Introduction
Hemodialysis (HD) and peritoneal dialysis (PD) are
the primary dialysis modalities used for patients with end-stage
renal disease (ESRD). PD is a simple, ‘low-tech’ form of renal
replacement therapy that is less expensive than conventional
in-center hemodialysis in numerous parts of the world (1). Since 1980, there has been a rapid
increase in the number of patients receiving PD (2). In 2008, the number of patients
receiving PD globally was ~196,000, which accounted for 11% of the
total number of those on dialysis. In total, 59% were treated in
developing countries and 41% in developed countries (3). The number of patients worldwide who
were treated with PD increased from 1997 to 2008, with a 2.5-fold
increase in the prevalence of patients receiving PD in developing
countries (3). However, due to
continuous exposure to non-biocompatible PD solution, the
peritoneal membrane undergoes structural and functional
alterations, which ultimately lead to peritoneal fibrosis and
therefore limit the long-term clinical application of PD (4,5).
The use of glucose-based peritoneal dialysis fluid
(PDF) has a number of disadvantages, including high concentrations
of hyperosmotic glucose and glucose degradation products (GDPs),
which may activate various inflammatory cytokines and growth
factors that result in damage to the peritoneum and the development
of fibrosis in peritoneal mesothelial cells (PMCs). Recent studies
have shown that high glucose peritoneal dialysis solutions may
result in peritoneal fibrosis (6,7).
However, further research is required into the induction of
peritoneal fibrosis by glucose degradation products.
Heat sterilization of glucose-based PDFs produces
GDPs, which accelerate the formation of advanced glycation
end-products (AGEs) within the peritoneal cavity. The accumulation
of AGEs is observed in peritoneal mesothelial and submesothelial
layers in patients on continuous ambulatory PD (CAPD), in
association with peritoneal thickening (8,9).
However, the role of AGEs in the development of peritoneal fibrosis
has remained elusive.
HSP70, a member of the heat-shock family of
proteins, is an ATP-dependent molecular chaperone. It participates
in numerous signaling pathways that are involved in growth control,
cell survival and developmental processes (10). However, the role of HSP70 in
AGEs-induced epithelial-to-mesenchymal transition (EMT) of
peritoneal mesothelial cells remains to be elucidated. The present
study aimed to investigate the mechanisms of AGEs-induced EMT,
changes in the expression of HSPs, as well as the role of HSP70 in
peritoneal mesothelial cells during EMT.
Materials and methods
Reagents
AGEs were obtained from (Sigma-Aldrich, St. Louis,
MO, USA). Dulbecco’s modified Eagle’s medium (DMEM)/F12 and fetal
bovine serum (FBS) were obtained from Gibco Laboratories (NY, New
York, USA). Dimethyl sulfoxide (DMSO) and Triton X-100 were
purchased from Sigma-Aldrich. Mouse monoclonal immunglobulin (Ig)G
anti-β-actin (1:400) and rabbit polyclonal IgG anti-phospho
(p)-Smad3,4 (1:400) antibodies were obtained from Santa Cruz
Biotechnology, Inc. (Dallas, TX, USA). Mouse monoclonal IgG1
anti-α-smooth muscle actin (α-SMA; 1:400) and mouse monoclonal IgG1
anti-E-cadherin (1:400) antibodies were obtained from BD
Biosciences (Heidelberg, Germany), mouse monoclonal IgG anti-HSP70
(1:400) was obtained from Sigma-Aldrich. An enhanced
chemiluminescence (ECL) kit was obtained from Pierce Biotechnology,
Inc. (Rockford, IL, USA). The reverse transcription-polymerase
chain reaction (RT-PCR) kit was obtained from Toyobo Co., Ltd.
(Osaka, Japan). The flow cytometer (FACSCalibur), was obtained from
Becton-Dickinson (Franklin Lankes, NJ, USA). All reagents used were
of trace element analysis grade. All water used was glass
distilled.
Isolation of RPMCs and
identification
A total of 4 Sprague-Dawley rats (aged, 12–16 weeks;
weight, 0.2–0.4 kg) of both genders were used for the following
experiments. All animal procedures were approved by the ethics
committee of Xinxiang Medical University (Henan, China) and were
performed in compliance with the European Economics Community
regulations (O.J. of E.C.L358 12/18/1986) and the National
Institute of Health standards (11). Rat peritoneal mesothelial cells
(RPMCs) were isolated and cultured. Briefly, surgically resected
omenta from sacrificed Sprague-Dawley rats were digested with
0.125% trypsin (Sigma-Aldrich) for 25 min at 37°C, followed by
neutralization with DMEM/F12 medium supplemented with 10% FBS. The
suspension was centrifuged at 60 × g for 5 min at 4°C and the
pellet was resuspended in DMEM/F12 with 10% FBS. Following
incubation for 1–3 days at 37°C, the medium was changed for the
first time. Cells were then transferred to serum-free DMEM/F12
medium for overnight starvation prior to subsequent experiments.
RPMCs were observed under a phase contrast inverted microscope
(CKX41; Olympus Corp., Tokyo, Japan) and identified using
immunocytochemistry. RPMCs were treated with various concentrations
of AGEs (0, 0.6, 1.2 and 2.5 mg/ml) for 24 h.
Immunocytochemistry (ICC)
Cells were grown on 96-well imaging plates (BD
Biosciences) and fixed using 4% w/v paraformaldehyde in 1X
phosphate-buffered saline (PBS; NaCl, KCl,
Na2HPO4, K2HPO4,
CaCl2, MgCl2•6H2O,
ddH2O) with Ca2+ and Mg2+ for 30
min at room temperature. Cells were washed with PBS and a blocking
buffer [4% v/v normal donkey serum (NDS)/normal goat serum (NGS),
1% w/v bovine serum albumin (BSA; Sigma-Aldrich) and 0.1% Triton-X
100 in PBS, pH 7.4] was applied for 30 min at room temperature. The
following primary antibodies were diluted in Ab solution (2% v/v
NDS/NGS, 0.5% w/v BSA and 0.05% Triton-X 100 in PBS) and applied to
cells for incubation overnight at 4°C: Cytokeratin (sc-57004;
1:400), vimentin (sc-373717; 1:400), VIII-related antigen
(sc-366000; 1:200) and CD45 (sc-28369; 1:200) (Santa Cruz
Biotechnology, Inc.). Following washing with PBS, the secondary
antibodies (FITC-conjugated and tetramethylrhodamine-conjugated
goat anti-mouse IgG antibodies; Santa Cruz Biotechnology, Inc.)
were diluted in Ab solution and applied at room temperature for 1
h. Cells were subsequently washed and Hoescht stain (Sigma-Aldrich)
was applied prior to the addition of antifade reagent (CitiFluor
Ltd., London, UK), which was then left to permeate at room
temperature for 1 h prior to imaging with the GPJ9-TS100-F
fluorescence microscope (Nikon Corp., Tokyo, Japan). RPMCs were
positive for cytokeratin and vimentin and negative for factor
VIII-related antigen and leukocyte CD45 antigen.
Detection of intracellular reactive
oxygen species (ROS) levels
In order to determine ROS generation within
AGEs-treated cells, FACS analysis was performed. Cells were stained
with 5 μg/ml DCF-DA for 30 min and subjected to flow
cytometry using a Becton-Dickinson FACSCalibur prior to analysis by
Cell Quest software, version 5.2.1 (Becton-Dickinson).
RNA interference plasmid construction and
transient transfection
Small interfering RNA (siRNA) corresponding to the
rat HSP70 cDNA was generated by cloning the synthesized
oligonucleotide into pSilencer 2. 1-U6-neo plasmid by Genechem Co.
(Shanghai, China). The following sequences for anti-Hsp70 were
used: Forward, 5′-UUACCUGGCUCUUUGCUGCUGCUCC-3′ and reverse,
5′-GGAGCAGCAGCAAAGAGCCAGGUAA-3′. The siRNA-HSP70 targeting
sequences matched exactly with partial sequences of the rat HSP70
gene, but not with any other known genes. The control siRNA did not
match any known rat gene. According to the manufacturer’s
instructions, transient transfections were performed using
Lipofectamine 2000 (Invitrogen Life Technologies, Carlsbad, CA,
USA). These experiments were performed in six-well culture plates.
Transfection efficiency averaged between 50–60%. Cells were allowed
to recover in medium for 24 h following transfection. The cultures
were then treated with agents and subjected to further
analysis.
HSP70 overexpression plasmid construction
and transient transfection
According to the manufacturer’s instructions,
plasmids expressing HSP70-cDNA gene were transfected into RPMCs
using Lipofectamine 2000. These experiments were performed in
six-well culture plates. Transfection efficiency averaged between
50–60%.
Preparation of total cellular
proteins
RPMC lysates were prepared using 20 mM Tris-HCl, pH
7.4; 150 mM NaCl; 1% Triton X-100; 2.5 mM EDTA; 2.5 mM EGTA; and
1:200 protease inhibitor (Calbiochem, La Jolla, CA, USA). Lysates
were kept on ice for 30 min and centrifuged at 10,000 × g for 10
min at 4°C. Protein concentration was determined using a
bicinchoninic acid protein assay kit (Pierce Biotechnology, Inc.)
according to the manufacturer’s instructions.
Western blot analysis
Samples containing 10 μg protein were
electrophoresed and then transferred to nitrocellulose membranes
(Millipore, Billerica, MA, USA). The nitrocellulose membrane was
cut according the molecular weight of protein and incubated with
primary antibodies. The following primary antibodies were used:
Anti-α-SMA (1:400), anti-β-actin (1:400), anti-E-cadherin (1:400),
anti-HSP70 (1:400) and anti-phospho Smad3,4 (1:400), and incubated
overnight at 4°C. The horseradish peroxidase-conjugated secondary
IgG antibodies (goat anti-mouse and goat anti-rabbit; 1:5000; Santa
Cruz Biotechnology, Inc.) were subsequently appliedfor 1 h at 4°C.
Detection was performed using the ECL kit. The quantity of each
protein was measured relative to that of β-actin. The results were
quantified by Quantity One Software v4.62 (Bio-Rad Laboratories,
Hercules, CA, USA).
Fluorescence microscopy
RPMCs were seeded in six-well plates for 24 h. Cells
were washed once with ice-cold PBS and fixed with 4%
paraformaldehyde (Sigma-Aldrich) for 30 min at 4°C. Cells were then
washed with PBS three times and incubated with 1% Triton X-100 for
10 min. Cells were blocked at nonspecific antibody binding sites by
incubating with 10% goat serum (Gibco Laboratories) in PBS
containing 0.3% Triton X-100 and 0.5% BSA for 30 min at room
temperature, followed by incubation with a mouse monoclonal
antibody against HSP70 (1:400) overnight. A fluorescein
isothiocyanate-conjugated goat anti-mouse IgG antibody (1:100 in
PBS) was added and incubated for 0.5 h at room temperature. Hoechst
33342 (Sigma-Aldrich) was added to the cells for 15 min. Cells were
washed three times with PBS and visualized under fluorescence
microscopy (GPJ9-TS100-F; Nikon Corp.).
RT-PCR
Total RNA from cultured cells was isolated using
TRIzol reagent (Invitrogen Life Technologies). RNase-free DNase I
was used in order to eliminate genomic DNA contamination in the RNA
samples. The 260/280 absorbance ratio was measured for verification
of the purity of RNA. The sequences of the HSP70 and β-actin genes
were obtained from the GenBank database (www.ncbi.nlm.nih.gov/genbank/), and specific primers
for them were designed over an exon-exon junction using Primer
Premier 5.0 (Premier Biosoft, Palo Alto, CA). The following primers
were used: Forward, 5′-AGCGGGAAATCGTCGGTG-3′, and reverse,
5′-GGGTACATGGTGGTGCCG-3′ for β-actin; forward,
5′-ACCAACTATTGCTTCAGCTC-3′ and reverse, 5′-CTTGCAGGAGCGCACGATCA-3′
for transforming growth factor-β (TGF-β); and forward,
5′-TACATATGGCCAAAGCCGCGGCAGTCG-3′ and reverse,
5′-TGCTCGAGATCTACCTCCTCAATGGTGGG-3′ for HSP70. PCR reactions were
performed using a Gene Amp PCR system 9700 (PerkinElmer, Inc.,
Waltham, MA, USA) and amplified as follows: cDNA templates were
initially heat-denatured at 94°C for 3 min followed by 94°C for 1
min, annealing at 58–65°C for 1 min, extension at 72°C for 1 min
(35 cycles) and a final extension cycle at 72°C for 10 min. The
amplified products were separated by electrophoresis on a 2%
agarose gel (Sigma-Aldrich) and visualized by ethidium bromide
staining (Sigma-Aldrich). Image density was quantified using a
FluoroImager SI (GE Healthcare, Little Chalfont, UK).
Statistical analysis
Values are expressed as the mean ± standard error of
the mean. Statistical significance was established by analysis of
variance and individual comparisons were then made using Tukey’s
multiple comparison test. P<0.05 was considered to indicate a
statistically significant difference. Statistical analysis was
performed using SPSS Version 18 (International Business Machines,
Armonk, NY, USA).
Results
Effect of AGEs on EMT in PMCs
In order to elucidate the mechanism underlying the
induction of EMT by AGEs, the expression of E-cadherin and α-SMA in
RPMCs was measured under different conditions. The PMCs were
cultured with 1.2 mg/ml BSA or 1.2 mg/ml AGEs for 24 h. The results
demonstrated that treatment with AGEs decreased the expression of
the E-cadherin protein and increased that of the α-SMA protein,
compared with those in the control or BSA group (P<0.01;
Fig. 1A–C). Western blotting
showed a dose-dependent change in the expression of EMT-associated
proteins in AGE-treated PMCs (Fig.
1D–F). The results indicated that AGEs induced EMT and led to
increased EMT events in PMCs.
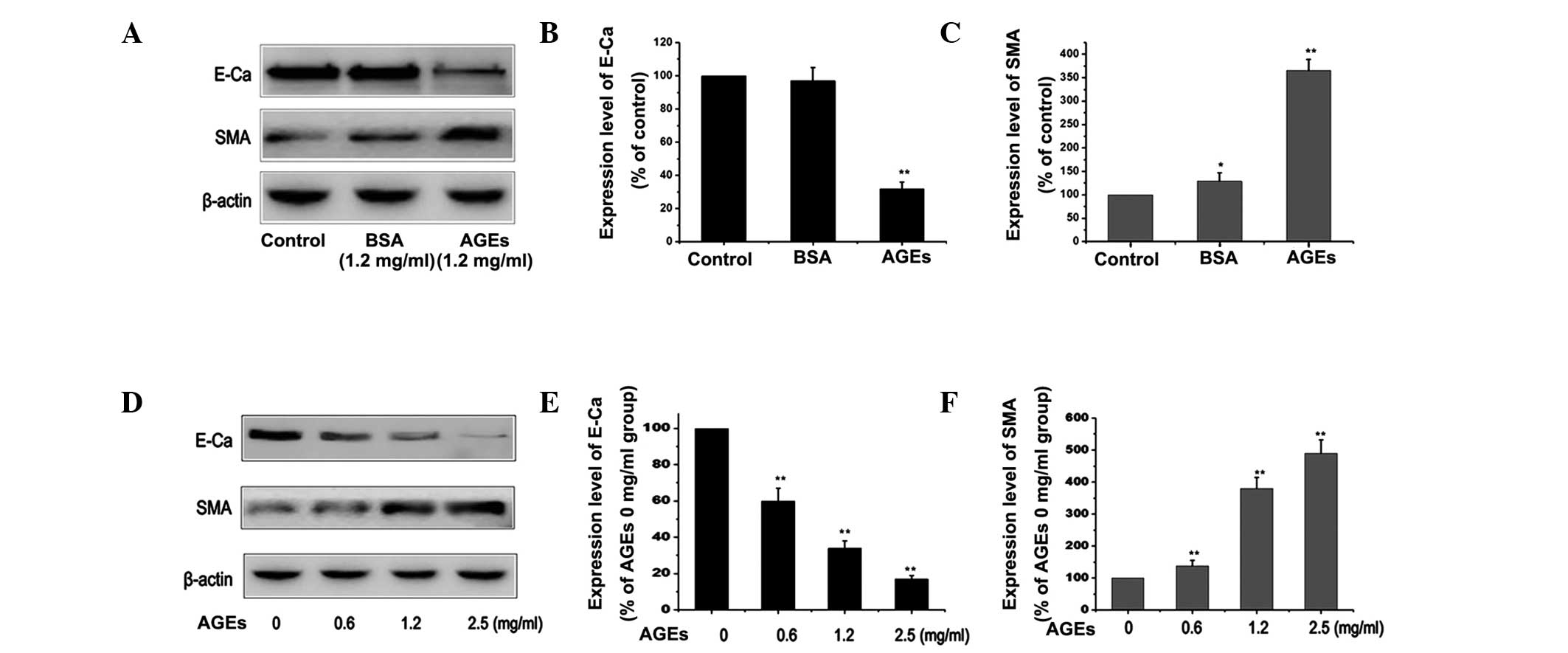 | Figure 1Effect of AGEs on the expression of
E-Ca and α-SMA in RPMCs. (A) RPMCs were treated with 1.2 mg/ml
bovine serum albumin or 1.2 mg/ml AGEs for 24 h in order to induce
EMT. The expression of E-Ca and α-SMA was measured by western
blotting. (B and C) Quantitative results from western blotting
experiments. Values are presented as the mean ± SEM of three
independent experiments. β-actin was used as a loading control.
**P<0.01 vs. control, *P<0.05 vs.
control. (D) RPMCs were treated with varying concentration of AGEs
(0, 0.6, 1.2 and 2.5 mg/ml) for 24 h in order to induce EMT. The
expression of E-Ca and α-SMA were detected by western blotting. (E
and F) Quantitative results from western blotting. Values are
presented as the mean ± SEM of three independent experiments.
β-actin was used as loading control. **P<0.01 vs. 0 h
group. AGEs, advanced glycation end-products; α-SMA, α-smooth
muscle actin; RPMCs, rat peritoneal mesothelial cells; EMT,
epithelial-to-mesenchymal transition; SEM, standard error of the
mean; E-Ca, E-Cadherin; BSA, bovine serum albumin. |
AGEs activate the TGF-β/Smad pathway in
RPMCs
The TGF-β/Smad signaling pathways have been reported
to be involved in EMT (12). The
present study aimed to investigate whether TGF-β/Smad signaling in
RPMCs is involved in AGEs-induced EMT. RPMCs were treated with 1.2
mg/ml BSA or 1.2 mg/ml AGEs for 24 h in order to induce EMT, and
the expression of TGF-β mRNA and p-Smad3,4 were detected by RT-PCR
and western blotting, respectively. Under controlled conditions
(1.2 mg/ml BSA for 24 h), administration of BSA did not increase
the levels of TGF-β mRNA and the p-Smad protein. By contrast,
following exposure to AGEs, levels of TGF-β mRNA and p-Smad3,4
protein were found to be markedly increased compared with those in
the control or BSA group (Fig. 2A
and E). Fig. 2C and H show a
dose-dependent change in the expression of TGF-β mRNA and p-Smad3,4
in PMCS treated with AGEs, as detected by RT-PCR and western
blotting. These results supported the hypothesis that AGEs
effectively activate the TGF-β/Smad signaling pathways.
 | Figure 2Effect of AGEs on the TGF-β/Smad
pathway in RPMCs. (A) RPMCs were treated with 1.2 mg/ml BSA or 1.2
mg/ml AGEs for 24 h in order to induce EMT and the expression of
TGF-β mRNA was detected by RT-PCR. (B) Quantitative results of
RT-PCR for A. **P<0.01 vs. control. (C) RPMCs were
treated with different concentrations of AGEs (0, 0.6, 1.2 and 2.5
mg/ml) for 24 h in order to induce EMT and the expression of TGF-β
mRNA was detected by RT-PCR. (D) Quantitative results of RT-PCR for
C. **P<0.01 vs. 0 h group. (E) RPMCs were treated
with 1.2 mg/ml BSA or 1.2 mg/ml AGEs for 24 h in order to induce
EMT and the expression of p-Smad3 and p-Smad4 were detected by
western blotting. (F and G) Quantitative results of D.
**P<0.01 vs. control. (H) RPMCs were treated with
different concentrations of AGEs (0, 0.6, 1.2 and 2.5 mg/ml) for 24
h in order to induce EMT and the expression of p-Smad3 and p-Smad4
were detected by western blotting. (I and J) Quantitative results
of western blotting in H. **P<0.01 vs. 0 h group and
*P<0.05 vs. 0 h group. Values are presented as the
mean ± standard error of the mean of three independent experiments.
AGEs, advanced glycation end-products; TGF-β, transforming growth
factor-β; RPMcs, rat peritoneal mesothelial cells; BSA, bovine
serum albumin; EMT, epithelial-to-mesenchymal transition; RT-PCR,
reverse transcription polymerase chain reaction. |
AGEs activate the ROS/mitogen-activated
protein kinases (MAPK)-extracellular signal-regulated kinases (ERK)
pathway in RPMCs
The ROS/MAPK-ERK signaling pathway has been reported
to be involved in EMT (13,14).
The present study aimed to investigate whether ROS/MAPK-ERK
signaling in RPMCs is involved in AGEs-induced EMT. RPMCs were
treated with 1.2 mg/ml BSA or 1.2 mg/ml AGEs for 24 h in order to
induce EMT. Cells were then stained with DCF-DA in order to detect
intracellular ROS production. The expression of p-ERK was detected
by western blotting. Following culture with AGEs, intracellular
levels of ROS and p-ERK were found to be markedly increased
compared with those in the control or BSA group (Fig. 3A and C). Fig. 3E shows a dose-dependent change in
the expression of p-ERK in PMCs treated with AGEs, as detected by
western blotting. These results supported the hypothesis that AGEs
effectively activated the ROS/MAPK-ERK signaling pathway.
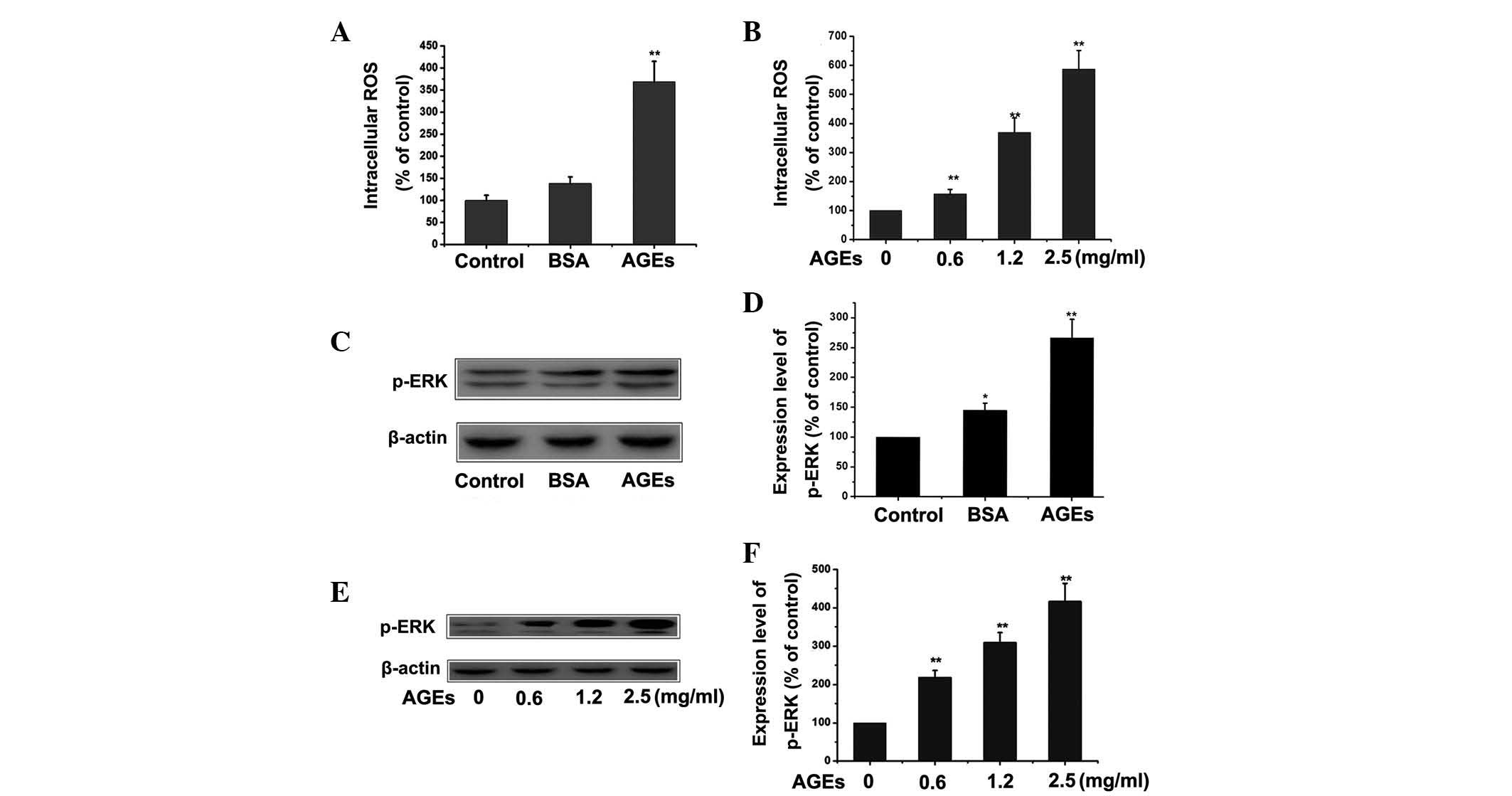 | Figure 3Effect of AGEs on the ROS/MAPK-ERK
pathway in RPMCs. (A) RPMCs were treated with 1.2 mg/ml BSA or 1.2
mg/ml AGEs for 24 h and the cells were stained with DCF-DA in order
to detect the intracellular ROS production in RPMCs.
**P<0.01 vs. control. (B) RPMCs were treated with
different concentrations of AGEs (0, 0.6, 1.2 and 2.5 mg/ml) for 24
h and the cells were stained with DCF-DA in order to detect
intracellular ROS production. **P<0.01 vs. control.
(C) RPMCs were treated with 1.2 mg/ml BSA or 1.2 mg/ml AGEs for 24
h and the expression of p-ERK was detected by western blotting. (D)
Quantitative results of western blotting from
C.**P<0.01 vs. control; *P<0.05 vs.
control. (E) RPMCs were treated with different concentration of
AGEs (0, 0.6, 1.2 and 2.5 mg/ml) for 24 h and the expression of
p-ERK was detected by western blotting. (F) Quantitative results of
western blotting in E. **P<0.01 vs. control. Values
are presented as the mean ± standard error of the mean of three
independent experiments performed. β-actin was used as loading
control. AGEs, advanced glycation end-products; ROS, reactive
oxygen species; MAPK, mitogen-activated protein kinases; p-ERK,
phosphorylated extracellular signal-regulated kinases; RPMCs, rat
peritoneal mesothelial cells; BSA, bovine serum albumin. |
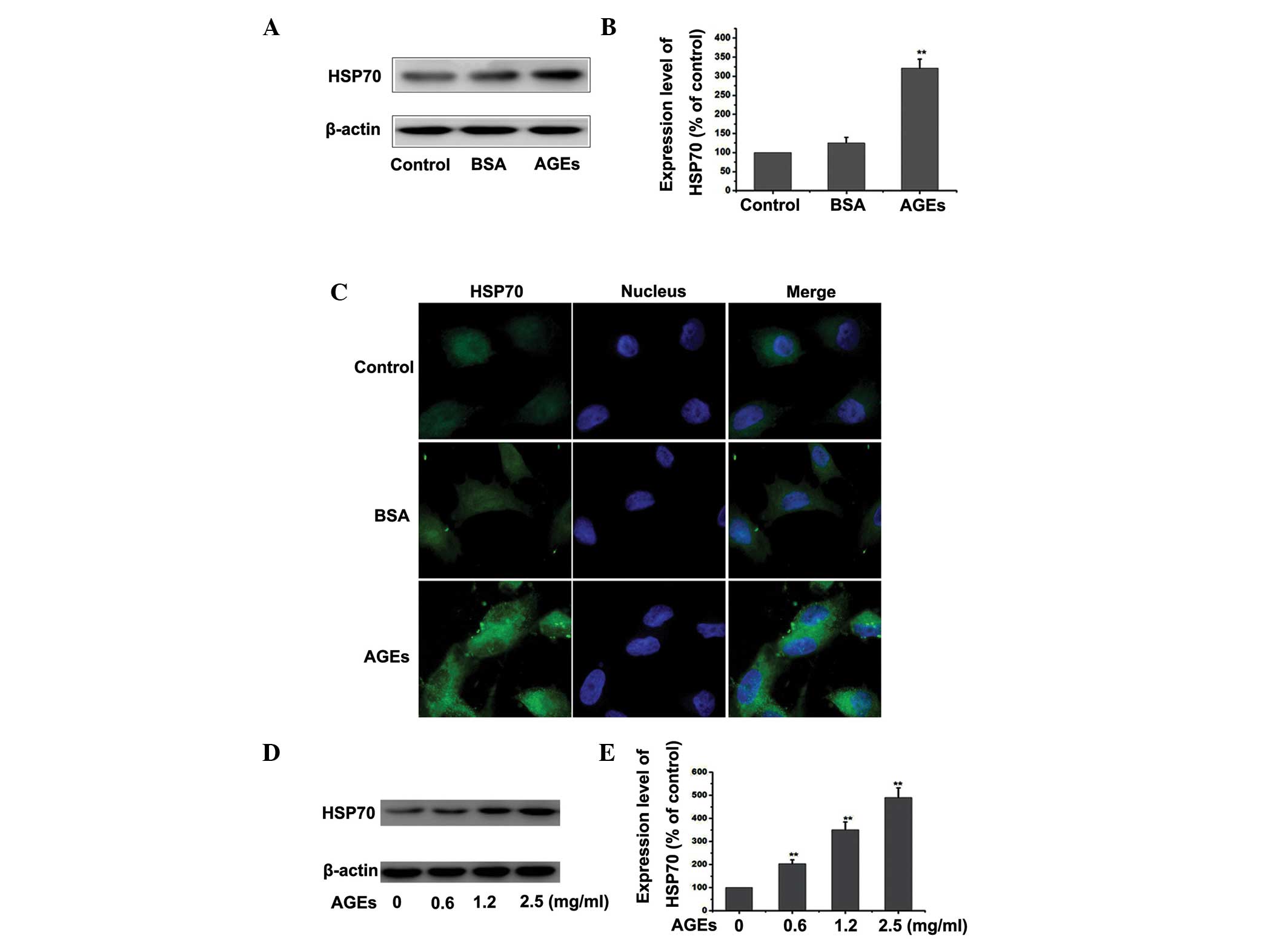
AGEs upregulate the expression of HSP70
in RPMCs
HSP70 may be upregulated by numerous stresses within
cells, including heat, oxidative stress and chemical injury
(15). Therefore, the present
study explored whether treatment of PMCs with AGEs altered the
expression of HSP70. Cells were treated with 1.2 mg/ml BSA or 1.2
mg/ml AGEs for 24 h and the expression of HSP70 was detected by
western blotting and fluorescence microscopy. As shown in Fig. 4A–C, treatment with AGEs increased
the levels of HSP70. This induction was sustained following AGEs
stimulation, in comparison with the control or the BSA group. As
shown in Fig. 4D and E, there was
a dose-dependent increase in the expression of HSP70 in PMCs
treated with AGEs, as detected by western blotting. The results
demonstrated that AGEs upregulated the expression of HSP70 in
RPMCs.
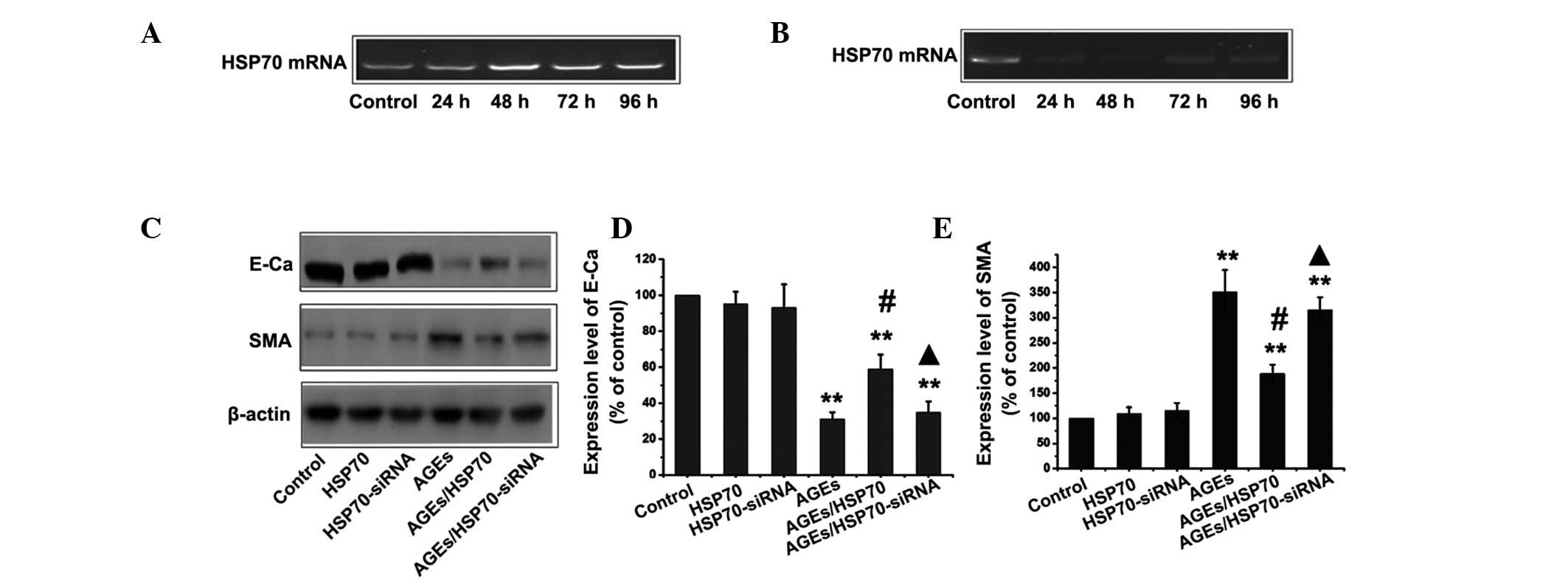
HSP70 inhibits AGEs-induced EMT in
RPMCs
In order to investigate whether HSP70 protects
against AGEs-induced EMT, PMCs were transfected with a plasmid
expressing the HSP70-cDNA gene or siRNA-HSP70. RT-PCR analysis was
performed on isolated total RNA in order to measure the level of
HSP70 mRNA following HSP70-pcDNA3.1/myc-HisA and HSP70-siRNA
transfection for various time periods (Fig. 5A and B). When cells were
transfected with HSP70-pcDNA3.1/myc-HisA, the expression of HSP70
was significantly increased compared with that in the control
groups and HSP70 levels peaked at 48 h. By contrast, HSP70
expression was decreased compared with that in the control group
following transfection with siRNA-HSP70. The lowest expression of
HSP70 was found 48 h following transfection with siRNA-HSP70. The
control group, HSP70-overexpressing group and HSP70-siRNA groups
were then treated with or without 1.2 mg/ml AGEs for 24 h,
following which the levels of the E-cadherin and α-SMA proteins
were assessed by western blotting. As shown in Fig. 5C, overexpression of HSP70
significantly decreased AGEs-induced EMT, as evidenced by the
reduction in the upregulation of α-SMA and the ameliorated
expression of the epithelial protein E-cadherin. By contrast,
siRNA-mediated suppression of HSP70 exacerbated AGE-induced EMT. Of
note, treatment with siRNA plasmids expressing HSP70 alone did not
induce EMT-mediated events in RPMCs.
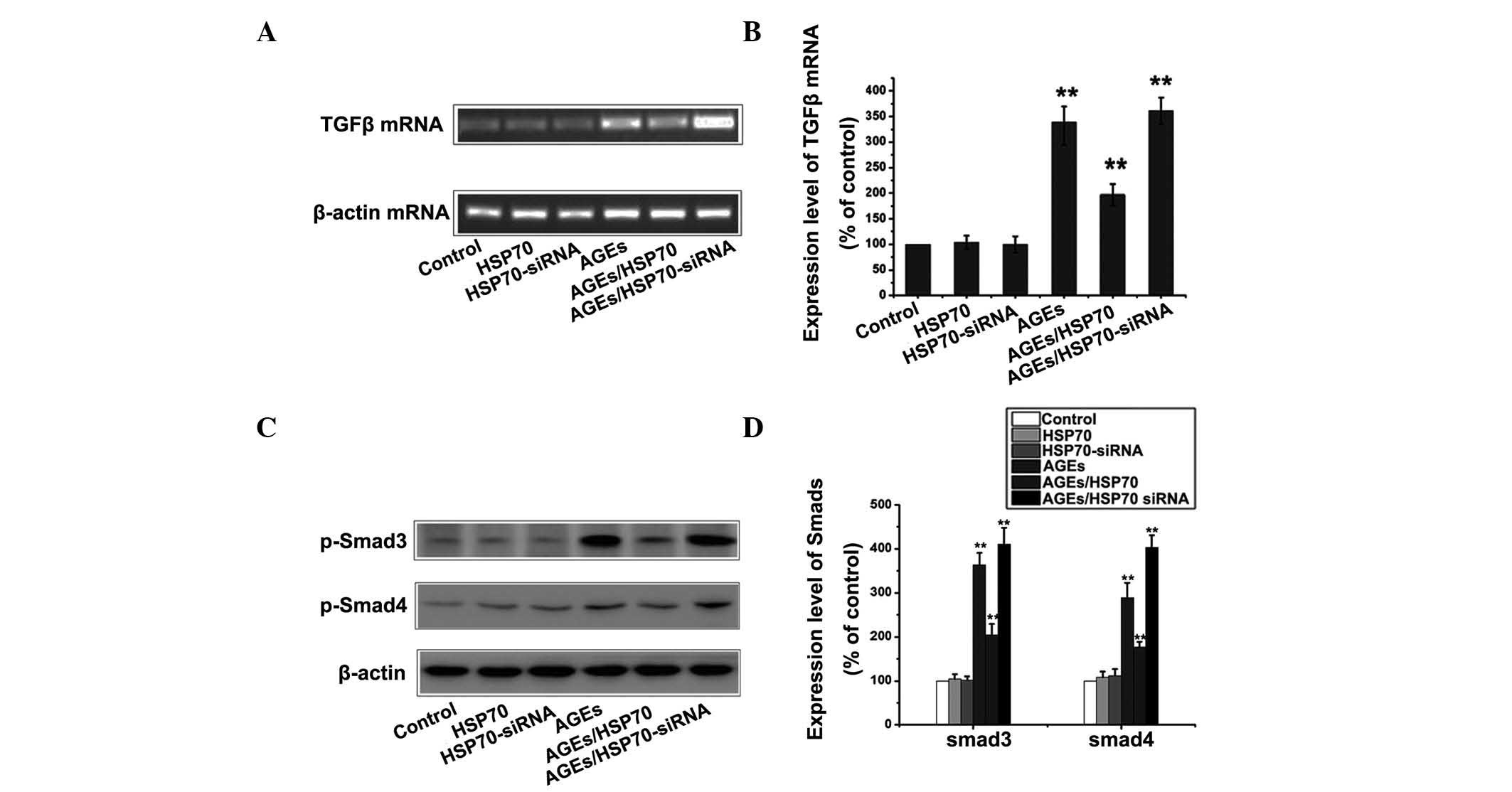
HSP70 inhibits the TGF-β/Smad pathway in
RPMCs treated with AGEs
In order to investigate whether HSP70 activates or
inhibits TGF-β/Smad signaling in RPMCs during AGEs-induced EMT, the
control group, HSP70-overexpression group and HSP70-siRNA group
were treated with or without 1.2 mg/ml AGEs for 24 h, following
which the expression of TGF-β mRNA and p-Smad3,4 were detected by
RT-PCR and western blotting. As shown in Fig. 6, overexpression of HSP70
significantly inhibited the TGF-β/Smad signaling pathway, as
indicated by the reduction in the AGEs-induced upregulation of
TGF-β and p-Smad3,4. By contrast, siRNA-mediated suppression of
HSP70 exacerbated the AGEs-induced activation of the TGF-β/Smad
signaling pathway.
HSP70 inhibits the ROS/MAPK-ERK pathway
in AGEs-treated RPMCs
In order to investigate whether HSP70 activates or
inhibits ROS/MAPK-ERK signaling in RPMCs during AGEs-induced EMT,
the control group, HSP70-overexpression group and HSP70-siRNA group
were treated with or without 1.2 mg/ml AGEs for 24 h, following
which the expression of p-ERK and intracellular ROS production were
measured. As shown in Fig. 7,
overexpression of HSP70 significantly inhibited the ROS/MAPK-ERK
signaling pathway, as demonstrated by the reduction in the
AGEs-induced upregulation of intracellular ROS production and p-ERK
expression. By contrast, siRNA-mediated suppression of HSP70
further exacerbated AGEs-induced activation of the MAPK-ERK
signaling pathway.
Discussion
AGEs result from a series of non-enzymatical
reactions, forming Schiff base and Amadori compounds that are
generated from glucose and other reducing sugars. AGEs accumulate
in diverse pathological conditions, including atherosclerosis and
oxidative modifications that alter cellular structure, function and
inflammation (16,17). When peritoneal proteins are exposed
to high-glucose dialysate during long-term PD, a glycosylation
reaction occurs, producing AGEs. Diabetic kidney disease is a
primary cause of morbidity and mortality in diabetic patients.
Longitudinal hyperglycemia increases intracellular sugars and the
derivatives of these may be involved in glycation and AGE formation
(18). AGEs are normally excreted
in urine in healthy subjects with a high renal clearance. However,
this clearance markedly declines in patients with chronic renal
failure. Accumulation of AGEs is further increased in patients with
end stage renal disease on PD or HD as a result of increased AGE
formation (19). Therefore, the
present study aimed to investigate whether AGEs are involved in the
formation of peritoneal fibrosis.
EMT is important in the processes of cellular
transdifferentiation during embryonic development, tumour invasion
and metastasis as well as in the development of tissue fibrosis
(20). EMT is involved in
fibroblast genesis during organ fibrosis in adult tissues. A
previous study demonstrated that PMCs underwent EMT during PD
(21). The present study
investigated whether the effects of AGEs in the development of
peritoneal fibrosis are due to EMT. As shown in Fig. 1, treatment with AGEs reduced the
expression of the E-cadherin protein, increased the expression and
led to increased EMT events in PMCs. A previous study showed that
TGF-β/Smads signaling is important in the promotion of EMT
(22). The present study
demonstrated that a dose-dependent change in the expression of
TGF-β mRNA and p-Smad3,4 was induced by AGE treatment of PMCs.
These results support the hypothesis that AGEs effectively activate
the TGF-β/Smad signaling pathways. Although the molecular
regulation underlying the EMT has been extensively studied in other
cell systems, predominantly in tumor cells, the signaling pathways
involved in this process have remained to be fully elucidated. The
present study demonstrated that the ROS/MAPK-ERK signaling pathway
participated in the AGE-induced EMT in RPMCs.
The HSP70 protein family and their co-chaperones
constitute a complex network of folding machines which is utilized
by cells in numerous ways, allowing them to adapt to gradual
changes in their environment and to survive in what would otherwise
be lethal conditions (23). With
respect to the HSP70 proteins it is elusive whether their activity
inhibits EMT in RPMCs. The present study demonstrated that
stimulation by AGEs upregulated the expression of HSP70, which in
turn reduced EMT. The effects of HSP70 were investigated by
modulating HSP70 expression through siRNA knockdown and plasmid
overexpression. Overexpression of HSP70 significantly reduced
AGEs-induced EMT, while siRNA-mediated suppression of HSP70
exacerbated AGE-induced EMT. Furthermore, HSP70 inhibited the
TGF-β/Smad and ROS/MAPK-ERK signaling pathways, thereby
antagonizing EMT-associated events.
In conclusion, the present study provided a detailed
assessment of the capacity of HSP70 to weaken or inhibit
AGE-induced EMT in PMCs. AGE-induced EMT occurs via independent
mechanisms, involving the TGF-β/Smad signaling pathway and
ROS/MAPK-ERK activation. Furthermore, HSP70 was shown to inhibit
these two pathways. The potential role of HSP70 in EMT, as well as
the involvement of upstream and downstream signaling molecules,
requires further investigation in order to identify possible
therapeutic targets, which may inhibit EMT in peritoneal
fibrosis.
Abbreviations:
|
HD
|
hemodialysis
|
|
PD
|
peritoneal dialysis
|
|
ESRD
|
end-stage renal disease
|
|
RPMCs
|
rat peritoneal mesothelial cells
|
|
AGE
|
advanced glycation end-products
|
|
DMEM
|
Dulbecco’s modified Eagle’s medium
|
|
FITC
|
fluorescein isothiocyanate
|
|
RIPA
|
radioimmunoprecipitation assay
|
|
TGF
|
transforming growth factor
|
|
EMT
|
epithelial-to-mesenchymal
transition
|
|
SMA
|
smooth muscle cell actin
|
|
ROS
|
reactive oxygen species
|
|
MAPKs
|
mitogen-activated protein kinases
|
|
ERK
|
extracellular signal-regulated protein
kinases
|
References
|
1
|
Bargman JM: Advances in peritoneal
dialysis: a review. Semin Dial. 25:545–549. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chou CY, Liang CC, Kuo HL, et al:
Comparing risk of new onset diabetes mellitus in chronic kidney
disease patients receiving peritoneal dialysis and hemodialysis
using propensity score matching. PLoS One. 9:e878912014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jain AK, Blake P, Cordy P and Garg AX:
Global trends in rates of peritoneal dialysis. J Am Soc Nephrol.
23:533–544. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Williams JD, Craig KJ, Topley N, Von
Ruhland C, Fallon M, Newman GR, Mackenzie RK and Williams GT;
Peritoneal Biopsy Study Group: Morphologic changes in the
peritoneal membrane of patients with renal disease. J Am Soc
Nephrol. 13:470–479. 2002.PubMed/NCBI
|
|
5
|
Li XJ, Sun L, Xiao L and Liu FY: Gene
delivery in peritoneal dialysis related peritoneal fibrosis
research. Chin Med J (Engl). 125:2219–2224. 2012.
|
|
6
|
Zhang J, Bi M, Zhong F, Jiao X, Zhang D
and Dong Q: Role of CIP4 in high glucose induced
epithelial-mesenchymal transition of rat peritoneal mesothelial
cells. Ren Fail. 35:989–995. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Baroni G, Schuinski AF, Berticelli PT,
Silva MA, Gouveia DS, Pecoits Filho R and Meyer F: The influence of
simvastatin in induced peritoneal fibrosis in rats by peritoneal
dialysis solution with glucosis 4.25%. Acta Cir Bras. 27:350–356.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nakamura S and Niwa T: Advanced glycation
end-products and peritoneal sclerosis. Semin Nephrol. 24:502–505.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Witowski J, Bender TO, Gahl GM, Frei U and
Jörres A: Glucose degradation products and peritoneal membrane
function. Perit Dial Int. 21:201–205. 2001.PubMed/NCBI
|
|
10
|
Mayer MP and Bukau B: Hsp70 chaperones:
cellular functions and molecular mechanism. Cell Mol Life Sci.
62:670–84. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
National Research Council (US) Institute
for Laboratory Animal Research: Guide for the care and Use of
Laboratory Animals. National Academies Press; Washington (DC), WA,
USA: pp. 86–23. 1996
|
|
12
|
Neil JR, Johnson KM, Nemenoff RA and
Schiemann WP: Cox-2 inactivates Smad signaling and enhances EMT
stimulated by TGF-beta through a PGE2-dependent mechanisms.
Carcinogenesis. 29:2227–2235. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Elsum IA, Martin C and Humbert PO:
Scribble regulates an EMT polarity pathway through modulation of
MAPK-ERK signaling to mediate junction formation. J Cell Sci.
126:3990–3999. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xie L, Law BK, Chytil AM, Brown KA, Aakre
ME and Moses HL: Activation of the Erk pathway is required for
TGF-beta1-induced EMT in vitro. Neoplasia. 6:603–610. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mikuriya T, Sugahara K, Takemoto T, Tanaka
K, Takeno K, Shimogori H, Nakai A and Yamashita H:
Geranylgeranylacetone, a heat shock protein inducer, prevents
acoustic injury in the guinea pig. Brain Res. 1065:107–114. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Aso Y, Inukai T, Tayama K and Takemura Y:
Serum concentrations of advanced glycation endproducts are
associated with the development of atherosclerosis as well as
diabetic microangiopathy in patients with type 2 diabetes. Acta
Diabetol. 37:87–92. 2000. View Article : Google Scholar
|
|
17
|
Wang X, Desai K, Juurlink BH, de Champlain
J and Wu L: Gender-related differences in advanced glycation
endproducts, oxidative stress markers and nitric oxide synthases in
rats. Kidney Int. 69:281–287. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yamabe N, Kang KS, Park CH, Tanaka T and
Yokozawa T: 7-O-galloyl-D-sedoheptulose is a novel therapeutic
agent against oxidative stress and advanced glycation endproducts
in the diabetic kidney. Biol Pharm Bull. 32:657–664. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Thornalley PJ: Glycation free adduct
accumulation in renal disease: the new AGE. Pediatr Nephrol.
20:1515–1522. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Roche J, Nasarre P, Gemmill R, Baldys A,
Pontis J, Korch C, Guilhot J, Ait-Si-Ali S and Drabkin H: Global
decrease of histone H3k27 acetylation in ZEB1-induced epithelial to
mesenchymal transition in lung cancer cells. Cancers (Basel).
5:334–356. 2013. View Article : Google Scholar
|
|
21
|
Fang CC, Huang JW, Shyu RS, Yen CJ, Shiao
CH, Chiang CK, Hu RH and Tsai TJ: Fibrin-induced
epithelial-to-mesenchymal transition of peritoneal mesothelial
cells as a mechanism of peritoneal fibrosis: effects of
pentoxifylline. PLoS One. 7:e447652012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Smith AL, Iwanaga R, Drasin DJ, Micalizzi
DS, Vartuli RL, Tan AC and Ford HL: The miR-106b-25 cluster targets
Smad7, activates TGF-β signaling and induces EMT and tumor
initiating cell characteristics downstream of Six1 in human breast
cancer. Oncogene. 31:5162–5171. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Selim ME, Rashed el HA, Aleisa NA and
Daghestani MH: The protection role of heat shock protein 70
(HSP-70) in the testes of cadmium-exposed rats. Bioinformation.
8:58–64. 2012. View Article : Google Scholar : PubMed/NCBI
|