmonary fibrosis was induced in rats by
one-off instillation of BLM, and the potential effects of Tan IIA
on the BLM-induced fibrotic lesions of rat lungs were determined.
The present data indicated that administration of Tan IIA reduced
BLM-induced inflammatory cell infiltration, pro-inflammatory
cytokine release and excessive pulmonary collagen deposition in rat
lung tissues. In addition, COX-2-associated oxidative reaction and
iNOS-derived NO production in the BLM treated rats were also
inhibited by Tan IIA injection.
Following BLM administration, increased numbers of
inflammatory cells were observed in the BALF of rats. In addition,
the secretion of TNF-α, IL-1β and IL-6 in rat lung tissue samples
was enhanced following BLM treatment. Pulmonary edema and fibrosis
were also detected in BLM-treated rats. The aforementioned
observations confirmed the validity of the BLM-induced pulmonary
fibrotic lesion model. These pathological alterations induced by
BLM were significantly attenuated by Tan IIA injection. Although
similar anti-inflammatory and antifibrotic effects of Tan IIA have
been reported in previous studies (17,31–33),
to the best of our knowledge, this is the first study demonstrating
a therapeutic effect of Tan IIA in pulmonary fibrosis.
The lungs are constantly exposed to relatively
higher oxygen tensions than other organs. The exogenous oxidants
may increase oxidant production and activate inflammatory cells to
generate free radicals in the lungs (34). Oxidative stress is an imbalance
between the generation of ROS and the capacity to detoxify these
intermediates (35), and it has a
major role in the pathogenesis of pulmonary fibrosis (25). A previous study demonstrated that
Tan IIA inhibits angiotensin II-induced ROS formation in rat
cardiac fibroblasts (36).
Additionally, Tan IIA has also been demonstrated to exert
protective effects in rat kidneys by attenuating oxidative stress
injury (37). However, by
contrast, a study by Chiu and Su (38) has indicated that Tan IIA promotes
the production of ROS in human lung cancer cells (38). These earlier studies suggest
inconsistent effects of Tan IIA on oxidative reactions. To address
this issue, the expression levels of certain key factors involved
in oxidative stress were examined. It was noted that BLM-induced
increased expression of redox-responsive COX-2 and its enzymatic
product PGE2 in rat lungs was significantly inhibited by Tan IIA
injection. Additionally, BLM-induced elevation of the oxidative
stress biomarker, MDA (39), in
rat lungs were also reduced following Tan IIA treatment. The
present findings revealed an anti-oxidative effect of Tan IIA in
pulmonary fibrosis, which was supported by several previously
reported studies (40–42). However, the exact mechanisms
underlying the regulatory role of Tan IIA in the oxidative reaction
in pulmonary fibrosis require further investigation.
In addition to ROS, an overproduction of NO also has
an important role in various disease models, including BLM-induced
fibrotic lung disease (43). An
earlier study demonstrated that inhibition or knockout of iNOS
results in resistance to BLM-induced lesions in mice (44). Based on these results, it was
hypothesized that Tan IIA may inhibit iNOS expression and the
subsequent NO production in BLM-induced pulmonary fibrosis. The
results confirmed this hypothesis by demonstrating a significant
reduction of pulmonary iNOS expression and NO production levels in
BLM-treated rats following Tan IIA injection, corresponding to
previous lines of evidence (32,45).
In addition, considering evidence that the iNOS-derived NO affects
the lung inflammatory response in mice (46), Tan IIA may exert its
anti-inflammatory effect by modulating NO production during the
development of pulmonary fibrosis.
In conclusion, the present study demonstrates that
administration of Tan IIA reduces BLM-induced inflammatory cell
infiltration, pro-inflammatory cytokine release and excessive
collagen deposition in the rat lung. Additionally, abnormal
oxidative reactions and NO production in the BLM-treated rats are
inhibited by Tan IIA administration. The present findings therefore
suggest a potential protective effect of Tan IIA against pulmonary
fibrosis.
Acknowledgments
This study was supported by a grant from the
National Natural Science Foundation of China (no. 81273924).
References
|
1
|
Wilson MS and Wynn TA: Pulmonary fibrosis:
pathogenesis, etiology and regulation. Mucosal Immunol. 2:103–121.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
du Bois RM: Strategies for treating
idiopathic pulmonary fibrosis. Nat Rev Drug Discov. 9:129–140.
2010. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wynn TA and Ramalingam TR: Mechanisms of
fibrosis: therapeutic translation for fibrotic disease. Nat Med.
18:1028–1040. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bringardner BD, Baran CP, Eubank TD and
Marsh CB: The role of inflammation in the pathogenesis of
idiopathic pulmonary fibrosis. Antioxid Redox Signal. 10:287–301.
2008. View Article : Google Scholar
|
|
5
|
Kliment CR and Oury TD: Oxidative stress,
extracellular matrix targets, and idiopathic pulmonary fibrosis.
Free Radic Biol Med. 49:707–717. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fubini B and Hubbard A: Reactive oxygen
species (ROS) and reactive nitrogen species (RNS) generation by
silica in inflammation and fibrosis. Free Radic Biol Med.
34:1507–1516. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
No authors listed. Regulatory watch: First
drug for idiopathic pulmonary fibrosis approved in Japan. Nat Rev
Drug Discov. 7:966–967. 2008. View
Article : Google Scholar
|
|
8
|
Moran N: p38 kinase inhibitor approved for
idiopathic pulmonary fibrosis. Nat Biotechnol. 29:3012011.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhou L, Zuo Z and Chow MS: Danshen: an
overview of its chemistry, pharmacology, pharmacokinetics and
clinical use. J Clin Pharmacol. 45:1345–1359. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chiu SC, Huang SY, Chen SP, Su CC, Chiu TL
and Pang CY: Tanshinone IIA inhibits human prostate cancer cells
growth by induction of endoplasmic reticulum stress in vitro and in
vivo. Prostate Cancer Prostatic Dis. 16:315–322. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shan YF, Shen X, Xie YK, et al: Inhibitory
effects of tanshinone II-A on invasion and metastasis of human
colon carcinoma cells. Acta Pharmacol Sin. 30:1537–1542. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yu T, Zhou Z, Mu Y, de Lima Lopes G and
Luo KQ: A novel anti-cancer agent, acetyltanshinone IIA, inhibits
oestrogen receptor positive breast cancer cell growth by
down-regulating the oestrogen receptor. Cancer Lett. 346:94–103.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hong HJ, Liu JC, Cheng TH and Chan P:
Tanshinone IIA attenuates angiotensin II-induced apoptosis via Akt
pathway in neonatal rat cardiomyocytes. Acta Pharmacol Sin.
31:1569–1575. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gao S, Liu Z, Li H, Little PJ, Liu P and
Xu S: Cardiovascular actions and therapeutic potential of
tanshinone IIA. Atherosclerosis. 220:3–10. 2012. View Article : Google Scholar
|
|
15
|
Chen Y, Wu X, Yu S, et al: Neuroprotection
of tanshinone IIA against cerebral ischemia/reperfusion injury
through inhibition of macrophage migration inhibitory factor in
rats. PLoS One. 7:e401652012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu Y, Chen H and Jiang Y: Effect of
tanshinone IIA on CCl4-induced liver fibrosis in rats.
Zhong Yao Cai. 25:31–33. 2002.In Chinese.
|
|
17
|
Sun RF, Liu LX and Zhang HY: Effect of
tanshinone II on hepatic fibrosis in mice. Zhongguo Zhong Xi Yi Jie
He Za Zhi. 29:1012–1017. 2009.
|
|
18
|
Chunming J, Miao Z, Cheng S, et al:
Tanshinone IIA attenuates peritoneal fibrosis through inhibition of
fibrogenic growth factors expression in peritoneum in a peritoneal
dialysis rat model. Ren Fail. 33:355–362. 2011.In Chinese.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Walters DM and Kleeberger SR: Mouse models
of bleomycin-induced pulmonary fibrosis. Curr Protoc Pharmacol
Chapter. 5:462008.
|
|
20
|
Wu Z, Yang L, Cai L, et al: Detection of
epithelial to mesenchymal transition in airways of a bleomycin
induced pulmonary fibrosis model derived from an alpha-smooth
muscle actin-Cre transgenic mouse. Respir Res. 8:12007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ashcroft T, Simpson JM and Timbrell V:
Simple method of estimating severity of pulmonary fibrosis on a
numerical scale. J Clin Pathol. 41:467–470. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hübner RH, Gitter W, El Mokhtari NE, et
al: Standardized quantification of pulmonary fibrosis in
histological samples. Biotechniques. 44:507–511. 514–507. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Savas HA, Gergerlioglu HS, Armutcu F, et
al: Elevated serum nitric oxide and superoxide dismutase in
euthymic bipolar patients: impact of past episodes. World J Biol
Psychiatry. 7:51–55. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Dongaonkar RM, Laine GA, Stewart RH and
Quick CM: Balance point characterization of interstitial fluid
volume regulation. Am J Physiol Regul Integr Comp Physiol.
297:R6–R16. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cheresh P, Kim SJ, Tulasiram S and Kamp
DW: Oxidative stress and pulmonary fibrosis. Biochim Biophys Acta.
1832:1028–1040. 2013. View Article : Google Scholar :
|
|
26
|
Greenhough A, Smartt HJ, Moore AE, et al:
The COX‑2/PGE2 pathway: key roles in the hallmarks of cancer and
adaptation to the tumour microenvironment. Carcinogenesis.
30:377–386. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gawel S, Wardas M, Niedworok E and Wardas
P: Malondialdehyde (MDA) as a lipid peroxidation marker. Wiad Lek.
57:453–455. 2004.In Polish.
|
|
28
|
Liu L, Lu W, Ma Z and Li Z: Oxymatrine
attenuates bleomycin-induced pulmonary fibrosis in mice via the
inhibition of inducible nitric oxide synthase expression and the
TGF-β/Smad signaling pathway. Int J Mol Med. 29:815–822.
2012.PubMed/NCBI
|
|
29
|
Song JS, Kang CM, Kang HH, et al:
Inhibitory effect of CXC chemokine receptor 4 antagonist AMD3100 on
bleomycin induced murine pulmonary fibrosis. Exp Mol Med.
42:465–472. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chen YL, Zhang X, Bai J, et al: Sorafenib
ameliorates bleomycin-induced pulmonary fibrosis: potential roles
in the inhibition of epithelial mesenchymal transition and
fibroblast activation. Cell Death Dis. 4:e6652013. View Article : Google Scholar
|
|
31
|
Dong X, Dong J, Zhang R, Fan L, Liu L and
Wu G: Anti-inflammatory effects of tanshinone IIA on
radiation-induced microglia BV-2 cells inflammatory response.
Cancer Biother Radiopharm. 24:681–687. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Fan GW, Gao XM, Wang H, et al: The
anti-inflammatory activities of Tanshinone IIA, an active component
of TCM, are mediated by estrogen receptor activation and inhibition
of iNOS. J Steroid Biochem Mol Biol. 113:275–280. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Fang J, Xu SW, Wang P, et al: Tanshinone
II-A attenuates cardiac fibrosis and modulates collagen metabolism
in rats with renovascular hypertension. Phytomedicine. 18:58–64.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kinnula VL, Fattman CL, Tan RJ and Oury
TD: Oxidative stress in pulmonary fibrosis: a possible role for
redox modulatory therapy. Am J Respir Crit Care Med. 172:417–422.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Todd NW, Luzina IG and Atamas SP:
Molecular and cellular mechanisms of pulmonary fibrosis.
Fibrogenesis Tissue Repair. 5:112012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chan P, Liu JC, Lin LJ, et al: Tanshinone
IIA inhibits angiotensin II-induced cell proliferation in rat
cardiac fibroblasts. Am J Chin Med. 39:381–394. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhang X, He D, Xu L and Ling S: Protective
effect of tanshinone IIA on rat kidneys during hypothermic
preservation. Mol Med Rep. 5:405–409. 2012.
|
|
38
|
Chiu TL and Su CC: Tanshinone IIA induces
apoptosis in human lung cancer A549 cells through the induction of
reactive oxygen species and decreasing the mitochondrial membrane
potential. Int J Mol Med. 25:231–236. 2010.PubMed/NCBI
|
|
39
|
Nielsen F, Mikkelsen BB, Nielsen JB,
Andersen HR and Grandjean P: Plasma malondialdehyde as biomarker
for oxidative stress: reference interval and effects of life-style
factors. Clin Chem. 43:1209–1214. 1997.PubMed/NCBI
|
|
40
|
Jeon SJ, Son KH, Kim YS, Choi YH and Kim
HP: Inhibition of prostaglandin and nitric oxide production in
lipopolysaccharide-treated RAW 264.7 cells by tanshinones from the
roots of Salvia miltiorrhizabunge. Arch Pharm Res. 31:758–763.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Sun D, Shen M, Li J, et al:
Cardioprotective effects of tanshinone IIA pretreatment via kinin
B2 receptor-Akt-GSK-3beta dependent pathway in experimental
diabetic cardiomyopathy. Cardiovasc Diabetol. 10:42011. View Article : Google Scholar
|
|
42
|
Dong K, Xu W, Yang J, Qiao H and Wu L:
Neuroprotective effects of Tanshinone IIA on permanent focal
cerebral ischemia in mice. Phytother Res. 23:608–613. 2009.
View Article : Google Scholar
|
|
43
|
Hsu YC, Wang LF and Chien YW: Nitric oxide
in the pathogenesis of diffuse pulmonary fibrosis. Free Radic Biol
Med. 42:599–607. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Genovese T, Cuzzocrea S, Di Paola R, et
al: Inhibition or knock out of inducible nitric oxide synthase
result in resistance to bleomycin-induced lung injury. Respir Res.
6:582005. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
hen TH, Hsu YT, Chen CH, Kao SH and Lee
HM: Tanshinone IIA from Salvia miltiorrhizainduces heme oxygenase-1
expression and inhibits lipopolysaccharide-induced nitric oxide
expression in RAW 264.7 cells. Mitochondrion. 7:101–105. 2007.
View Article : Google Scholar
|
|
46
|
Speyer CL, Neff TA, Warner RL, et al:
Regulatory effects of iNOS on acute lung inflammatory responses in
mice. Am J Pathol. 163:2319–2328. 2003. View Article : Google Scholar : PubMed/NCBI
|