Introduction
Spinal cord injury (SCI) is a serious complication
of vertebral fractures. Primary SCI, caused by vertebral fracture,
results in an irreversible mechanical injury to the spinal cord
(1). Secondary SCI is often a
sequel to the primary injury that results from arterial disruption,
electrolyte imbalance, thrombosis or hypoperfusion due to shock.
Secondary SCI is associated with complex pathological and
physiological processes that have widespread consequences. For this
reason, secondary SCI may be more harmful to the nervous system
than the primary injury.
Secondary SCI is both preventable and reversible.
Physical therapy and the early application of drug therapy can have
a significant impact on patient prognosis. However, to date,
therapeutic options for secondary SCI management are limited and
the development of clinical and therapeutic modalities based on new
technology is the subject of ongoing research (2).
The roles of apoptosis and necrosis in secondary SCI
have been reported previously (2).
Neuronal and glial cell death does not appear to be due to direct
damage. Studies using animal models and human tissue samples have
revealed that cell damage is associated with the apoptosis of
neuronal and glial cells, and that these processes may be key
factors in secondary SCI (3,4).
The extent of apoptosis and cavity formation in the
spinal cord after secondary SCI are important factors that require
further investigation. These changes have been shown to decrease
when hemorrhaging is reduced by drug treatment (5). Other research has demonstrated that
inflammation occurs following secondary SCI, leading to the
abnormal apoptosis of neuronal and glial cells, thereby
exacerbating the situation.
Increases in the mRNA levels of the cytokine factor
monocyte chemoattractant peptide-1 (MCP-1) have been observed
following the occurrence of secondary SCI (6). This finding may shed light on the
mechanism that precipitates tissue inflammation (7).
In the present study, an astrocyte cell line from
rat brain tissue and a rat model of Nyström’s posterior spinal cord
compression injury were used to explore the association between
MCP-1 expression and secondary SCI.
Materials and methods
Cell culture
Whole brains harvested from newborn rats under
sterile conditions were placed in D-Hank’s solution containing 10%
fetal bovine serum and 5% DMEM-F12 (Gibco, Carlsbad, CA, USA). The
pallium was separated under a dissecting microscope and the
resulting tissue was exposed to trypsin. Cells were collected by
centrifugation and plated onto Petri dishes at a density of
1.5×107 cells per dish. The cells were cultured for 10
days at 37°C in the presence of 5% CO2 and 95%
O2. High purity astrocytes were prepared following
separation, purification and subculture.
Animal models
Healthy, closed population, male Sprague-Dawley (SD)
rats weighing 260–300 g were used for the animal models. The rats
were given free access to food and water in natural light at room
temperature. Improved Nyström surgery was performed in order to
produce the spinal cord injury by applying compression for 5 min
with a 35-g weight. Following surgery, the rats were raised
separately at room temperature. In the control group, the lamina of
the vertebral arch was removed to expose the dura mater. A total of
80 SD rats were used to establish the animal model. In total, 20
rats died; four during the breeding period, six during anesthesia
for surgery and ten during the observation period once the model
had been established. The 60 surviving rats were randomly divided
into five groups, with 12 rats in each group; group 1 (untreated
group), group 2 (sham surgery group), group 3 (rat spinal cord
compressed for 1 min), group 4 (rat spinal cord compressed for 5
min) and group 5 (rat spinal cord compressed for 10 min). All
animal studies were approved by the Institutional Animal Care and
Use Committee of the Second Military Medical University (Shanghai,
China) and all experimental procedures were carried out in
accordance with institutional guidelines.
RNA manipulation studies
A small interfering RNA expression vector, pSilencer
3.1-H1 puro (Ambion, Foster City, CA, USA) containing MCP-1 and
MCP-1β targeting sequences, and appropriate scrambled controls were
constructed according to the manufacturer’s instructions. In order
to select the optimum targeting sequences, the MCP-1 coding
sequences were submitted to the Ambion siRNA target finder website
(http://www.ambion.de/en/) for prediction. The
following two small interfering (siRNA) sequences and a scramble
siRNA, as a negative siRNA control, were used for the RNA
interference experiments: MCP-1, 5′-GAT CCC GTGCCCCACTCACCT GCTGCT
TCA AGA GAG CAGC AGG TGA GTG GGG CAC TTT TTT GGA AA-3′ and 5′-AGC
TTT TCC AAA AAA GTG CCC CAC TCA CCT GCTG CTC TCT TGA AGC AGC
AGGTGAGTGGGGCACGG-3′; and scramble siRNA,
5′-ATGGACAGAATAAATGGACTT-3′. Constructed pSilencer-MCP-1 and
pSilencer-MCP-1β were transfected into the astrocyte cell line
using Lipofectin (Invitrogen, Carlsbad, CA, USA).
Protein and mRNA quantification
Changes in the expression of MCP-1 and MCP-1β were
confirmed by the measurement of mRNA and protein levels. Total RNA
was extracted from cell line samples using TRIzol reagent
(Invitrogen) according to the manufacturer’s instructions. Reverse
transcription polymerase chain reaction (RT-PCR) was used to
evaluate the mRNA expression levels. The primers used were as
follows (100 nM each): MIP-1β upstream,
5′-TCTGCGATTCAGTGCTGTCAGC-3′ and downstream,
5′-GATTTGCCTGCCTTTTTTGGTC-3′; MCP-1 upstream,
5′-CCTGTTGTTCACAGTTGCTGCC-3′ and downstream,
5′-TCTACAGAAGTGCTTGAGGTGGTTG-3′; and β-actin upstream,
5′-ATGGTGGGTATGGGTCAGAA-3′ and downstream,
5′-GCTGTGGTGGTGAAGCTGTA-3′. Cell extracts were prepared in NP-40
lysis buffer (0.1% NP-40, 150 mM NaCl, 1 mM EDTA, 50 mM Tris pH
8.0, 1 mM sodium vanadate, 1 mM phenylmethylsulfonyl fluoride)
supplemented with a protease inhibitor cocktail (Sigma, St. Louis,
MO, USA; P-8340). Protein concentrations were determined using a
Bradford assay (Bio-Rad, Hercules, CA, USA). Equivalent amounts of
protein (10–30 μg) were electrophoretically resolved using
SDS-PAGE on 6–10% gels. MCP-1 and MCP-1β primary antibodies were
used for western blotting to detect the protein expression
levels.
Immunohistochemistry
The astrocyte cell line and rat tissues were
subjected to immunohistochemistry. Paraffin-embedded sections were
cleared and incubated at 37°C for 10 min with 0.1% Pronase (Roche,
Mannheim, Germany; #165 921) in 0.1% CaCl2 at pH 7.8.
The suspension was blocked by exposure to 3% hydrogen peroxide in
TBST for 10 min. The tissues were further washed and blocked with a
biotin blocking system (Dako, Carpinteria, CA, USA; X0590),
followed by washing and exposure to 10% normal rabbit serum for 10
min at room temperature. The cells were then incubated for 1 h at
room temperature with polyclonal rabbit anti-mouse MCP-1 antibody
(1:800 diluted; Santa Cruz Biotechnology, Inc., Dallas, TX, USA).
This was followed by incubation for 30 min at room temperature with
biotinylated rabbit anti-mouse serum (1:100 diluted; Dako Denmark
A/S, Glostrup, Denmark) and finally by incubation for 30 min at
room temperature with Strep-ABC complex (1:100 diluted; Dako
Denmark A/S). The sections were developed for 20 min at room
temperature using an AEC substrate kit (Vector Lab, Burlingame, CA,
USA; SK-4200), counterstained with hematoxylin and, following
drying, were mounted with Dako aqueous mount (Dako Denmark
A/S).
Terminal deoxynucleotidyl transferase
dUTP nick end labeling (TUNEL) assay
TUNEL is a common method for detecting the DNA
fragmentation that results from apoptotic signaling cascades. The
technique was applied to an astrocyte cell smear and tissue samples
using an in situ cell death detection kit (Roche). Cell
smear preparation, tissue sample treatment and TUNEL analysis were
performed according to the manufacturer’s instructions. Cells were
mounted under a glass coverslip and analyzed with a fluorescence
microscope to observe apoptosis.
Results and Discussion
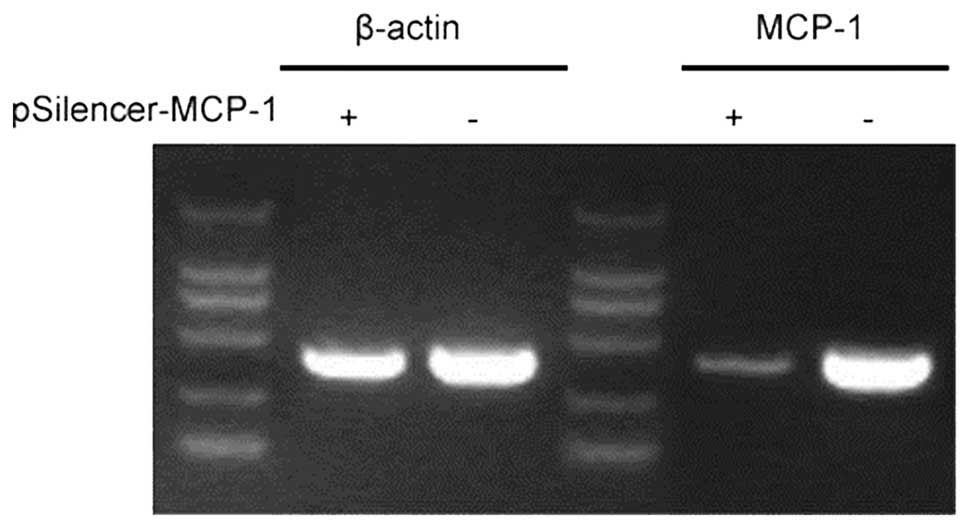
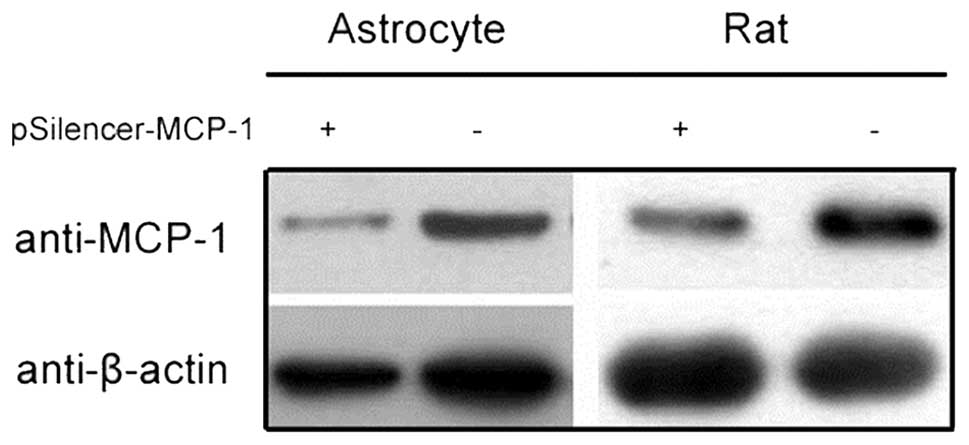
RNA inhibition (RNAi) plasmid inhibits
MCP-1 expression
MCP-1 plays an important role in the inflammatory
response induced by secondary SCI (6,8).
RNAi decreased the expression of MCP-1 in animal models and
astrocyte cell lines (Figs. 1 and
2). This finding provides a basis
for further studies investigating the effect of MCP-1 expression on
SCI.
Inflammatory response after secondary
SCI
Astrocytes are a major cell type found in the
cerebrum. These cells constitute an inner microenvironment
essential for neuron development. They also contain a number of
neurotransmitters, which facilitate neuronal conduction. It has
been reported that astrocytes in the region of the blood-brain
(spinal cord) barrier are involved in inflammatory immune responses
in the brain and spinal cord. These cells excrete inflammatory
chemokines involved in the recruitment of peripheral blood
monocytes, macrophages and T lymphocytes (9–11).
It has previously been demonstrated that an
inflammatory response occurs during the first few hours after SCI.
This involves activation of the remaining astrocytes and the
infiltration of peripheral inflammatory cells; the majority of
these are neutrophils, granulocytes, monocytes and macrophages
(12). Glial cells and vascular
endothelial cells in the central nervous system produce
inflammatory cytokines (13).
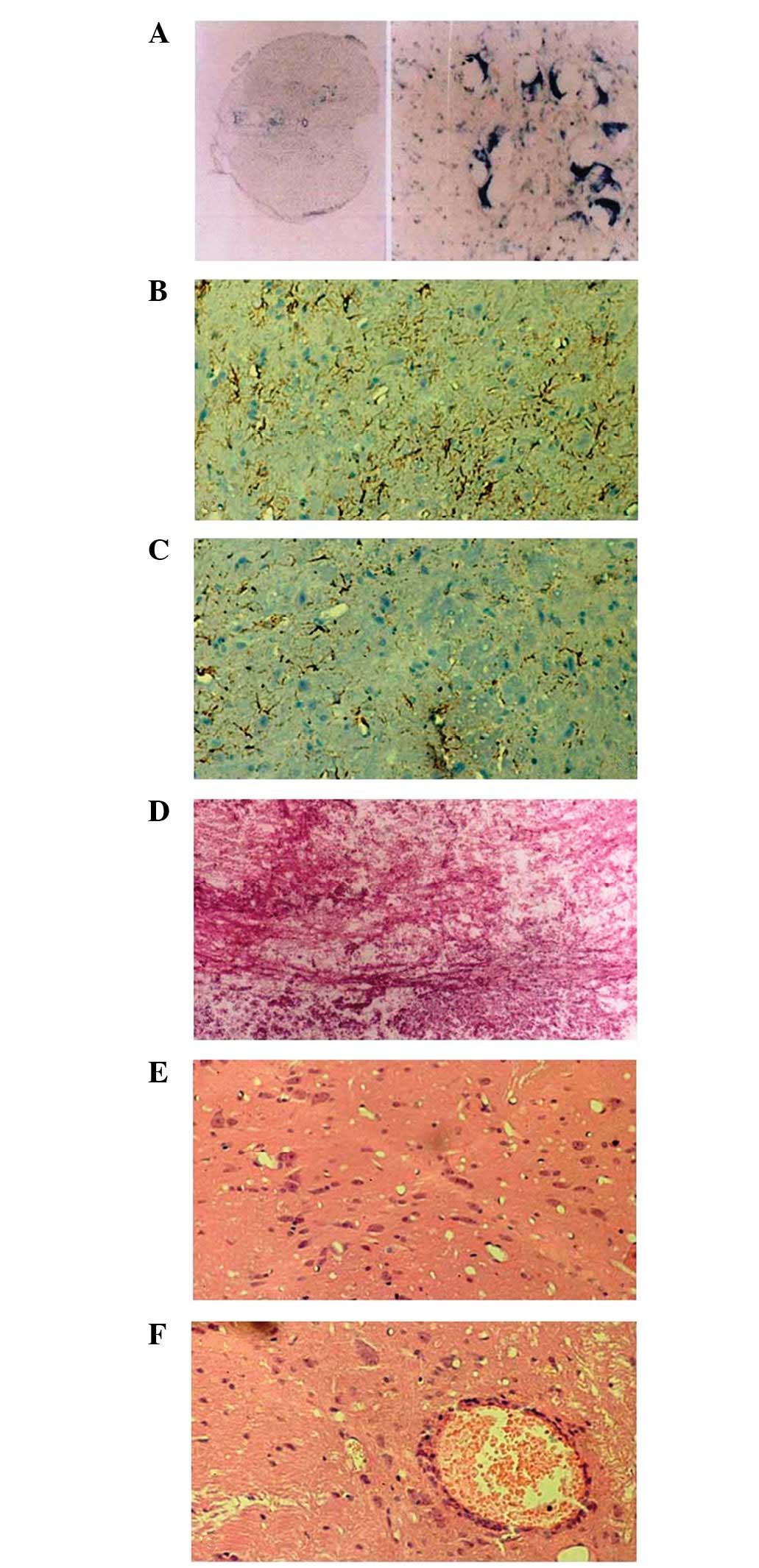
Changes that occurred at different stages of injury
in the rat SCI model are shown in Fig.
3. Sections from the animal model revealed that the most severe
SCI occurred after 24 h, where the necrosis of glial cells, a
reduction in the number of neurons and disorder of white matter
structure with only a few nerve cells remaining around the area of
damage were observed. Injured neurons were detected, mainly around
small vessels.
Following 2 weeks of SCI, glial fibers gradually
appeared in the grey matter, accompanied by small cystic
degeneration. The central canal began to reconstruct, changes in
white matter structure were observed and the abundant proliferation
of glial cells was detected.
Inhibiting MCP-1 reduces inflammatory
changes
Inflammatory cytokines, including IL-1β and TNF-α,
are hypothesized to cause the accumulation and increased expression
of MCP-1 in rat astrocytes, and these changes may be involved in
important signaling pathways (14). In the same experiments, abnormally
high expression levels of these cytokines were shown to be
correlated with the development of inflammation.
In rat models of cerebral cortical nitrocellulose
membrane needle-stick injury, elevated MCP-1 expression has been
linked to astrocyte activation and the infiltration of monocytes
and macrophages (15). It has been
demonstrated that mechanical injury to the central nervous system
causes astrocytes to produce MCP-1 (11).
The expression of inflammatory chemokines MCP-1 and
MIP-1β were significantly increased in the secondary SCI rat model
(Fig. 4), which is consistent with
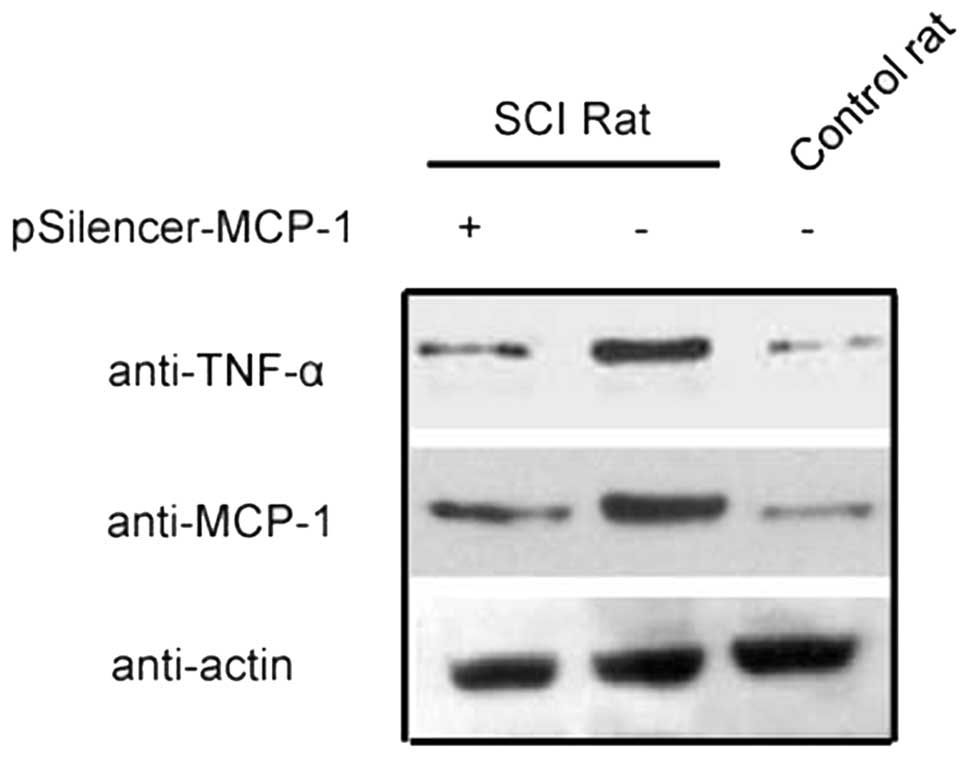
previous studies (6,16).
In this study, MCP-1 and MIP-1β expression increased
after SCI and monocyte and macrophage infiltration also increased
in the damaged areas. After antisense gene intervention, the
expression levels of MCP-1 and MIP-1β were reduced and the
infiltration of monocytes and macrophages decreased.
The expression of TNF-α in response to spinal injury
decreased after RNAi inhibited the expression of MCP-1 (Fig. 5), demonstrating that the
inflammatory response induced by SCI is associated with MCP-1
expression.
The observation of rat tissue sections revealed that
significant changes occurred in the activity of monocytes and
macrophages following the inhibition of MCP-1 expression.
Reduction in MCP-1 expression inhibits
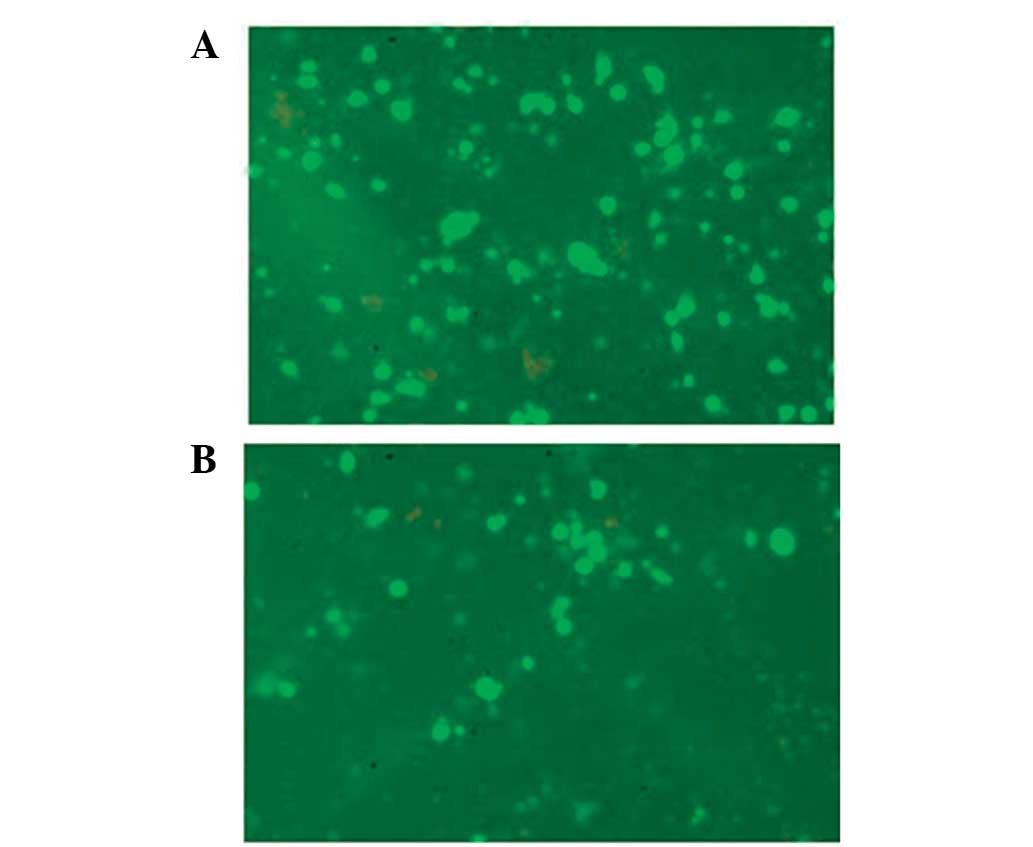
neural cell apoptosis induced by secondary SCI
Following the emergence of secondary SCI, apoptosis
induced by a series of inflammatory responses following the primary
SCI resulted in neural cell necrosis. We demonstrated that the
number of TUNEL-positive cells reached a peak at 24 h after the
primary SCI (Table I). The
TUNEL-positive cells were mainly glial cells and were predominantly
distributed in grey matter. The number of TUNEL-positive glial
cells in the white matter reached a peak ~72 h later. However, in
the RNAi group, the number of TUNEL-positive cells in the grey and
white matter were lower than the control group following inhibition
of MCP-1 expression by RNAi (Table
I).
 | Table IPositive cells at various time-points
after spinal cord injury, as determined by a TUNEL assay. |
Table I
Positive cells at various time-points
after spinal cord injury, as determined by a TUNEL assay.
| Time after spinal
cord injury | Number of positive
cells (mean ± SD)
|
|---|
Grey matter
| White matter
|
|---|
| RNAi | Control | RNAi | Control |
|---|
| 1 h | 0±1 | 0±1 | 0±1 | 0±1 |
| 8 h | 40±10 | 42±8a | 7±2 | 9±1 |
| 24 h | 10±3 | 95±12a | 6±1 | 35±5a |
| 72 h | 5±3 | 50±6a | 9±4 | 413±10a |
Apoptosis in secondary SCI is a process in which
numerous regulatory factors are involved, with the caspase family
as the core component. Low caspase-3 expression is usually detected
in the spinal cord nerve cells of normal rats; however, its
expression has been found to increase several hours after SCI,
reaching a peak after 2–3 days (17). This time profile is consistent with
the apoptotic process. Furthermore, it has been observed that
caspase-1 activity is inhibited after the emergence of SCI in mice
lacking dynorphin, suggesting that caspase-1 protects the nervous
tissues following the occurrence of SCI (18).
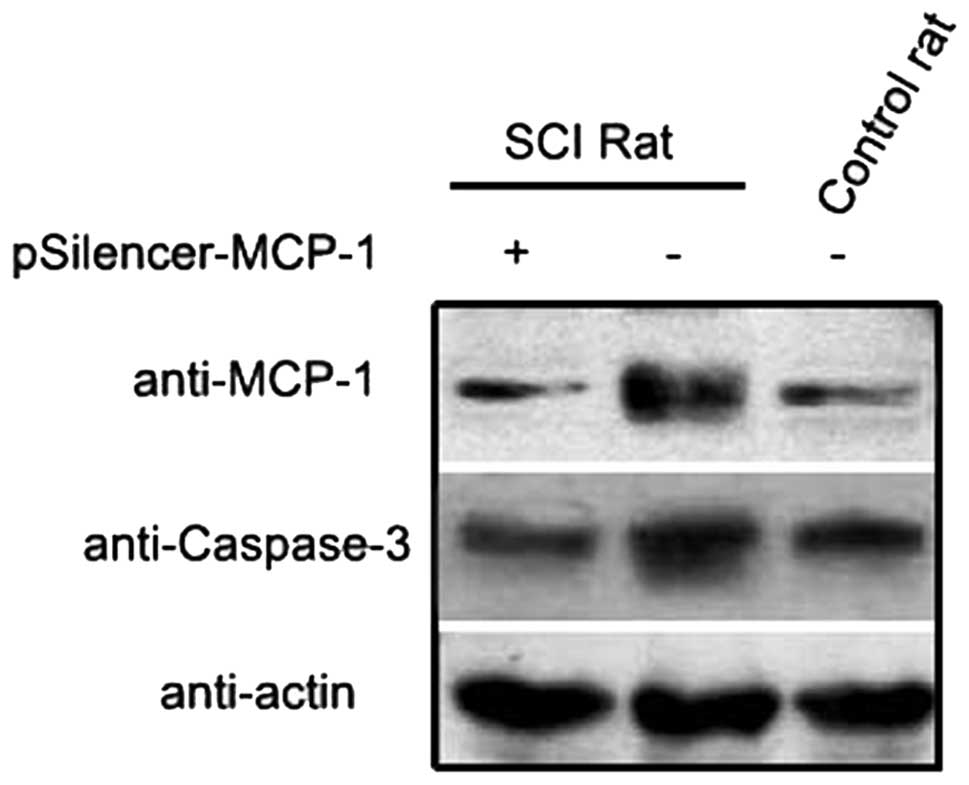
In the present study, following SCI, caspase-3
expression levels decreased compared with the control group after
the inhibition of MCP-1 expression (Fig. 6). Immunohistochemistry revealed
that injury to neuronal cells and astrocytes decreased, suggesting
that a reduction in MCP-1 expression inhibits the aggravation of
apoptosis, thus protecting nerve cells during the secondary SCI
process.
In conclusion, our results suggest that inhibiting
inflammation alleviates nerve cell injury caused by apoptosis and
offers an important approach to the future treatment of secondary
SCI. The use of RNAi to inhibit MCP-1 expression, alleviate spinal
cord injury and protect nerve cells offers a feasible treatment
strategy for patients with this condition. Further research is
required to investigate the pathogenesis of secondary SCI and to
explore the clinical use of RNAi.
Acknowledgments
This study was supported by the Young Science Fund
Projects of National Natural Science Fund (no. 30801155). We
appreciate the valuable advise from other members of our
laboratories.
References
|
1
|
Fehlings MG and Perrin RG: The role and
timing of early decompression for cervical spinal cord injury:
update with a review of recent clinical evidence. Injury. 36(Suppl
2): B13–B26. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Barinaga M: New view of spinal cord
injury. Science. 274:14661996. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Li GL, Farooque M and Olsson Y: Changes of
Fas and Fas ligand immunoreactivity after compression trauma to rat
spinal cord. Acta Neuropathol. 100:75–81. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Emery E, Aldana P, Bunge MB, et al:
Apoptosis after traumatic human spinal cord injury. J Neurosurg.
89:911–920. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Whalley K, O’Neill P and Ferretti P:
Changes in response to spinal cord injury with development:
vascularization, hemorrhage and apoptosis. Neuroscience.
137:821–832. 2006. View Article : Google Scholar
|
|
6
|
McTigue DM, Tani M, Krivacic K, et al:
Selective chemokine mRNA accumulation in the rat spinal cord after
contusion injury. J Neurosci Res. 53:368–376. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pan JZ, Ni L, Sodhi A, Aguanno A, Young W
and Hart RP: Cytokine activity contributes to induction of
inflammatory cytokine mRNAs in spinal cord following contusion. J
Neurosci Res. 68:315–322. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Stammers AT, Liu J and Kwon BK: Expression
of inflammatory cytokines following acute spinal cord injury in a
rodent model. J Neurosci Res. 90:782–790. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gourmala NG, Buttini M, Limonta S, Sauter
A and Boddeke HW: Differential and time-dependent expression of
monocyte chemoattractant protein-1 mRNA by astrocytes and
macrophages in rat brain: effects of ischemia and peripheral
lipopolysaccharide administration. J Neuroimmunol. 74:35–44. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Miyagishi R, Kikuchi S, Takayama C, Inoue
Y and Tashiro K: Identification of cell types producing RANTES,
MIP-1 alpha and MIP-1 beta in rat experimental autoimmune
encephalomyelitis by in situ hybridization. J Neuroimmunol.
77:17–26. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Glabinski AR, Balasingam V, Tani M, et al:
Chemokine monocyte chemoattractant protein-1 is expressed by
astrocytes after mechanical injury to the brain. J Immunol.
156:4363–4368. 1996.PubMed/NCBI
|
|
12
|
Bartholdi D and Schwab ME: Expression of
pro-inflammatory cytokine and chemokine mRNA upon experimental
spinal cord injury in mouse: an in situ hybridization study. Eur J
Neurosci. 9:1422–1438. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lee SC, Liu W, Dickson DW, Brosnan CF and
Berman JW: Cytokine production by human fetal microglia and
astrocytes. Differential induction by lipopolysaccharide and IL-1
beta. J Immunol. 150:2659–2667. 1993.PubMed/NCBI
|
|
14
|
Thompson WL and Van Eldik LJ: Inflammatory
cytokines stimulate the chemokines CCL2/MCP-1 and CCL7/MCP-3
through NFkB and MAPK dependent pathways in rat astrocytes
[corrected]. Brain Res. 1287:47–57. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Majumder S, Zhou LZ and Ransohoff RM:
Transcriptional regulation of chemokine gene expression in
astrocytes. J Neurosci Res. 45:758–769. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lee YL, Shih K, Bao P, Ghirnikar RS and
Eng LF: Cytokine chemokine expression in contused rat spinal cord.
Neurochem Int. 36:417–425. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang J, Zheng Q, Zhao M and Guo X:
Neurocyte apoptosis and expressions of caspase-3 and Fas after
spinal cord injury and their implication in rats. J Huazhong Univ
Sci Technolog Med Sci. 26:709–712. 2006. View Article : Google Scholar
|
|
18
|
Adjan VV, Hauser KF, Bakalkin G, et al:
Caspase-3 activity is reduced after spinal cord injury in mice
lacking dynorphin: differential effects on glia and neurons.
Neuroscience. 148:724–736. 2007. View Article : Google Scholar : PubMed/NCBI
|