Introduction
The fallopian tube is the tubular organ connecting
the periovarian space to the uterus, and performs several
interrelated functions. It captures the cumulus-oocyte complex,
maintains viability, and constrain the spermatozoa to the
fertilization site. Moreover, it provides the biological
environment for fertilization, contributes to removal or addition
of oocyte coatings, and nourishes the egg (1).
The Von Hippel-Lindau gene (VHL) was isolated
by positional cloning and identified as a tumor suppressor gene
since 1993 (2). VHL, which
is a classical tumor suppressor gene, can regulate the mRNA
stability of a number of target genes through selective degradation
of RNA-bound proteins, including the endothelial growth factor
(EGF), transforming growth factor-α (TGF-α), hypoxia-inducible
factor 1α (HIF-1α), and carbonic anhydrase 9 (3,4). VHL
disease is an inherited neoplastic disease characterized by a
predisposition to develop vascularized tumors or cysts in numerous
tissues (5). Moreover, mutations
in the VHL gene are associated with cancer development of a
number of organs, including kidney, lung, breast, ovary, and cervix
(6). The VHL protein (pVHL) has
been ascribed several distinct biochemical activities, and was
shown to be involved in the regulation of the cell cycle (7). pVHL is a multifunctional protein,
related to the inhibition of cell growth, angiogenesis, fibronectin
matrix assembly, activation of p53, and proteolysis; nearly all
these processes contribute to its tumor suppressor function
(8). In addition, pVHL is also a
component of the mechanism that transduces local differences in
oxygen tension at the fetal-maternal interface to the
cytotrophoblasts, affecting their biologic behavior (9). However, whether the VHL gene
is expressed in the human fallopian tube is presently unclear.
This study was carried out to investigate the
expression of VHL at the mRNA and protein level in human healthy
fallopian tube samples, isolated from the proliferative and the
secretory stages of the menstrual cycle, and to elucidate whether
VHL expression levels change during the different stages of the
menstrual cycle.
Materials and methods
Tissue samples
Samples of human healthy fallopian tube tissues were
obtained by abdominal hysterectomy with salpingectomy for benign
uterine disease. Twenty-seven fresh tissue samples of the
proliferative (n=14) and the secretory phase (n=13) of the
menstrual cycle were collected. One part of each sample was
immediately frozen in liquid nitrogen or stored at −80°C until
further use for reverse transcription-polymerase chain reaction
(RT-PCR) and western blotting. The other part of the samples was
fixed in 10% formalin and embedded in paraffin for
immunohistochemistry (IHC) analysis.
Patients with healthy fallopian tube tissues were
operated for reasons unrelated to tubal dysfunction, and their mean
± standard deviation (SD) age was 36 (4.38) years (range, 32–43
years). Their menstrual day was coincident with the histological
diagnosis of the stage of the endometrium according to the criteria
of Noyes et al (10).
Patients were excluded if they had received exogenous steroid
treatment or chemotherapy within the last 6 months before surgery.
All patients signed informed consent letters, and the study
protocol was approved by the Local Ethics Committee of Jinan
University (Guangzhou, China).
RNA isolation and RT-PCR
Total RNA was extracted from 27 fresh fallopian tube
tissues using an RNA isolation kit by Omega Bio-Tek Inc. (Norcross,
GA, USA) according to the manufacturer’s protocol. The mRNA
concentration and purity were determined by 1.0% agarose gel
electrophoresis, and the optical density (OD)260/280
ratio was >1.8. Aliquots of mRNA (20 μg) from each sample
were reverse transcribed using oligo (dT)18 primer and the M-MLV
reverse transcriptase (Promega Corp., Madison, WI, USA). The
housekeeping gene glyceraldehyde 3-phosphate dehydrogenase
(GAPDH) was used as an internal control to normalize the
results for variations in the input cDNA amount or the RT
efficiency. The forward primer for the amplification of the
VHL gene (PCR product, 300 bp) was
5′-GTCGAAGAGTACCGCCCTGAAG-3′ and the reverse,
5′-GTGTCCCTGCATCTCTGAAGAG-3′. PCR amplification was carried out in
the following conditions: initial denaturation at 95°C for 10 min,
followed by 40 cycles of denaturation at 94°C for 15 sec, annealing
at 60°C for 15 sec, and extension at 72°C for 1 min. The PCR
products were verified by electrophoresis on a 1.5% agarose gel,
and densitometry analysis was carried using the Bio-Rad Gel Doc
2000 Imaging system (Bio-Rad Laboratories, Hercules, CA, USA).
Densitometrical values were used to calculate the relative
expression ratio VHL/GAPDH.
Western blotting
The fresh fallopian tube tissues were homog-enized
and lysed on ice using cell lysis buffer (Beyotime Institute of
Biotechnology, Shanghai, China) and a protease inhibitor cocktail.
Following centrifugation at 10,000 x g for 15 min at 4°C, protein
concentrations were determined using the Bicinchoninic Acid Protein
Assay kit (Pierce Biotechnology, Inc., Rockford, IL, USA). Total
proteins were denatured in Laemmli buffer (Bio-Rad Laboratories),
fractionated using 10% one-dimensional sodium dodecyl
sulfate-polyacrylamide gel electrophoresis and transferred onto
nitrocellulose membranes. Blots were blocked for 2 h in TBST
solution (20 mmol/l Tris pH 7.6, 137 mmol/l sodium chloride, 0.1%
Tween-20) containing 10% non-fat dry milk, and incubated with
primary antibodies targeting human pVHL (1:1,000; Cell Signaling
Technology, Inc., Danvers, MA, USA) overnight at 4°C, or β-actin
(1:3,000; Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) for
1 h at room temperature with rocking. Subsequently, the blots were
washed three times for 10 min each in TBST, followed by incubation
for 1 h at room temperature with anti-rabbit and anti-mouse
horseradish peroxidase-linked species-specific immunoglobulin
(IgG). The bound antibodies were detected with the enhanced
chemiluminescence (ECL) system BeyoECL Plus (Beyotime Institute of
Biotechnology). Band intensities were quantified by scanning
densitometry, using the Bio-Rad Quantity One software (Bio-Rad
Laboratories).
IHC
Paraffin-embedded sections of fallopian tissues at
the proliferative (n=14) and secretory (n=13) phase were examined
by IHC. Positive staining was evaluated with the standard
streptavidin-biotin system (Mai-xin Bio, Fuzhou, China). The
4-μm thick sections were deparaffinized in xylene, hydrated
through graded alcohol, and incubated in antigen retrieval solution
(0.01-mol/l sodium citrate buffer, pH 6.0) at 60°C for 16 min.
Endogenous peroxidase activity was blocked by incubating the
samples in 3% hydrogen peroxide for 10 min. Non-specific antibody
binding was blocked by incubation with normal goat serum for 10
min. Mouse anti-human VHL monoclonal antibody (1:100; Mai-xin Bio)
was used as the primary antibody, and the sections were incubated
with the antibody overnight at 4°C. The secondary antibodies were
biotinylated anti-mouse IgGs, and the reaction was developed with
the streptavidin-peroxidase system. The diaminobenzidine
Substrate-Chromogen system (Mai-xin Bio) was used as the
color-developing substrate. The samples were observed under a
fluorescence microscope (E400; Nikon, Tokyo, Japan).
Statistical analysis
Statistical analysis was performed with the SPSS
17.0 software (SPSS, Inc., Chicago, IL, USA). Data are expressed as
mean ± SD and were analyzed with one-way analysis of variance
(ANOVA). P<0.05 was considered to indicate a statistically
significant difference.
Results
RT-PCR analysis
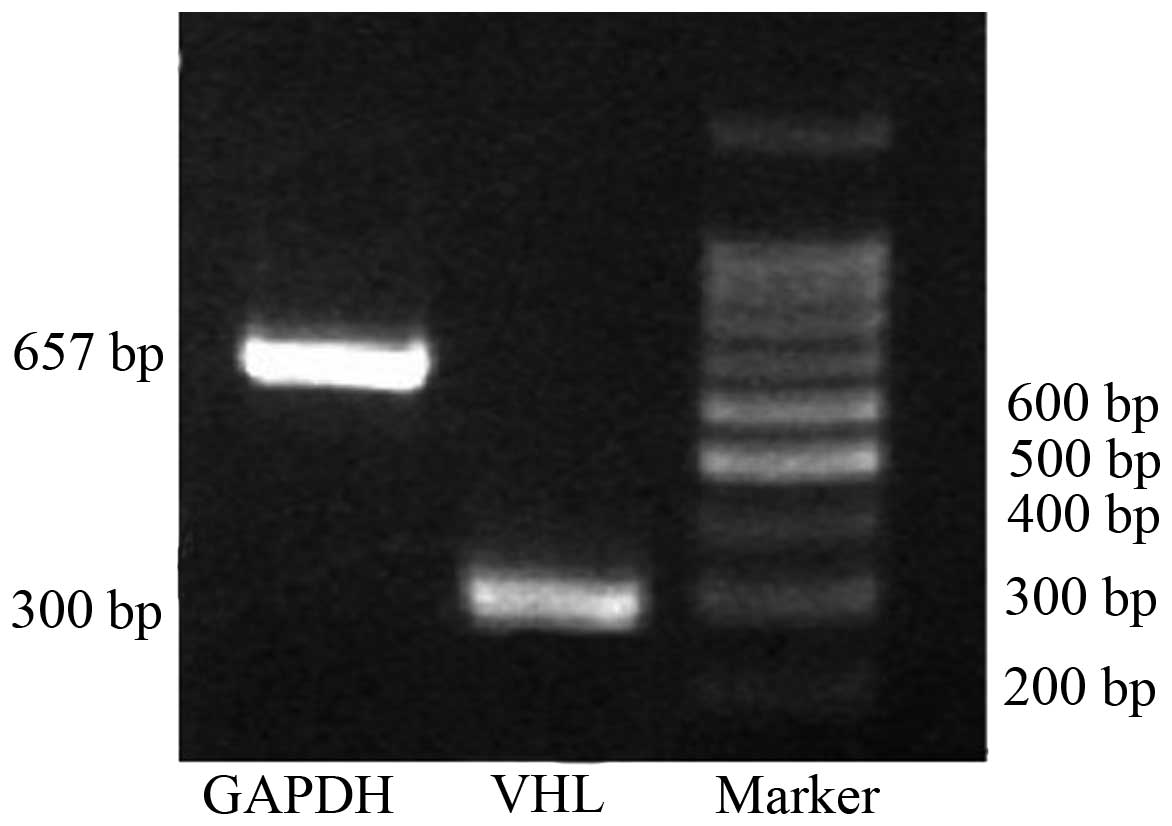
VHL expression was analyzed in the human
fallopian tube by RT-PCR. The GAPDH mRNA was first detected
in twenty-seven fresh fallopian tube samples, and those that were
positive were then used for detection of the VHL mRNA level
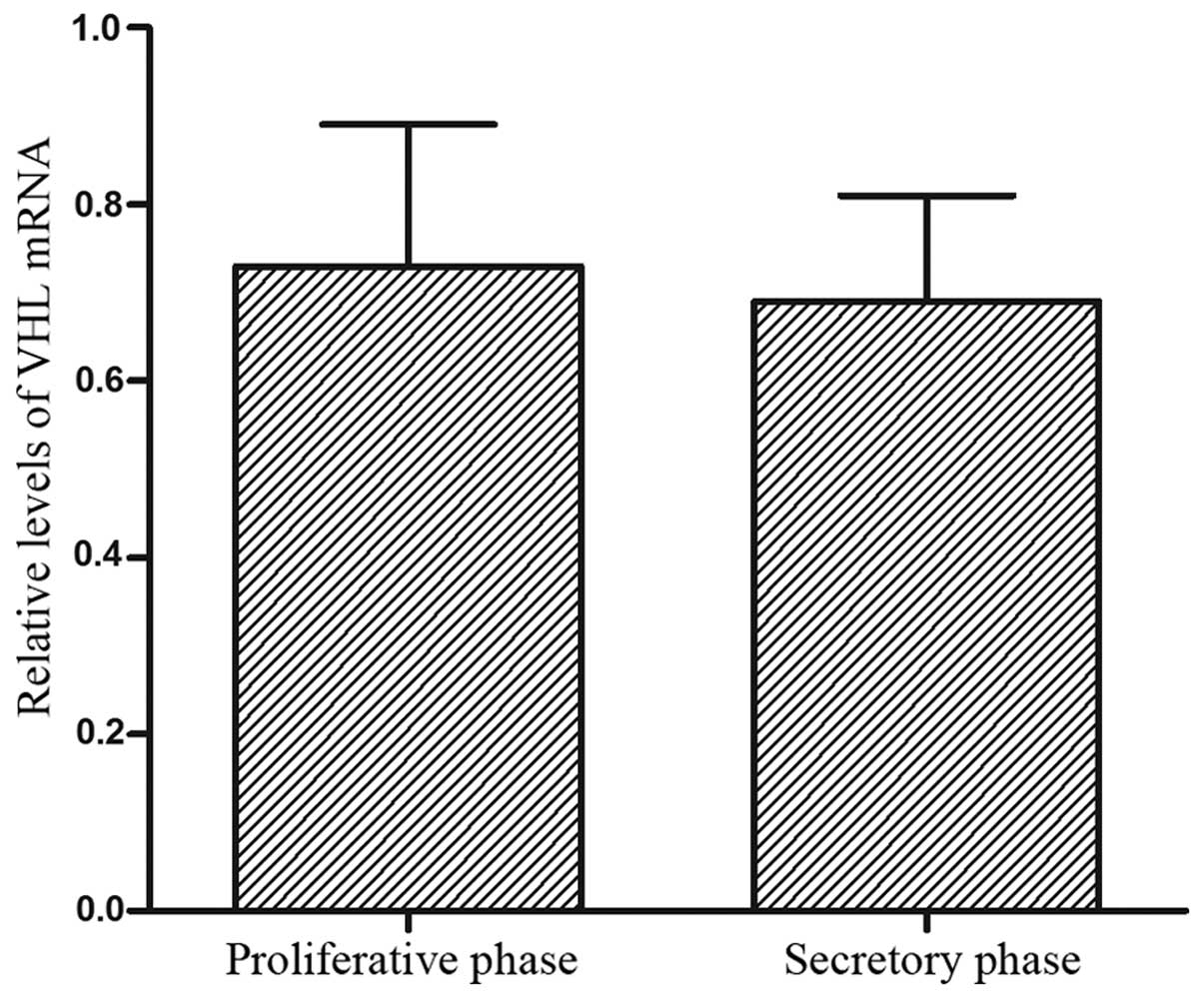
(Fig. 1). The VHL mRNA
levels in the human fallopian tubes are presented in Fig. 2. The VHL mRNA level in the
fallopian tube was higher at the proliferative phase compared to
the secretory phase of the menstrual cycle. There was no difference
in the expression of VHL between the proliferative and the
secretory phase (P>0.05).
Western blotting
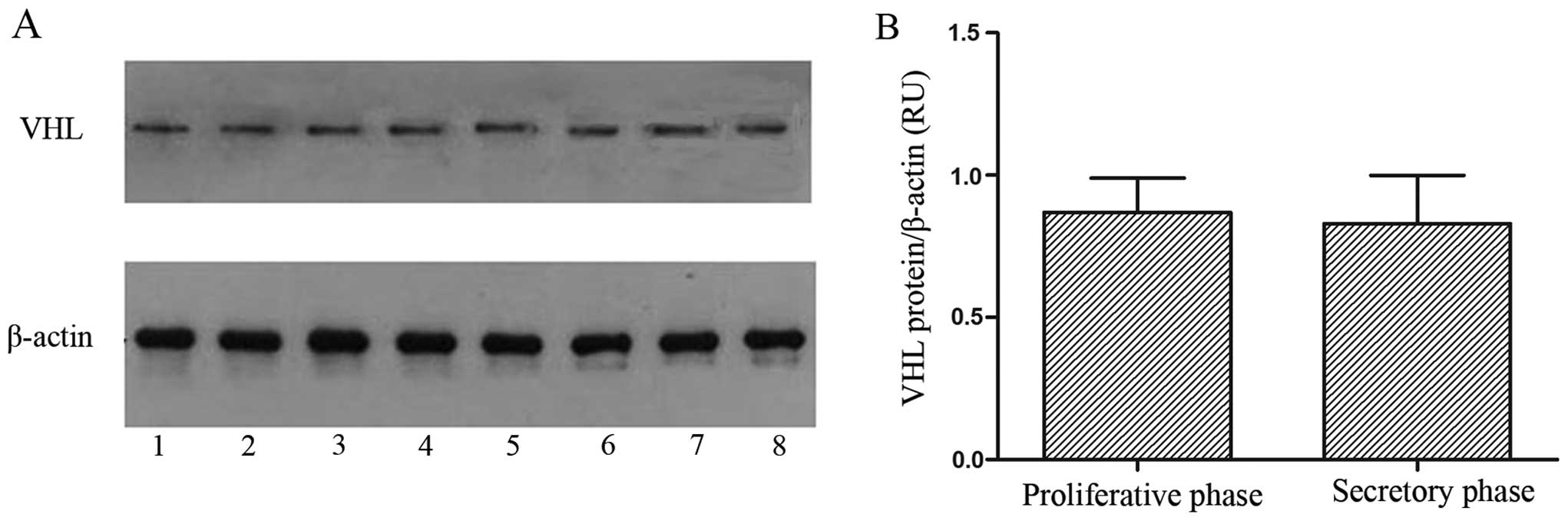
The pVHL and β-actin protein expression levels were
revealed by western blotting analysis in the fresh fallopian tube
tissues. A single band for each protein was observed in all fresh
fallopian tube tissues (Fig. 3A).
The pVHL levels in the 27 fresh fallopian tube samples are shown in
Fig. 3B. Although pVHL expression
in fallopian tubes was increased at the proliferative phase
compared to the secretory one, this difference was not
statistically significant (P>0.05).
IHC
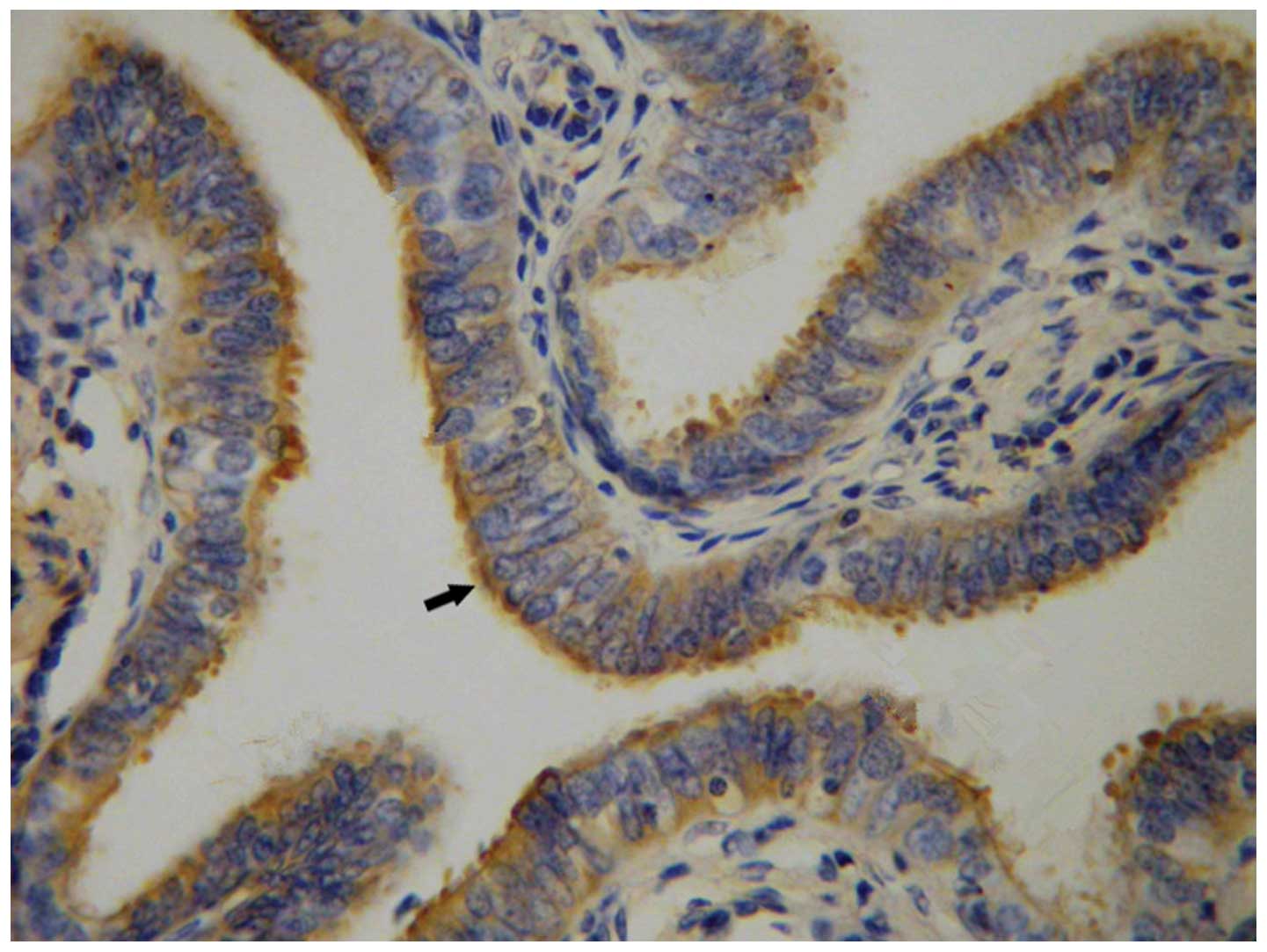
Positive staining corresponding to pVHL was detected
by IHC in the cytoplasm of ciliated cells of the fallopian tube
epithelium (Fig. 4). Positive
staining for pVHL was detected in the 27 ciliated cells of the
fallopian tube tissues.
Discussion
The VHL gene locates at the short arm of the
human chromosome 3. It is ubiquitously expressed in a variety of
organs, with particularly high levels of expression in the
urogenital system, brain, kidney, sensory ganglia, and bronchial
epithelium (11). pVHL, encoded by
the VHL gene, is a multifunctional protein essential for
endothelial extracellular matrix deposition, and inhibits cell
motility (12). Moreover, pVHL can
promote the assembly of actin and vinculin, and induce cell
differentiation and growth arrest through integration of cell-cell
and cell-extracellular matrix (ECM) signaling (13).
It was reported that pVHL is associated with
embryogenesis and placenta development. The fallopian tube is the
organ that provides the microenvironment for gamete transport,
fertilization and preimplantation of the embryo. However, whether
the VHL gene is expressed in human fallopian tubes has not
been reported to date. pVHL can control ciliogenesis by stabilizing
microtubule growth of the ciliated cells (14). In this study, pVHL-positive
staining was detected in the cytoplasm of ciliated epithelial cells
of human fallopian tubes. These results indicate that pVHL may be
involved in the development of the ciliated epithelial cells and
the cell differentiation of cilia in the human fallopian tube.
The epithelium of human fallopian tube undergoes
morphological and functional changes during the menstrual cycle.
The number of tubal epithelial cells is low during the secretory
phase of the menstrual cycle, and increase from the proliferative
phase until the periovulatory period. At the periovulatory period,
both secretory and ciliated cells are of equal size, with the
secretory cells forming domes between the tufts of cilia (15). At approximately the time of
ovulation, the secretory cells reach their peak activity,
consequently reducing in height relative to the ciliated cells in
tubal epithelium (16). The
secretory cells can differentiate into ciliated cells, although
this may be also considered as prima facie evidence for the
existence of a multipotent precursor that can differentiate into
other cell types via an intermediate cell type (17). A previous study indicated that pVHL
can regulate the formation of primary cilia in the renal epithelium
(18). Restoration of pVHL
expression in VHL-negative human cancer cell lines was shown to
enhance the frequency of ciliated cells, and knockdown of
VHL in immortalized mouse kidney epithelial cells resulted
in loss of primary cilia; these results suggest that VHL is
necessary for the formation of the primary cilium (14,19).
VHL-induced cell differentiation in renal epithelial cells was also
reported (13). In this study, we
demonstrated that the VHL gene is expressed in the human
fallopian tube during the menstrual cycle, and that its expression
at the proliferative phase is higher than the secretory phase,
although the difference was not significant in our data. These
results suggest that the VHL gene may regulate the
differentiation of ciliated and secretory cells.
pVHL, as a tumor suppressor protein, regulates the
proper extracellular fibronectin matrix assembly and the cell
cycle. The fallopian tube epithelium can secrete fibronectin, and
localizes at the luminal surface of the cells, especially on the
tips of the cilia (20).
Fibronectin is a regulator of various cellular activities, by
promoting cell migration and spread, and extracellular matrix
assembly. A previous study indicated that pVHL binds to
fibronectin, and that VHL expression is associated with the
deposition of assembled fibronectin (21). Overexpression of pVHL may increase
fibronectin expression post-transcriptionally, as well as the
secretion of extracellular fibronectin (22). Additionally, VHL gene
mutations associated with disease result in fibronectin assembly
defects, and pVHL-deficient cells fail to perform proper
extracellular fibronectin matrix assembly (23). Therefore, pVHL physically interacts
with fibronectin under physiological conditions, and this
interaction can influence the ability of cells to assemble the
extracellular fibronectin matrix. In the present study, the pVHL
expression in the human fallopian tubes was not different between
the proliferative and the secretory phase of the menstrual cycle.
These results indicate that pVHL may not affect the fallopian tube
during menstrual cycle through the interaction with
fibronectin.
Integrins are αβ heterodimeric transmembrane
proteins that provide a link between cells and the surrounding
matrix through tightly regulated interactions with ligands.
Integrins have also been used as molecular markers of endometrial
receptivity. Sülz et al reported that a similar ‘window of
implantation’ may exist in the fallopian tube, based on their
observation of regulated expression of β3 integrin in the human
fallopian tube epithelium (24). A
previous study demonstrated that VHL induces cell differentiation
through cell-cell and ECM signaling (13). Cell-ECM signaling is mediated by
integrins, and integrin signaling influences cell survival,
proliferation and differentiation, which indicates that integrins
may contribute to VHL-mediated cell differentiation (25). VHL-mediated tight junction and
adherens junction assembly was associated to the downregulation of
integrins (26). In addition, the
presence of β1-integrin fibrillar adhesions in VHL(+) cells at late
confluence allowed the cells to firmly anchor to the substrate, and
thus may be an important mechanism in controlling cell migration
(27). In our study, the
expression levels of VHL mRNA and protein in the fallopian tubes
were not significantly different during the two stages of the
menstrual cycle studied. This result indicates that VHL may
regulate the differentiation of ciliated cells in the fallopian
tube through integrin signaling.
The environment in the fallopian tube can affect the
fertilization potential of the sperm and the embryo development.
The transport of the spermatozoa and the pre-embryo is deemed to be
aided by muscular contractions in the wall of the fallopian tube
and the cilia in the tubal mucosa. Regulation of muscular activity
and formation of cilia in the fallopian tube were shown to be
affected by sex steroids, nitric nerves, and prostaglandins (PG)
(28). PGE2 can increase HIF-1α
levels (29). Moreover, the HIF1-α
subunit was identified and targeted for rapid proteasome-dependent
degradation by the VHL E3 ubiquitin ligase complex at normal oxygen
concentrations (30). Under normal
conditions, the HIF1-α protein is rapidly degraded via the
VHL-ubiquitin-proteasome pathway (31). Therefore, VHL gene
expression in the fallopian tube may be associated with the
function of the fallopian tube.
In conclusion, this study aimed to investigate VHL
gene and protein expression in the human fallopian tube tissues
during the menstrual cycle. Expression levels of VHL mRNA and
protein were not significantly different between the proliferative
and the secretory phase. Our result may enhance the current
understanding on the mechanism of fallopian tube associated-disease
and functions. Additional studies are needed to investigate whether
the VHL mRNA and protein expression levels can be the target of
novel therapies against fallopian tube associated-disease.
References
|
1
|
Croxatto HB: Physiology of gamete and
embryo transport through the fallopian tube. Reprod Biomed Online.
4:160–169. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Latif F, Tory K, Gnarra J, Yao M, Duh FM,
Orcutt ML, Stackhouse T, Kuzmin I, Modi W, Geil L, et al:
Identification of the von Hippel-Lindau disease tumor suppressor
gene. Science. 260:1317–1320. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kamada M, Suzuki K, Kato Y, Okuda H and
Shuin T: von Hippel-Lindau protein promotes the assembly of actin
and vinculin and inhibits cell motility. Cancer Res. 61:4184–4189.
2001.PubMed/NCBI
|
|
4
|
Lu N, Hui H, Yang H, Zhao K, Chen Y, You
QD and Guo QL: Gambogic acid inhibits angiogenesis through
inhibiting PHD2-VHL-HIF-1α pathway. Eur J Pharm Sci. 49:220–226.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ohh M, Park CW, Ivan M, Hoffman MA, Kim
TY, Huang LE, Pavletich N, Chau V and Kaelin WG: Ubiquitination of
hypoxia-inducible factor requires direct binding to the beta-domain
of the von Hippel-Lindau protein. Nat Cell Biol. 2:423–427. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhou MI, Wang H, Foy RL, Ross JJ and Cohen
HT: Tumor suppressor von Hippel-Lindau (VHL) stabilization of
Jade-1 protein occurs through plant homeodomains and is VHL
mutation dependent. Cancer Res. 64:1278–1286. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Schraml P, Struckmann K, Hatz F, Sonnet S,
Kully C, Gasser T, Sauter G, Mihatsch MJ and Moch H: VHL mutations
and their correlation with tumour cell proliferation, microvessel
density, and patient prognosis in clear cell renal cell carcinoma.
J Pathol. 196:186–193. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shuin T, Yamasaki I, Tamura K, Okuda H,
Furihata M and Ashida S: Von Hippel-Lindau disease: molecular
pathological basis, clinical criteria, genetic testing, clinical
features of tumors and treatment. Jpn J Clin Oncol. 36:337–343.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rajakumar A, Doty K, Daftary A, Markovic N
and Conrad KP: Expression of von Hippel Lindau (pVHL) protein in
placentae from normal pregnant women and women with preeclampsia.
Placenta. 27:411–421. 2006. View Article : Google Scholar
|
|
10
|
Noyes RW, Hertig AT and Rock J: Dating the
endometrial biopsy. Am J Obstet Gynecol. 122:262–263.
1975.PubMed/NCBI
|
|
11
|
Richards FM, Schofield PN, Fleming S and
Maher ER: Expression of the von Hippel-Lindau disease tumour
suppressor gene during human embryogenesis. Hum Mol Genet.
5:639–644. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tang N, Mack F, Haase VH, Simon MC and
Johnson RS: pVHL function is essential for endothelial
extracellular matrix deposition. Mol Cell Biol. 26:2519–2530. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Davidowitz EJ, Schoenfeld AR and Burk RD:
VHL induces renal cell differentiation and growth arrest through
integration of cell-cell and cell-extracellular matrix signaling.
Mol Cell Biol. 21:865–874. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Schermer B, Ghenoiu C, Bartram M, Müller
RU, Kotsis F, Höhne M, Kühn W, Rapka M, Nitschke R, Zentgraf H, et
al: The von Hippel-Lindau tumor suppressor protein controls
ciliogenesis by orienting microtubule growth. J Cell Biol.
175:547–554. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lyons R, Saridogan E and Djahanbakhch O:
The reproductive significance of human Fallopian tube cilia. Hum
Reprod Update. 12:363–372. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Crow J, Amso NN, Lewin J and Shaw RW:
Morphology and ultrastructure of fallopian tube epithelium at
different stages of the menstrual cycle and menopause. Hum Reprod.
9:2224–2233. 1994.PubMed/NCBI
|
|
17
|
Comer MT, Leese H and Southgate J:
Induction of a differentiated ciliated cell phenotype in primary
cultures of Fallopian tube epithelium. Hum Reprod. 13:3114–3120.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Esteban MA, Harten SK, Tran MG and Maxwell
PH: Formation of primary cilia in the renal epithelium is regulated
by the von Hippel-Lindau tumor suppressor protein. J Am Soc
Nephrol. 17:1801–1806. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lutz MS and Burk RD: Primary cilium
formation requires von hippel-lindau gene function in renal-derived
cells. Cancer Res. 66:6903–6907. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Makrigiannakis A, Karamouti M, Petsas G,
Makris N, Nikas G and Antsaklis A: The expression of receptivity
markers in the fallopian tube epithelium. Histochem Cell Biol.
132:159–167. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ohh M, Yauch RL, Lonergan KM, Whaley JM,
Stemmer-Rachamimov AO, Louis DN, Gavin BJ, Kley N, Kaelin WG Jr and
Iliopoulos O: The von Hippel-Lindau tumor suppressor protein is
required for proper assembly of an extracellular fibronectin
matrix. Mol Cell. 1:959–968. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhou Q, Pardo A, Königshoff M, Eickelberg
O, Budinger GR, Thavarajah K, Gottardi CJ, Jones J, Varga J and
Selman M: Role of von Hippel-Lindau protein in fibroblast
proliferation and fibrosis. FASEB J. 25:3032–3044. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lonser RR, Glenn GM, Walther M, Chew EY,
Libutti SK, Linehan WM and Oldfield EH: von Hippel-Lindau disease.
Lancet. 361:2059–2067. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sülz L, Valenzuela JP, Salvatierra AM,
Ortiz ME and Croxatto HB: The expression of alpha (v) and beta3
integrin subunits in the normal human Fallopian tube epithelium
suggests the occurrence of a tubal implantation window. Hum Reprod.
13:2916–2920. 1998. View Article : Google Scholar
|
|
25
|
Guo W and Giancotti FG: Integrin
signalling during tumour progression. Nat Rev Mol Cell Biol.
5:816–826. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ji Q and Burk RD: Downregulation of
integrins by von Hippel-Lindau (VHL) tumor suppressor protein is
independent of VHL-directed hypoxia-inducible factor alpha
degradation. Biochem Cell Biol. 86:227–234. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Esteban-Barragán MA, Ávila P,
Álvarez-Tejado M, Gutiérrez MD, García-Pardo Á, Sánchez-Madrid F
and Landázuri MO: Role of the von Hippel-Lindau tumor suppressor
gene in the formation of β1-integrin fibrillar adhesions. Cancer
Res. 62:2929–2936. 2002.
|
|
28
|
Mastroianni L Jr: The fallopian tube and
reproductive health. J Pediatr Adolesc Gynecol. 12:121–126. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wanggren K, Lalitkumar P, Stavreus-Evers
A, Stabi B and Gemzell-Danielsson K: Prostaglandin E2 and F2alpha
receptors in the human Fallopian tube before and after mifepristone
treatment. Mol Hum Reprod. 12:577–585. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Semenza GL: Hypoxia-inducible factor 1
(HIF-1) pathway. Sci STKE. 2007:pp. cm82007, View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Maxwell PH, Wiesener MS, Chang GW,
Clifford SC, Vaux EC, Cockman ME, Wykoff CC, Pugh CW, Maher ER and
Ratcliffe PJ: The tumour suppressor protein VHL targets
hypoxia-inducible factors for oxygen-dependent proteolysis. Nature.
399:271–275. 1999. View
Article : Google Scholar : PubMed/NCBI
|