Introduction
Hyperpermeability of the microvasculature is one of
the most damaging pathological features of diabetes mellitus
(1). A number of mechanisms have
been proposed to explain endothelial hyperpermeability, including
hemodynamic changes (2), oxidative
stress (3) and the accelerated
production of kinins, histamine or serotonin (4,5).
Advanced glycation end products (AGEs) may also
increase vascular permeability (6,7),
possibly by activating AGE receptors (RAGEs), which are expressed
by endothelial cells. AGEs are formed through nonenzymatic
reactions between sugars and amine residues on proteins, lipids and
nucleic acids (8). In diabetes,
AGEs are known to accumulate in the microvasculature of the kidney
and, therefore, may contribute to the pathophysiology of diabetes
(8,9). Dynamic regulation of glomerular
endothelial cell (GEnC) permeability is pivotal for maintaining the
selectivity of glomerular filtration and for preventing proteinuria
(10). Indeed, clinical studies
have revealed a correlation between serum AGEs and the progression
of microalbuminuria (11),
suggesting that AGEs may induce pathological increases in
glomerular permeability in diabetes mellitus. The impact of AGEs on
the pathophysiology of diabetic nephropathy remains to be
elucidated.
Matrix metalloproteinases (MMPs) are a family of
zinc-dependent proteinases that degrade the extracellular matrix
(12). The concentrations and
activities of MMP-2 and MMP-9 are increased by high glucose in
vivo (13) and in vitro
(14). Furthermore, the level of
MMP activity is associated with the degree of albuminuria (15–19),
suggesting an important role of MMP-2/9 in the pathogenesis of
diabetic nephropathy. The elevated expression of MMP-2/9 in
diabetes may facilitate an increase in vascular permeability by
degrading tight junction (TJ) proteins with concomitant disruption
of the TJ complex (13).
Enhanced GEnC permeability by the MMP-mediated
degradation of TJ proteins has not been demonstrated previously.
The present study examined the activity of MMP-2 and MMP-9 in the
GEnCs following exposure to exogenous AGEs and the correlation
between MMP activity in response to the changes in GEnC’s
permeability and TJ protein expression. The results indicated a
possible role of MMP-mediated TJ proteolysis in diabetic kidney
dysfunction.
Materials and methods
Chemicals and antibodies
AGEs conjugated to bovine serum albumin (BSA) and
Calbiochem® MMP-2/MMP-9 Inhibitor II (BiPS) were
purchased from Merck KGaA (Darmstadt, Germany). Antibodies to
occludin, claudin-5 and Alexa fluor® 546 were purchased
from Invitrogen Life Technologies (Carlsbad, CA, USA) and GAPDH was
purchased from Proteintech Group, Inc. (Chicago, IL, USA).
Dylight® 488 was purchased from Multiscience Biotech
Co., Ltd (Hangzhou, China). Millicell®-ERS was purchased
from Millipore (Bedford, MA, USA). Fluorescein isothiocyanate
(FITC)-dextran was purchased from Sigma-Aldrich (St. Louis, MO,
USA).
Cell culture
GEnC cells were purchased from ATCC (Boulevard, MA,
USA). Rat GEnC cultures were established and characterized as
previously described (20).
Briefly, GEnCs were grown in RPMI-1640 medium (Gibco-BRL, Carlsbad,
CA, USA) supplemented with 10% fetal bovine serum (Gibco-BRL) and
10% NuSerum (Sigma-Aldrich) in a cell incubator at 37°C under 5%
CO2. When the cells reached 75–80% confluence, they were
incubated in low-serum medium and treated with AGEs at different
doses (20, 40 and 80 μl) and for various durations (6, 12
and 24 h). In certain experiments, the MMP2/9 inhibitor BiPS (50
μM) was also added. The cells and medium were collected 24 h
after the initial treatment.
Western blot analysis
The endothelial cells were treated with lysis buffer
and centrifuged at 15,000 × g for 10 min at 4°C. The supernatant
was collected for western blotting or stored at −80°C for future
analysis. Equal quantities of protein were loaded onto a gel for 15
% SDS-PAGE (Sigma-Aldrich) in order to separate the proteins.
Separated proteins were transferred onto polyvinylidene difluoride
membranes (Millipore, Lyon, France) and incubated with mouse
anti-claudin-5 monoclonal antibody (1:3,000; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA) and mouse anti-Occuludin
monoclonal antibody (1:2,000; Santa Cruz Biotechnology, Inc.)
overnight at 4°C. Following washing with phosphate-buffered saline
(PBS), the appropriate horseradish peroxidase-conjugated rabbit
anti-mouse polyclonal secondary antibody (1:1,000; Santa Cruz
Biotechnology, Inc.,) was added for 1 h at room temperature.
Protein immunoreactivity was detected using the enhanced
chemiluminescence reaction kit (ECL; Pierce, WI, USA), with the
Tanon-4200 chemiluminescence reaction detection system (Tiangen
Biot Co. Ltd, Beijing China). Band intensities were quantified by
Quantity One 2.0 software (Bio-Rad, Hercules, CA, USA).
Transendothelial electrical resistance
(TEER) and paracellular permeability assays
The electrical resistance across the confluent cell
monolayer, TEER, was measured using the Millicell®-ERS
system (Millipore) according to the manufacturer’s instructions.
Briefly, the cells were grown to post-confluence on transwell
filters (Corning Costar®, Inc., Union City, CA, USA) and
treated with AGEs or AGEs + BiPS. The shorter electrode was placed
within the Millicell culture plate insert and the longer electrode
was placed in the outer well. The resistance of a culture or cell
free plate (blank) was measured at three time-points from different
points across the inner and outer wells until a stable value was
measured each time. The resistance of the blank was subtracted from
that measured with endothelial cells (net resistance). The unit
area TEER (KΩ·cm2) was calculated by multiplying the net
resistance by the area of the culture plate insert.
For the FITC-dextran flux assays, the cells were
grown to post-confluence on transwell filters and 20 μl
FITC-dextran solution was added to the inner well insert at a final
concentration of 1 mg/ml. The medium was collected from the outer
well, which was continuous with the basolateral compartment after
24 h. The quantity of diffused FITC-dextran was read using a
fluorometer (SpectraMax M5, Molecular Devices, Tokyo, Japan) at
wavelengths of 520 nm emission and 485 nm excitation.
Immunofluorescence staining
The endothelial cells were grown on glass cover
slips to 70–80% confluence and then cultured in AGEs with or
without BiPS for 24 h. The treated cells were fixed in 4%
formaldehyde solution (Sigma-Aldrich) for 10 min, washed in PBS,
permeabilized in 0.2% Triton X-100 (Sigma-A ldrich) plus PBS,
blocked with 5% goat serum for 30 min and incubated with rabbit
anti-claudin-5 polyclonal antibody (1:100; Santa Cruz
Biotechnology, Inc.) and mouse anti-Occuludin monoclonal antibody
(1:500; Santa Cruz Biotechnology, Inc.) overnight at 4°C in
humidified chambers. The fixed cells were then probed with a
fluorophore-tagged secondary antibody (1:500) for 1 h at room
temperature. The slides were washed in PBS and visualized using a
laser scanning confocal microscope (Zeiss LSM510 Meta; Carl Zeiss,
Stuttgart, Germany).
Gelatin zymography
To assess gelatinase activity, the cell medium was
collected and centrifuged at 12,000 × g for 10 min at 4°C. The
supernatant was collected and concentrated 20-fold using Amicon
Centricon centrifugal filters (Millipore). The concentrated media
was separated electrophoretically on 10% polyacrylamide gels
containing 0.1% gelatin. Following electrophoresis, gels were
washed for 90 min in 2.5% (v/v) Triton X-100 and incubated in
enzyme buffer containing 50 mM Tris-HCl, (pH 7.5; Sigma-Aldrich),
150 mM NaCl, 5 mM CaCl2 and 1 M ZnCl2 for 40
h with gentle agitation at 37°C. The gel was then stained with 0.2%
Coomassie brilliant blue R-250 (Sigma-Aldrich) in a mixture of
methanol, acetic acid and water (2:1:7) for 1 h and then destained
in destaining solution. The band density was quantified by Quantity
One 2.0 software (Bio-Rad) following negative image reversal.
Statistical analysis
All the experiments were performed at least three
times. The results are presented as the mean ± standard deviation
of independent experiments. Statistically significant differences
between treatment groups were determined by one-way analysis of
variance using SPSS 19.0 software (International Business Machines,
Armonk, NY, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
Effects of AGEs on the expression of
occludin and claudin-5 in the GEnCs
Elevated serum AGE levels have been linked to
hyperpermeability of the microvasculature in diabetes mellitus. The
altered expression of TJ proteins by endothelial cells may induce
these permeability changes; therefore, the expression levels of the
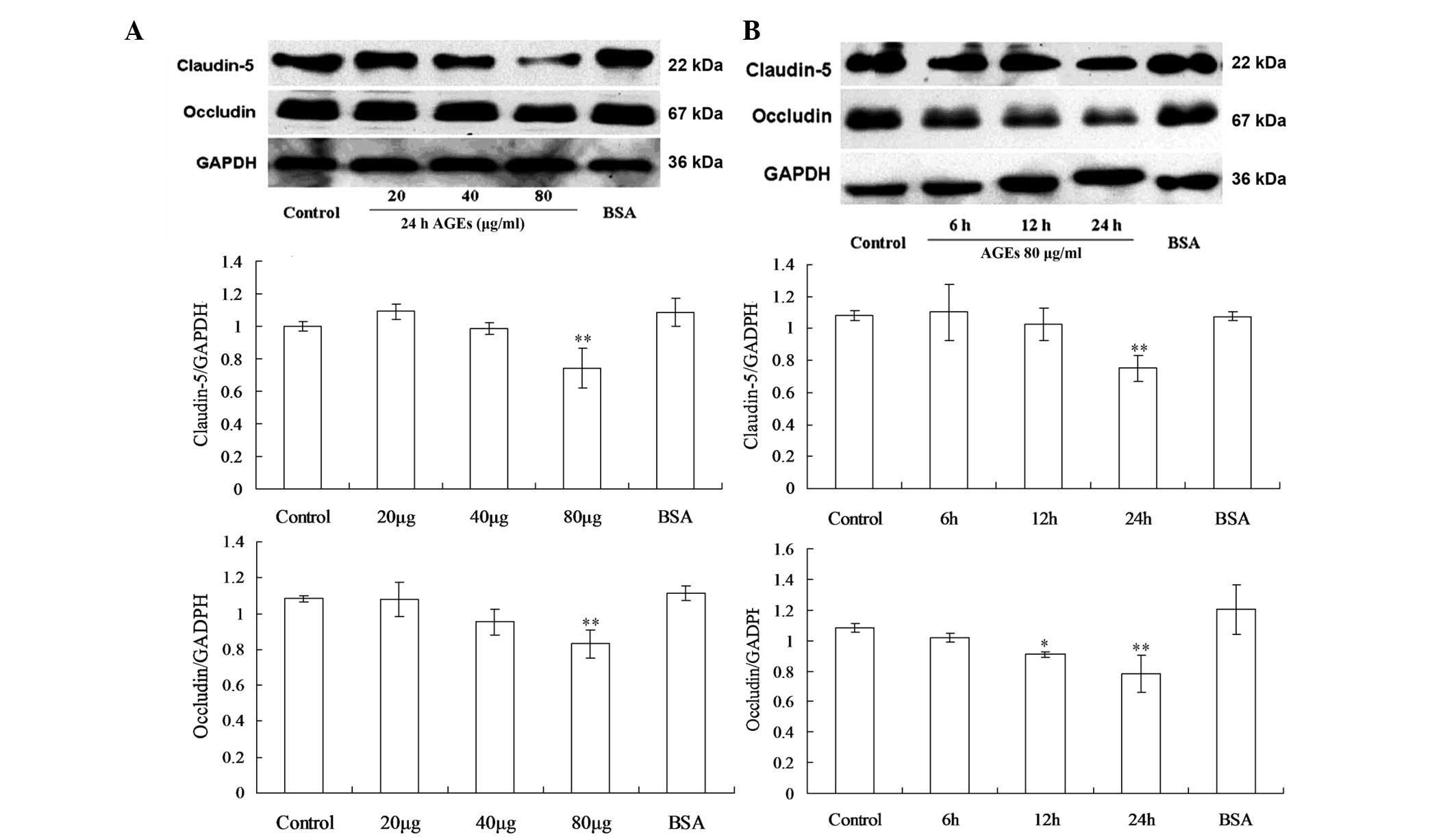
TJ proteins occludin and claudin-5 following AGEs exposure at
different doses and treatment time periods were examined (Fig. 1). In a dose-response study, GEnCs
were exposed to various concentrations of AGEs for 24 h and the
total content of occludin and claudin-5 in the cell lysate was
measured by western blot analysis as described previously. The
results revealed that exposure of GEnCs to 80 μg/ml AGEs
caused a significant decrease of occludin and claudin-5, while
exposure to 40 μg/ml or less of AGEs did not cause
significant changes (Fig. 1A). To
examine the effect of treatment duration, endothelial cells were
exposed to 80 μg/ml AGEs for 6, 12, or 24 h. The expression
of occludin began to decrease after 12 h and reached the lowest
level after 24 h, while claudin-5 did not decrease significantly
unless treated for the full 24 h. (Fig. 1B).
Effects of AGEs on the activities of
MMP-2 and MMP-9 in the GEnCs
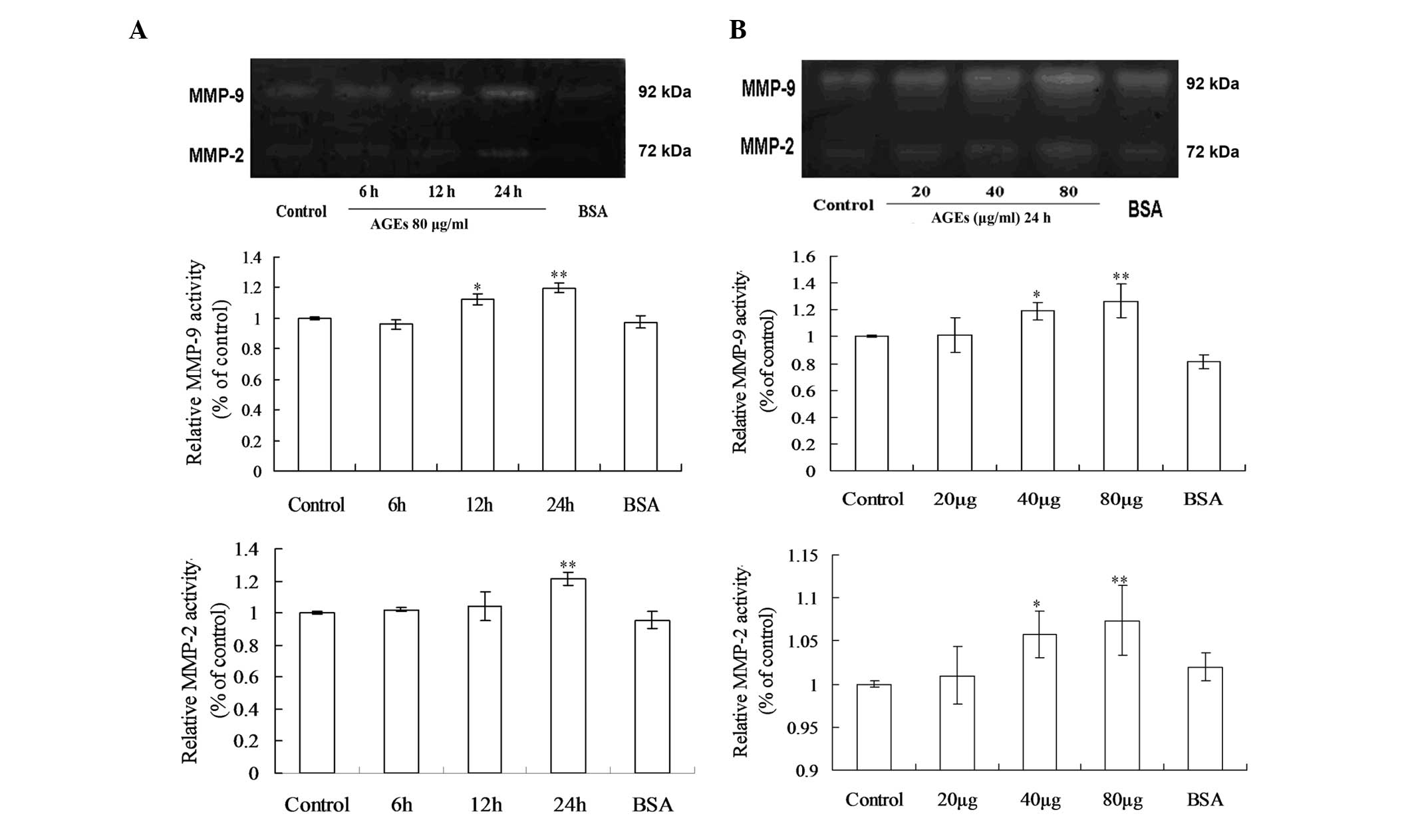
The effects of AGEs on MMP-2 and MMP-9 activity were
investigated using gelatin zymography analysis at different doses
and durations (Fig. 2). In the
time course studies, the results demonstrated that when endothelial
cells were exposed to 80 μg/ml AGEs for 6, 12 and 24 h,
there were significant increases in MMP-9 activity after 12 h
exposure and the activities peaked when treatment was extended to
24 h, while the maximal induction of MMP-2 activity occurred at 24
h at the same concentration of AGEs (Fig. 2A). The dose-response analysis
results demonstrated that the proteolytic activities of MMP-2 and
MMP-9 were significantly higher at 40 μg/ml and 80
μg/ml AGEs for 24 h (Fig.
2B).
GEnC permeability increases in response
to treatment with AGEs and is inhibited by BiPS
To assess the effect of AGE treatment on GEnC
permeability, the TEER of the confluent GEnC monolayers was
measured. After 24 h exposure to 80 μg/ml AGEs, the GEnCs
exhibited a significant decrease in TEER (Fig. 3A). The transendothelial diffusion
of FITC-dextran (4 kDa) after 24 h stimulation with AGEs was also
examined, as this molecule may diffuse across the paracellular
spaces if endothelial TJs are disrupted. The results of
FITC-dextran filtration measurements mirrored the TEER results of
the present study; FITC-dextran translocation across the GEnC
monolayer was higher in the AGE-treated cultures than that in
untreated cultures and BSA did not alter basal permeability of
GEnCs (Fig. 3B). To determine the
effect of MMP-2 and MMP-9 on AGE-induced GEnC hyperpermeability,
BiPS, an inhibitor of MMP-2/MMP-9, was co-administered with the
AGEs. The results revealed that TEER levels and FITC-dextran
filtration did not change significantly when the AGE-stimulated
cells were co-treated with BiPS compared with those of the control
group. These findings supported the role of MMP-2 and MMP-9 as key
mediators of AGE-induced hyperpermeability in GEnCs.
BiPS inhibits the disruption of TJs
between cells induced by AGEs
To examine molecular changes in the TJs following
AGE stimulation, the surface expression levels of occludin and
claudin-5 were measured using immunofluorescence (Fig. 4). In the untreated control
cultures, simultaneous fluorescent labeling of occludin and
claudin-5 revealed continuous lines surrounding the individual cell
peripheries and between cell-cell borders. Exposure to AGEs caused
this labeling to become weaker and discontinuous or fractured
compared with untreated control cultures, particularly in the case
of occludin. When AGEs were co-administered with BiPS, however, the
labeling again appeared as unbroken lines surrounding the cell
peripheries and few fractures (TJ gaps) were observed. Furthermore,
immunostaining was more intense compared with the cells treated
with AGEs alone, indicating that the surface expression of occludin
and claudin-5 was rescued by the MMP-2/9 inhibitor.
BiPS eliminates the effect of AGEs on
occludin, claudin-5 and MMP-2/9
Subsequently, the association between TJ protein
expression and MMP-2/9 activity in the AGE-treated cultures was
examined in the presence and absence of BiPS (Fig. 5). The expression levels of occludin
and claudin-5 were significantly reduced following AGE treatment,
while neither occludin nor claudin-5 expression were significantly
different from those in controls in cultures co-treated with AGEs
and BiPS (Fig. 5A). Concomitant
with the AGE-induced reduction in occludin and claudin-5, MMP-2/9
activity was elevated, while co-administration with BiPS again
reversed this AGE-induced increase in MMP-2/9 activity (Fig. 5B).
Discussion
In the present study, it was demonstrated that
exogenous AGEs significantly increased the permeability of GEnC
monolayers, upregulated the activity of MMP-2/9 and decreased the
expression of the TJ proteins occludin and claudin-5. The organic
MMP-2/9 inhibitor BiPS reversed the AGE-induced hyper-permeability
of GEnCs and the decrease in occludin/claudin-5 expression,
suggesting that AGEs increased transendothelial permeability by
increasing MMP-2/9 activity with subsequent MMP-mediated
degradation of the TJs.
Hyperpermeability is a common vascular complication
associated with diabetes. Several processes have been implicated in
vascular pathogenesis, including the accumulation of AGEs in
endothelial cells (6,7). Consistent with previous studies, it
was identified that AGEs increased the permeability of GEnCs as
evidenced by lower transendothelial electrical resistance and
higher transendothelial FITC-dextran permeability.
Immunofluorescence imaging revealed that the rows of TJs between
the adjacent endothelial cells became fractured or discontinuous,
thus creating low resistance gaps, while immunostaining also
demonstrated weaker surface occludin and claudin-5 expression in
the TJs following exposure. These effects were inhibited by BiPS,
suggesting that AGEs disrupt TJs between the cells, at least in
part, by MMP-mediated proteolysis.
Increased MMP activity can increase vascular
permeability (21,22); therefore, the activity of MMP-2/9
following AGE administration was examined. Indeed, the activity of
MMP-9 was markedly enhanced by the AGEs and this response was dose-
and time-dependent. Previously, MMP-9 was observed to decrease the
expression of occludin and claudin-5, leading to an increased
permeability across the blood-brain barrier and consequent
retinopathy (13,22,23).
Therefore, MMP-9 may be an important factor regulating
microvasculature permeability in numerous tissues. In addition to
MMP-9, MMP-2 also increased following AGE exposure, although to a
lesser extent and later compared with that of MMP-9. Although AGEs
upregulated the activity of MMP-2 and MMP-9, leading to TJ protein
degradation, whether MMP-2, MMP-9, or the two together, mediated
the proteolysis of TJ protein remains to be elucidated. Bojarski
et al (24) demonstrated
that occludin contains a putative extracellular MMP cleavage site
that may allow degradation by MMPs. However, degradation by MMPs
and resistance to MMP-mediated proteolysis have been demonstrated
for claudin-5 (25,26); therefore, further studies are
required to determine the substrate specificities of the individual
MMPs on specific TJ proteins.
TJs provide the material foundation to restrict
vascular permeability to molecules that can either diffuse across
cell membranes or be carried across the membranes by specific
membrane transporters (27–29).
Thus, disruption of TJs is likely to increase vascular permeability
to molecules, which are not normally transported by membrane
permeability or active transport, resulting in the pathological
appearance of serum constituents in urine. Occludin is one of the
main components of TJs in the majority of tissues (30), while claudin-5 is only expressed in
GEnCs in the kidney (31). The two
proteins are critical in maintaining the endothelial barrier
(32–34); therefore, the expression of
occludin and claudin-5 was examined following exposure to AGEs in
the presence or absence of BiPS. Immunoblotting analysis suggested
that AGEs reduced the total cellular content of occludin and
claudin-5, likely due to protein degradation, while expression
levels in the cultures co-treated with AGEs and the MMP-2/9
inhibitor BiPS were not different from those in the untreated
control cultures, again indicating that AGEs reduced the expression
of occludin and claudin-5 by increasing MMP-2/9-mediated
proteolysis.
In conclusion, the present study demonstrated that
AGEs increased the permeability of GEnCs by an MMP-mediated
disruption of intercellular TJs. The disruption of TJs was most
likely mediated by degradation of the TJ proteins occludin and
claudin-5 by MMP-9, MMP-2 or the two in combination. This effect of
AGEs was eliminated by an MMP-2/9 inhibitor. Accumulation of AGEs
from chronic hyperglycemia may increase microvascular permeability
by disrupting the TJs between cells, leading to proteinuria and
other symptoms of diabetic nephropathy.
Acknowledgments
This study was supported by the National Natural
Science Foundation of China (grant no. 30800408).
References
|
1
|
Bhonsle HS, Korwar AM, Chougale AD, Kote
SS, Dhande NL, Shelgikar KM and Kulkarni MJ: Proteomic study
reveals down-regulation of apolipoprotein A1 in plasma of poorly
controlled diabetes: a pilot study. Mol Med Rep. 7:495–498.
2013.
|
|
2
|
Tooke JE: Microvasculature in diabetes.
Cardiovasc Res. 32:764–771. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bonnardel-Phu E, Wautier JL, Schmidt AM,
Avila C and Vicaut E: Acute modulation of albumin microvascular
leakage by advanced glycation end products in microcirculation of
diabetic rats in vivo. Diabetes. 48:2052–2058. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Svensjo E, Arfors KE, Raymond RM and Grega
GJ: Morphological and physiological correlation of
bradykinin-induced macromolecular efflux. Am J Physiol.
236:H600–H606. 1979.PubMed/NCBI
|
|
5
|
Bi YL, Wu MF, Lu LX, Du F, Sun XT, Tang SF
and Xu GT: Functions of corneal endothelial cells do not change
after uptake of superparamagnetic iron oxide nanoparticles. Mol Med
Rep. 7:1767–1772. 2013.PubMed/NCBI
|
|
6
|
Leto G, Pricci F, Amadio L, Iacobini C,
Cordone S and Diaz-Horta O: Increased retinal endothelial cell
monolayer permeability induced by the diabetic milieu: Role of
advanced non-enzymatic glycation and polyol pathway activation.
Diabetes Metab Res Rev. 17:448–458. 2001. View Article : Google Scholar
|
|
7
|
Bonnardel-Phu E, Wautier JL and Vicaut E:
Advanced glycation end products are involved in microvascular
permeability changes observed in microcirculation of diabetic rats
in vivo. J Mal Vasc. 25:122–127. 2000.PubMed/NCBI
|
|
8
|
Soulis-Liparota T, Cooper ME, Dunlop M and
Jerums G: The relative roles of advanced glycation, oxidation and
aldose reductase inhibition in the development of experimental
diabetic nephropathy in the sprague-dawley rat. Diabetologia.
38:387–394. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rojas A and Morales MA: Advanced glycation
and endothelial functions: A link towards vascular complications in
diabetes. Life Sci. 76:715–730. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Camici M: Renal glomerular permselectivity
and vascular endothelium. Biomed Pharmacother. 59:30–47. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Beisswenger PJ, Makita Z, Curphey TJ,
Moore LL and Jean S and Jean S: Formation of immunochemical
advanced glycosylation end products precedes and correlates with
early manifestations of renal and retinal disease in diabetes.
Diabetes. 44:824–829. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Thrailkill KM, Clay Bunn R and Fowlkes JL:
Matrix metalloproteinases: Their potential role in the pathogenesis
of diabetic nephropathy. Endocrine. 35:1–10. 2009. View Article : Google Scholar :
|
|
13
|
Giebel SJ, Menicucci G, McGuire PG and Das
A: Matrix metalloproteinases in early diabetic retinopathy and
their role in alteration of the blood-retinal barrier. Lab Invest.
85:597–607. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Death AK, Fisher EJ, McGrath KC and Yue
DK: High glucose alters matrix metalloproteinase expression in two
key vascular cells: Potential impact on atherosclerosis in
diabetes. Atherosclerosis. 168:263–269. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Thrailkill KM, Bunn RC, Moreau CS,
Cockrell GE, Simpson PM and Coleman HN: Matrix metalloproteinase-2
dysregulation in type 1 diabetes. Diabetes Care. 30:2321–2326.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang L, Wu J, Zhang W, Zhi Y, Wu Y, Jiang
R and Yang R: Effects of aspirin on the ERK and PI3K/Akt signaling
pathways in rats with acute pulmonary embolism. Mol Med Rep.
8:1465–1471. 2013.PubMed/NCBI
|
|
17
|
Ahuja TS, Gopalani A, Davies P and Ahuja
H: Matrix metalloproteinase-9 expression in renal biopsies of
patients with hiv-associated nephropathy. Nephron Clin Pract.
95:c100–c104. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jung JY, Oh JH, Kim YK, Shin MH, Lee D and
Chung JH: Acute UV irradiation increases heparin sulfate
proteoglycan levels in human skin. J Korean Med Sci. 27:300–306.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lauhio A, Sorsa T, Srinivas R, Stenman M,
Tervahartiala T and Stenman UH: Urinary matrix metalloproteinase
-8, -9, -14 and their regulators (try-1, try-2, tati) in patients
with diabetic nephropathy. Ann Med. 40:312–320. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Peng H, Wang C, Ye ZC, Chen YR, Zhang J,
Chen ZJ, Yu XQ and Lou TQ: How increased vegf induces glomerular
hyperpermeability: A potential signaling pathway of rac1
activation. Acta Diabetol. 47:57–63. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Huang W, Eum SY, András IE, Hennig B and
Toborek M: PPARalpha and PPARgamma attenuate HIV-induced
dysregulation of tight junction proteins by modulations of matrix
metalloproteinase and proteasome activities. FASEB J. 23:1596–1606.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yang Y, Estrada EY, Thompson JF, Liu W and
Rosenberg GA: Matrix metalloproteinase-mediated disruption of tight
junction proteins in cerebral vessels is reversed by synthetic
matrix metalloproteinase inhibitor in focal ischemia in rat. J
Cereb Blood Flow Metab. 27:697–709. 2007.
|
|
23
|
Gu Z, Cui J, Brown S, Fridman R, Mobashery
S, Strongin AY and Lipton SA: A highly specific inhibitor of matrix
metalloproteinase-9 rescues laminin from proteolysis and neurons
from apoptosis in transient focal cerebral ischemia. J Neurosci.
25:6401–6408. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bojarski C, Weiske J, Schoneberg T,
Schroder W, Mankertz J, Schulzke J, Florian P, Fromm M, Tauber R
and Huber O: The specific fates of tight junction proteins in
apoptotic epithelial cells. J Cell Sci. 117:2097–2107. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sun X, Han F, Yi J, Hou N and Cao Z: The
effect of telomerase activity on vascular smooth muscle cell
proliferation in type 2 diabetes in vivo and in vitro. Mol Med Rep.
7:1636–1640. 2013.PubMed/NCBI
|
|
26
|
Liu W, Hendren J, Qin XJ, Shen J and Liu
KJ: Normobaric hyperoxia attenuates early blood-brain barrier
disruption by inhibiting MMP-9-mediated occludin degradation in
focal cerebral ischemia. J Neurochem. 108:811–820. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gonzalez-Mariscal L, Tapia R and Chamorro
D: Crosstalk of tight junction components with signaling pathways.
Biochim Biophys Acta. 1778:729–756. 2008. View Article : Google Scholar
|
|
28
|
Anderson JM and Van Itallie CM: Physiology
and function of the tight junction. Cold Spring Harb Perspect Biol.
1:a0025842009. View Article : Google Scholar :
|
|
29
|
Schneeberger EE and Lynch RD: The tight
junction: A multifunctional complex. Am J Physiol Cell Physiol.
286:C1213–C1228. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Feldman GJ, Mullin JM and Ryan MP:
Occludin: Structure, function and regulation. Adv Drug Deliv Rev.
57:883–917. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kiuchi-Saishin Y, Gotoh S, Furuse M,
Takasuga A, Tano Y and Tsukita S: Differential expression patterns
of claudins, tight junction membrane proteins, in mouse nephron
segments. J Am Soc Nephrol. 13:875–886. 2002.PubMed/NCBI
|
|
32
|
Paris L, Tonutti L, Vannini C and Bazzoni
G: Structural organization of the tight junctions. Biochim Biophys
Acta. 1778:646–659. 2008. View Article : Google Scholar
|
|
33
|
Harhaj NS and Antonetti DA: Regulation of
tight junctions and loss of barrier function in pathophysiology.
Int J Biochem Cell Biol. 36:1206–1237. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Xu ZC, Zhang Q and Li H: Differentiation
of human hair follicle stem cells into endothelial cells induced by
vascular endothelial and basic fibroblast growth factors. Mol Med
Rep. 9:204–210. 2014.
|