Introduction
Osmidrosis is one of the most common complaints in
the departments of plastic surgery at the First Affiliated Hospital
of Nanjing Medical University (Nanjing, China). The apocrine
glands, which are located in the axilla, are the predominant cause
of axillary odor (1). This is due
to the fat-like secretion produced by the apocrine glands, which is
broken down into volatile odorous substances by bacteria (2).
Certain factors affect and/or contribute to the
formation of axillary odor, including genetics, gender, age,
developmental stage, diet, treatment history and family history
(3–5). However, the mechanisms underlying the
development of osmidrosis remain to be elucidated. A previous study
demonstrated that genetic and environmental factors are considered
the most significant factors in the development of osmidrosis
(6). The effect of genetics as a
vital contributing factor is also of interest. Increasing evidence
has indicated that the single nucleotide polymorphism (SNP),
rs17822931 (538 G>A), of the ABCC11 gene located on human
chromosome 16q12.1 is associated with axillary osmidrosis (3). ABCC11 is expressed and
localized in the apocrine glands and has a key function in the
secretion of odorants and their precursors (7). A previous study revealed that ~98.7%
of individuals with osmidrosis have the GG or GA genotype (7), which is a significantly larger
proportion compared with the overall population and suggests that
the G allele of A BCC11 may be important in the expression
of axillary odor. Apolipoprotein D (ApoD), a 29 kDa glycoprotein,
is the primary protein component of high-density lipoprotein in
human plasma (8). It has been
demonstrated that ApoD is a physiological carrier of odor
precursors in vivo and its sequence is also expressed in the
apocrine glands (9), indicating a
role for ApoD in the transition of axillary odor precursors.
Furthermore, it has been revealed that levels of ApoD are increased
in individuals with osmidrosis (4). Associations between the rs17822931
SNP of ABCC11, ApoD and axillary odor have been observed.
Notably, the ethnic distribution of the ABCC11 G allele is
similar to that of high-level apocrine ApoD (10). To date, few studies have
investigated the correlation between A BCC11 and
ApoD. Therefore, the present study aimed to further examine
the association between the A BCC11 genotype at rs17822931
and the mRNA expression levels of ApoD in the apocrine
glands of patients with osmidrosis.
Materials and methods
Patients and samples
All procedures were performed according to protocols
approved by the Ethical Review Board of the First Affiliated
Hospital of Nanjing Medical University (Nanjing, China) between
November 2012 and August 2013. A total of 33 patients exhibiting
symptoms of axillary odor and receiving surgery in the Department
of Plastic and Burn Surgery (First Affiliated Hospital of Nanjing
Medical University) were included in the study following the
provision of written informed consent. Peripheral blood (2 ml) was
drawn from all subjects using into EDTA-containing tubes (BD
vacutainer; BD Biosciences, Franklin Lakes, NJ, USA) and stored at
−20°C to obtain genomic DNA. The gender, age, height, weight,
dietary preferences, treatment history and family history of each
patient were recorded. To evaluate the odor severity, the patients
were required to bathe 1 day prior to their procedure and rest in
an examination room (22°C) for 30 min prior to surgery. The entire
axilla was then exposed for odor evaluation, which was performed by
a researcher and plastic surgeon. Four distinct stages were
outlined according to the distance from which the odor was sensed,
which are outlined in Table I.
When there was a disagreement in stage designation, the stage was
evaluated by an additional researcher and the median was used. All
the patients received surgery involving micro-incision subcutaneous
trimming under local anesthesia (Lidocaine, 2% diluted with saline
to 1%; Shanghai Fuxing Chaohui Pharmaceutical Co., Ltd., Shanghai,
China). The subcutaneous tissues, including the apocrine glands of
the axilla, were removed during surgery, immediately frozen in
liquid nitrogen (Changzhou Changyu Practical Gas Co., Ltd.,
Changzhou, China) and transferred for storage at −80°C.
 | Table IStages of odor severity. |
Table I
Stages of odor severity.
| Stage | Distance from odor
detection (cm) |
|---|
| One | <15 |
| Two | ≥15 and <30 |
| Three | ≥30 and <100 |
| Four | ≥100 |
Base-quenched probe genotyping of the
ABCC11 (538 G>A) polymorphism
Individual genomic DNA was extracted from 250
μl samples of peripheral blood using a 3S Blood DNA
Isolation kit (Shenergy Biocolor Co., Shanghai, China), according
to the manufacturer’s instructions. Sequence data for the human
ABCC11 gene was obtained from the National Center for
Biotechnology Information (NCBI) database (http://www.ncbi.nlm.nih.gov/gene/85320#reference-sequences).
Primer Premier 5.0 (Premier Biosoft International, Palo Alto, CA,
USA) was used to design a pair of primers and a probe to
distinguish the nucleotide (538 G>A). The primer and probe
sequences are shown in Table II.
All the specific primers and probe were synthesized and
fluorescence-modified by Sangon Biotech, Co., Ltd. (Shanghai,
China). Taq DNA polymerase, 4X deoxyribonucleotide
triphosphates (dNTPs), 10X polymerase chain reaction (PCR) buffer
(100 mM Tris-HCl, pH 8.3 at 25°C; 500 mM KCl; 15 mM MgCl2; and
0.01% gelatin) and MgCl2 were purchased from Shenergy
Biocolor Co. Briefly, PCR was performed as using 2 μl
genomic DNA template, 2.5 μl 10X PCR buffer, 1.5 μl
25 mM MgCl2, 0.5 μl 4X dNTPs, 1.25 U Taq
DNA polymerase, 0.1 μl 100 μM of each primer and 0.2
μl 10 μM probes in a final reaction volume of 25 μl.
Thermal cycling for ABCC11 (538G>A) was performed on a
LightCycler (Version 480II; Roche Diagnostics, Basel, Switzerland)
under the following conditions: 5 min of initial denaturation at
95°C, followed by 40 cycles at 95°C for 1 sec (temperature
transition rate 44°C/sec), 62°C for 25 sec and 72°C for 10 sec. The
analytical melting program involved melting the PCR products at
95°C for 1 min, 35°C for 2 min and increasing the temperature to
70°C at a transition rate of 0.06°C/sec, with continuous
acquisition of fluorescence data using the LightCycler, as
previously described (11). The
sequences of the homozygous G genotype and the base-quenched probe
formed an exact match resulting in a higher melting temperature
(TM) to enable the different genotypes to be distinguished
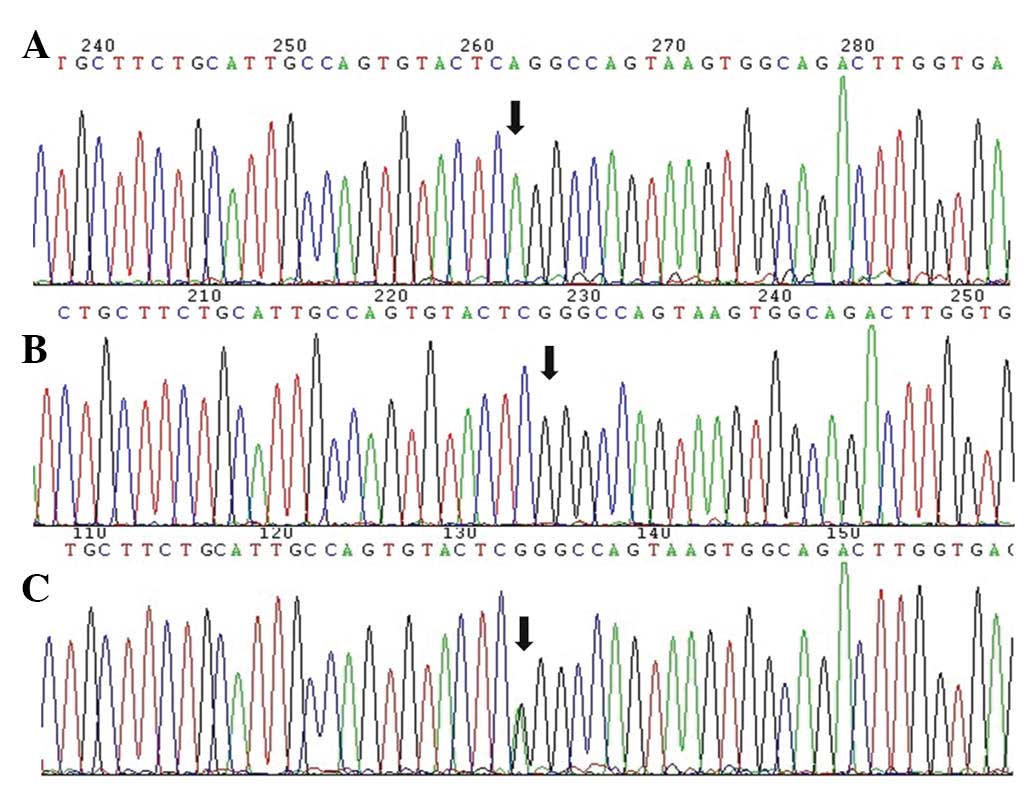
(12). Subsequently, seven samples
were randomly selected and sequenced on an automatic sequencer
(Model 3730, Applied Biosystems, Invitrogen Life Technologies,
Shanghai, China) to verify the genotyping results.
 | Table IISequences of primers and probe of
ABCC11 gene (538 G>A). |
Table II
Sequences of primers and probe of
ABCC11 gene (538 G>A).
| Primer/probe | Primer sequence
(5′–3′) |
|---|
| Forward primer |
gattccaccagttccattatcctct |
| Reverse primer |
cccccaaacctcaccaagtct |
| Probe | agtgtactcgggccagta-FAM |
Reverse transcription-PCR analyses of the
mRNA expression of ApoD
The total RNA of apocrine gland tissues were
extracted using the Total RNAPurification kit (Sangon Biotech Co.,
Ltd, Shanghai, China), according to the manufacturer’s
instructions, no prior steps were required. The quality of the RNA
samples was evaluated by measuring the absorbance at 260/280 nm
(Biophotometer; Eppendorf, Hamburg, Germany). Total RNA (2
μg) was reverse transcribed to cDNA using a RevertAid
FirstStrand cDNA Synthetic kit (Thermo Scientific, Waltham, MA,
USA) according to the manufacturer’s instructions. Sequence data
for human ApoD mRNA was obtained from the NCBI database.
Primer Premier 5.0 software was used to design the primers and
probes for the human ApoD and GAPDH genes. The
primers and probes are shown in Table III. GAPDH was used as the
reference gene. Amplifications were performed in a 7300 Real-time
PCR system (Applied Biosystems Life Technologies, Foster City, CA,
USA). The optimum reaction conditions were obtained using 2.5
μl 10X PCR buffer, 2.5 μl 25 mm MgCl2, 0.5
μl 10 mm 4X dNTPs, 0.25 μl 5 U/μl Taq
DNA polymerase, 0.1 μl 100 μM sense primer, 0.1
μl 100 μM antisense primer, 0.1 μM probe and 2
μl template cDNA. Finally, 16.95 μl ddH2O
was added to the reaction mixture. Standard cycling conditions were
as follows: 95°C for 3 min, followed by 40 cycles at 95°C for 5 sec
(temperature transition rate 4.4°C/sec), 60°C for 27 sec and 40°C
for 1 min. Changes in transcriptional expression were estimated
using the 2−ΔΔCT method (13). Significant differences in gene
transcription were evaluated using Student’s t-test.
 | Table IIIPrimers and fluorescent probes for
quantitative polymerase chain reaction. |
Table III
Primers and fluorescent probes for
quantitative polymerase chain reaction.
|
Gene/primer/probe | Sequence (5′–3′) |
|---|
| ApoD | |
| Forward |
ccagtcaccaagacaggcatc |
| Reverse |
ctggagaagggacctggagc |
| Probe |
FAM-atcggctgattctgcatctggaaact-TAMRA |
| GAPDH | |
| Forward |
ggaaggtgaaggtcggagtc |
| Reverse |
cgttctcagccttgacggt |
| Probe |
FAM-tttggtcgtattgggcgcctg-TAMRA |
Statistical analysis
Statistical analyses were performed using SPSS
version 13.0 (SPSS, Inc., Chicago, IL, USA). P<0.05 was
considered to indicate a statistically significant difference.
Quantitative data was evaluated to determine whether the various
data sets were normally distributed. Age and BMI of patients were
expressed as mean ± standard deviation and ApoD mRNA levels were
expressed as median with interquartile range. Student’s t-test was
used for two-group comparisons of normally distributed data sets,
and abnormally distributed data sets were modified by square root
transformation prior to comparison. Fisher’s exact test was applied
to graded data.
Results
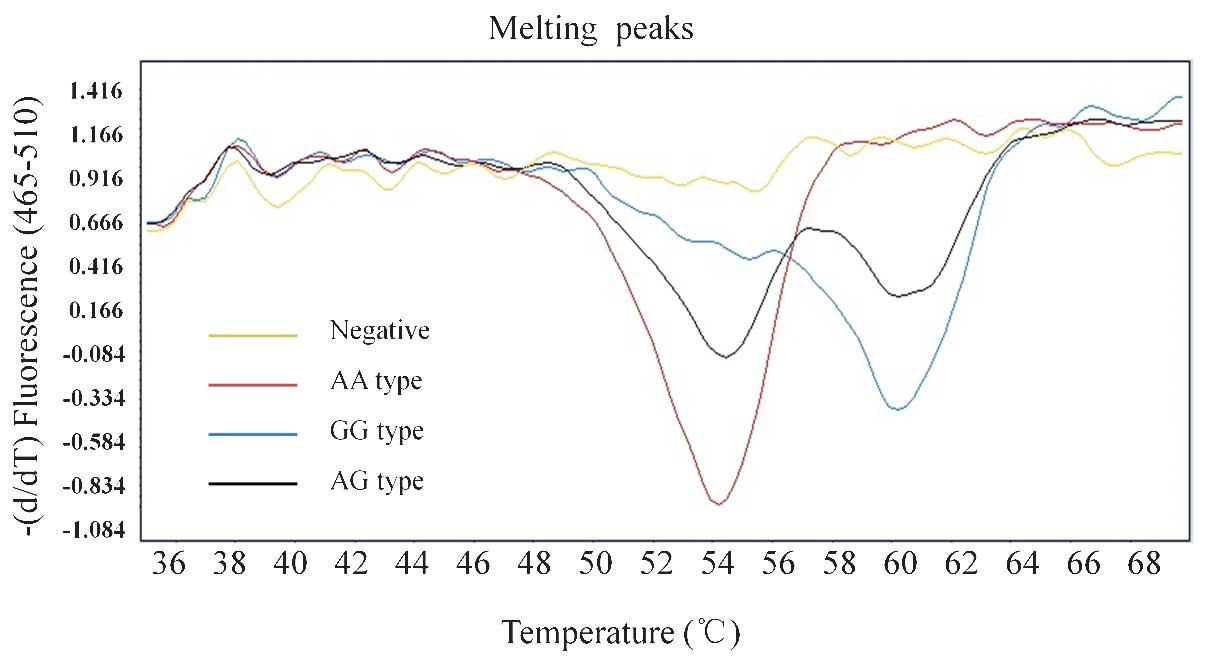
Genotyping of ABCC11 538 G>A
polymorphisms using the base-quenched probe method
The biallelic SNP marker (G/A) of A BCC11 538
G>A yielded three distinct genotypes, including G homozygote, A
homozygote or GA heterozygote. The homozygous G genotype and the
base-quenched probe had corresponding base pairs, resulting in a
high melting temperature (TM). When the homozygous A genotype was
present, a mismatch in the base pairs occurred and the TM was
reduced, as previously described (11). The heterozygous genotype was
characterized by two TM valleys (Fig.
1). In the 33 patients, the GG, GA and AA genotypes were
present in 1,27 and 5 individuals, respectively. As the allele G
was the dominant gene, the patients were divided into two groups:
Group I, comprising individuals with the GG or GA genotype and
Group II, comprising individuals with the AA genotype. Complete
concordance was observed between the results of the DNA sequencing
and the base-quenched probe method (Fig 2).
Differences in other factors between the
groups
As indicated in Table
IV, statistically significant differences (P<0.05) were
observed in gender, family history and odor severity between the
groups, whereas no significant differences (P>0.05) were
observed in age, body mass index (BMI) or diet between the
groups.
 | Table IVPatient characteristics. |
Table IV
Patient characteristics.
| Characteristic | Value |
|---|
| Number of patients
(n) | 33 |
| Gender, n (%) |
| Male | 9 (27.27) |
| Female | 24 (72.73) |
| By group
(male/female; n) | I (9/19)
II (0/5) |
| Age (years) |
| Mean | 24.44±6.76 |
| Range | 16–44 |
| By group | I
(24.22±7.01)
II (25.60±5.73) |
| BMI
(kg/m2) |
| Mean BMI | 21.35±3.00 |
| By group | I
(21.48±3.10)
II (20.70±2.57) |
| Family history, n
(%) |
| With
osmidrosis | 24 (72.73) |
| Without
osmidrosis | 9 (27.27) |
| By group
(with/without; n) | I (23/5), II
(1/4) |
| Dietary preference,
n (%) |
| Meat or
capsicum | 17 (51.52) |
| No meat or
capsicum | 16 (48.48) |
| By group (meat or
capsicum/none; n) | I (14/14)
II (3/2) |
| Odor severity
stage, n (%) |
| One | 5 (15.15) |
| Two | 8 (24.24) |
| Three | 11 (33.33) |
| Four | 9 (27.27) |
| By group
(one/two/three/four; n) | I
(2/7/10/9)
II (3/1/1/0) |
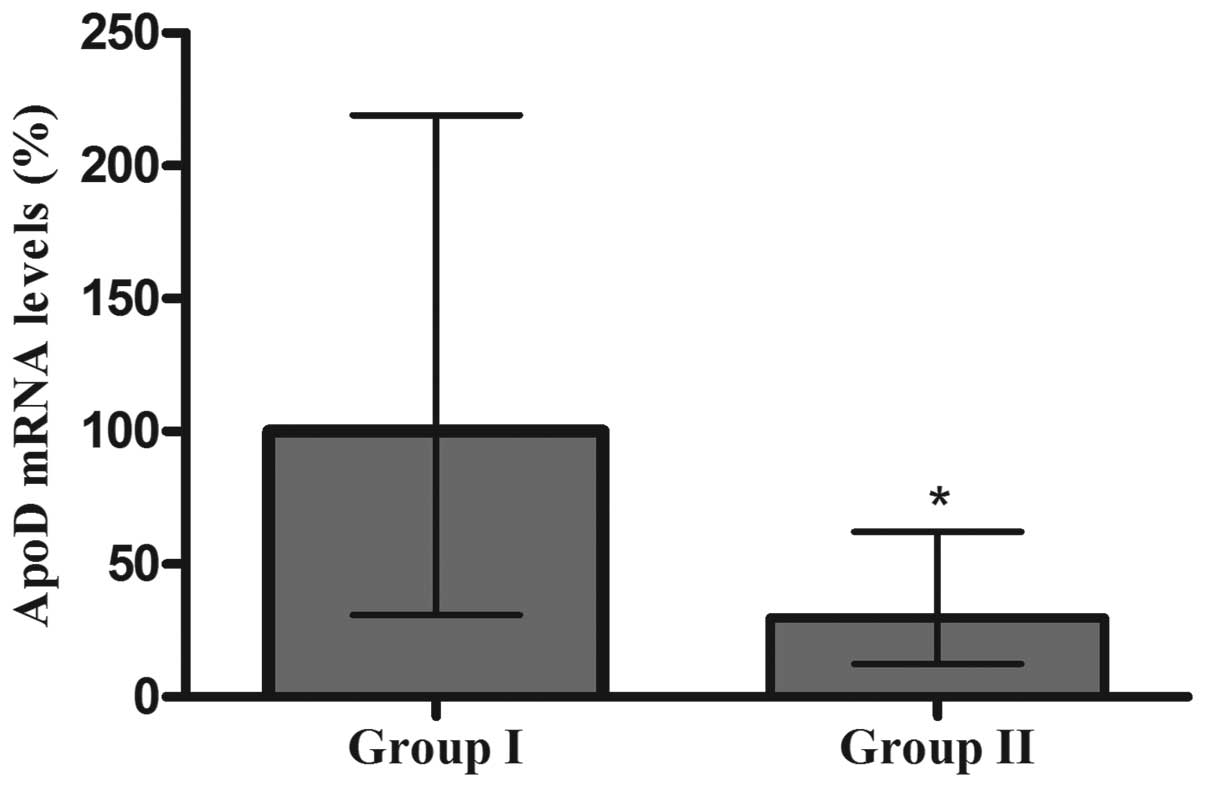
PCR analysis of the mRNA expression of
ApoD
As shown in Fig. 3,
the relative mRNA expression levels of ApoD were significantly
higher in the Group I (100.0%, interquartile ranged from 35.96% to
217.5%) compared with those in the Group II (28.38%, interquartile
ranged from 11.98% to 59.11%; P<0.05).
Discussion
Osmidrosis is a hereditary condition (14). It has been demonstrated that the
538 G>A (Gly180Arg) SNP of the ABCC11 gene is associated
with axillary odor in the Japanese population (7), in which individuals with the AA
genotype exhibited elimination of odor. This mutation has
previously been recommended as a diagnostic standard (7), however, odor is a subjective factor
and is readily affected by several other factors, including
hormones (4), the environment
(6) and dietary intake (5). As a physiological factor, the effects
of genetic mutations do not take these factors into account and,
therefore, the genetic diagnosis of osmidrosis may be
inaccurate.
Axillary odor is comprised of a mixture of numerous
substances, among which (E)-3-methyl-2-hexenoic acid (E-3M2H) is
one of the key odorous components (15). ApoD is involved in the
transportation of E-3M2H by covalently bonding to it, forming an
odor precursor (16). A previous
study revealed that ApoD functions as one of the key transport
proteins in the process of odor production and that ApoD is
expressed at significantly higher levels in patients with
osmidrosis compared with individuals without significant axillary
odor (4).
The SNP of the ABCC11 gene and the ApoD
transfer protein have previously been associated with axillary
odor; however, whether there is a correlation between the 538
G>A SNP and the expression of ApoD remains to be
elucidated (10). Therefore, the
present study further investigated the association between the 538
G>A SNP of ABCC11 and the mRNA expression levels of
ApoD in the apocrine glands. As the G allele is dominant,
the GG and GA genotypes are phenotypically identical (17). The patients were divided into two
groups according to genotype: Group I included individuals with GG
or GA genotypes and Group II includedindividuals with the AA
genotype. It has been reported that ApoD mRNA is exclusively
expressed in the apocrine glands of the axillary tissues (9); therefore, the ApoD mRNA
detected from axillary subcutaneous tissues accurately represents
the expression of ApoD by the apocrine glands. Additionally,
other possible influencing factors, including gender, age, BMI,
diet, odor severity and family history, were also evaluated. A
previous study suggested that ApoD may be a downstream
target of regulating androgen receptor signals (18). The apocrine glands are direct
targets of steroid hormone signaling and steroid hormones may
therefore affect their activity (19). In vivo, steroid hormone
levels vary amongst individuals at various time-points,
particularly between genders. Therefore, fluctuation in axillary
odor is frequently observed, emerging during teenage years,
remaining throughout young adulthood and gradually declining to
absence in individuals over 50 years old. However, steroid hormones
have been observed to affect the quantity and distribution of body
fat and have a negative correlation with obesity (20,21).
For these reasons, individuals requiring surgery and aged between
15 and 45 years old were selected, and variations in age, gender
and BMI were analyzed. Family history may reflect the heredity
characteristic of osmidrosis and introduce an additional genetic
interference factor, therefore, family history was also analyzed
for its potential association with the ABCC11 gene. In
addition, dietary intake, particularly red meat, may alter the type
and quantity of axillary fatty acid secretion in a short period and
lead to a change in odor severity (5). Therefore, these data were analyzed
and their potential effects on the experimental findings were
evaluated. Significant differences were detected in gender, family
history and severity of odor, but not in age, BMI or dietary intake
between the two groups. The variations in gender between the groups
may have been due to men’s ignorance of mild odor, resulting in the
male subjects having a moderate or severe odor, which led to a
selection bias. The differences in family history provided evidence
for the genetic relevance of osmidrosis, which was clear in the
results from patients with the G allele. The quantitative analysis
demonstrated that the mRNA expression of ApoD was higher in
patients with the G allele at 538 G>A compared with those
without the G allele, suggesting that this SNP may affect the
expression of ApoD. These results suggested that an SNP
leading to a G180R substitution in the corresponding protein may
decrease the transcription of ApoD through an unknown
complex pathway.
Acknowledgments
The authors would like to thank Mrs. Jun Zhang and
Miss. Li Qin for their technical assistance and critical editing of
the manuscript.
References
|
1
|
Bang YH, Kim JH, Paik SW, Park SH, Jackson
IT and Lebeda R: Histopathology of apocrine bromhidrosis. Plast
Reconstr Surg. 98:288–292. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zeng X, Leyden JJ, Brand JG, Spielman AI,
McGinley KJ and Preti G: An investigation of human apocrine gland
secretion for axillary odor precursors. J Chem Ecol. 18:1039–1055.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Martin A, Saathoff M, Kuhn F, Max H,
Terstegen L and Natsch A: A functional ABCC11 allele is essential
in the biochemical formation of human axillary odor. J Invest
Dermatol. 130:529–540. 2010. View Article : Google Scholar
|
|
4
|
Chen H, Li Y, Du J, Cao Y and Li X:
Increased JNK1 activity contributes to the upregulation of ApoD in
the apocrine secretory gland cells from axillary osmidrosis. Mol
Cell Biochem. 354:311–316. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Havlicek J and Lenochova P: The effect of
meat consumption on body odor attractiveness. Chem Senses.
31:747–752. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Penn DJ, Oberzaucher E, Grammer K, et al:
Individual and gender fingerprints in human body odour. J R Soc
Interface. 4:331–340. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nakano M, Miwa N, Hirano A, Yoshiura K and
Niikawa N: A strong association of axillary osmidrosis with the wet
earwax type determined by genotyping of the ABCC11 gene. BMC Genet.
10:422009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rassart E, Bedirian A, Do Carmo S, et al:
Apolipoprotein D. Biochim Biophys Acta. 1482:185–198. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zeng C, Spielman AI, Vowels BR, Leyden JJ,
Biemann K and Preti G: A human axillary odorant is carried by
apolipoprotein D. Proc Natl Acad Sci USA. 93:6626–6630. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Preti G and Leyden JJ: Genetic influences
on human body odor: from genes to the axillae. J Invest Dermatol.
130:344–346. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zheng L, Luo G, Zhang X, et al: A novel
method of detecting mitochondrial m1494C>T and m1555A>G
mutations in a single PCR reaction using base-quenched probe. Clin
Chim Acta. 411:2114–2116. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Luo G, Zheng L, Zhang X, Zhang J,
Nilsson-Ehle P and Xu N: Genotyping of single nucleotide
polymorphisms using base-quenched probe: a method does not
invariably depend on the deoxyguanosine nucleotide. Anal Biochem.
386:161–166. 2009. View Article : Google Scholar
|
|
13
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-delta delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
14
|
Qian JG and Wang XJ: Effectiveness and
complications of subdermal excision of apocrine glands in 206 cases
with axillary osmidrosis. J Plast Reconstr Aesthet Surg.
63:1003–1007. 2010. View Article : Google Scholar
|
|
15
|
Zeng X, Leyden JJ, Lawley HJ, Sawano K,
Nohara I and Preti G: Analysis of characteristic odors from human
male axillae. J Chem Ecol. 17:1469–1492. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Spielman AI, Zeng XN, Leyden JJ and Preti
G: Proteinaceous precursors of human axillary odor: isolation of
two novel odor-binding proteins. Experientia. 51:40–47.
1995.PubMed/NCBI
|
|
17
|
Inoue Y, Mori T, Toyoda Y, et al:
Correlation of axillary osmidrosis to a SNP in the ABCC11 gene
determined by the Smart Amplifcation Process (SmartAmp) method. J
Plast Reconstr Aesthet Surg. 63:1369–1374. 2010. View Article : Google Scholar
|
|
18
|
Appari M, Werner R, Wünsch L, et al:
Apolipoprotein D (APOD) is a putative biomarker of androgen
receptor function in androgen insensitivity syndrome. J Mol Med
(Berl). 87:623–632. 2009. View Article : Google Scholar
|
|
19
|
Beier K, Ginez I and Schaller H:
Localization of steroid hormone receptors in the apocrine sweat
glands of the human axilla. Histochem Cell Biol. 123:61–65. 2005.
View Article : Google Scholar
|
|
20
|
Santosa S and Jensen MD: Sex and sex
steroids: impact on the kinetics of fatty acids underlying body
shape. Horm Mol Biol Clin Investig. 20:15–23. 2014.PubMed/NCBI
|
|
21
|
Tchernof A, Mansour MF, Pelletier M, et
al: Updated survey of the steroid-converting enzymes in human
adipose tissues. J Steroid Biochem Mol Biol 14. 147C:56–69.
2014.
|