Introduction
Ankylosing spondylitis (AS) is a severe chronic
inflammatory disease that affects the sacroiliac joints and axial
skeleton. AS is characterized primarily by inflammatory back pain,
which may lead to structural and functional impairment, and
ultimately the development of a ‘bamboo-like’ spine (1–3). A
significant proportion of AS patients are young adults. As there is
currently no effective cure for this disease, morbidity from AS
results in a high burden on families, as well as society in general
(4,5).
The pathogenesis of AS is complex and remains poorly
understood. Hereditary factors are hypothesized to be the primary
etiological agent, although infection, metabolic disorders and
autoimmune disorders may also be involved (3,6). The
gene encoding human leukocyte antigen B27 (HLA-B27) is understood
to be the most important genetic factor associated with AS
(7). However, additional genes
associated with an increased susceptibility to this disease have
been identified. These include genes encoding HLA-B60 (8), killer cell immunoglobulin-like
receptors (9), interleukin family
members (10,11) and tumor necrosis factor α (TNF-α)
(12). However, these genetic
alterations do not fully explain the pathogenesis of AS, in
particular uncontrolled bone formation and subsequent spinal
fusion.
Uncontrolled bone formation occurs during the
pathogenesis of AS, resulting in spinal fusion and disability
(4). Thus, spinal fusion is
thought to be the most serious consequence of AS (13,14).
A previous study identified alterations in the supraspinal
ligaments of patients with AS, including irregular arrangements of
collagen fibrils, degeneration of fibroblasts, significant
increases in microvessel density and calcification of tissue
(15). This phenotype suggests a
tendency to osteogenesis in the supraspinal ligaments of those with
AS, indicating that further investigation of the molecular changes
present in supraspinal ligaments may improve understanding of the
pathogenesis of spinal fusion in patients with AS.
In order to assess the molecular changes associated
with spinal fusion, polymerase chain reaction (PCR)-based
suppression subtractive hybridization (SSH) was conducted, using
supraspinal ligaments of patients with AS and from healthy
controls. Differentially expressed genes (DEGs) between the two
groups were identified, and the mRNA and protein expression
patterns of six DEGs were determined.
Materials and methods
Clinical samples
Paraspinal tissues were obtained via spinal
orthopedic surgery from six patients (four males and two females;
mean age, 30.2 years) who were in the active stage of AS, according
to the criteria described by the American Rheumatism Association
(16). The X-ray imaging of
patients were carried out with a Definium 6000 (GE Healthcare,
Wauwatosa, WI, USA). Paraspinal tissues were also obtained during
reattachment surgery from three patients (two males and one female;
mean age, 31.5 years) who had sustained spinal fractures.
Supraspinous ligaments were carefully isolated from these tissue
samples, washed with phosphate-buffered saline (0.01 mM, pH 7.2;
Sangon Biotech Co., Ltd, Shaghai, China) and then immediately
stored in liquid nitrogen, or fixed in 4% paraformaldehyde solution
(Sangon Biotech Co., Ltd) and embedded in paraffin (Sangon Biotech
Co., Ltd). The study protocol was approved by the ethics committee
of Xinqiao Hospital (Chongqing, China). All patients provided
written informed consent for the experimental use of the obtained
materials.
Pathological assessment
Pathological assessment was performed as previously
described (1,17). Briefly, paraffin-embedded
supraspinous ligaments were cut into thin slices and stained with
hematoxylin and eosin (Sangon Biotech Co., Ltd). Following
dehydration, transparency and drying, all sections were evaluated
by a pathologist who was blinded to patient diagnosis using a
microscope (IX71, Olympus Corp., Tokyo, Japan) and Analysis FIVE
software version 5.0 (Olympus Corp.).
SSH
Frozen tissue samples were ground in liquid nitrogen
and total RNA was extracted using RNAiso plus (Takara, Dalian,
China). PolyA+ mRNA was isolated from total RNA using
PolyATract kits (Promega Corp., Madison, WI, USA). A 2-μg
aliquot of each mRNA sample was used as a template in order to
generate double-stranded cDNA (dscDNA) using a SMART PCR cDNA
synthesis kit (Clontech, Palo Alto, CA, USA).
SSH was performed using PCR-select cDNA subtraction
kits (Clontech) according to the manufacturer’s instructions. Three
independent SSH experiments using the AS and control samples were
conducted. Briefly, forward and reverse SSH were simultaneously
performed in each independent SSH experiment. During forward SSH,
the dscDNA of the AS samples was set as the Tester and the dscDNA
of the control samples as the Driver (Tester and Driver were set
according to the instructions). For reverse SSH, the Tester and
Driver dscDNAs were exchanged. Each dscDNA was digested with
RsaI (Clontech), and the blunt-ended products were ligated
with adaptor. The Tester and Driver were hybridized twice, with the
product used as a PCR template in order to amplify differentially
expressed cDNAs. Throughout the SSH procedure, the efficiency of
dscDNA synthesis, RsaI digestion, adaptor ligation and
subtractive hybridization were monitored according to the
manufacturer’s instructions.
Construction of subtracted cDNA
libraries
Differentially expressed cDNAs synthesized by SSH
were recycled, inserted into a pMD-19T vector (Takara) and
subsequently used to transform competent Escherichia coli
DH5α (Takara). The transformed cells were selected using
Luria-Bertani agar plates containing ampicillin, X-gal and IPTG
(Sangon Biotech Co., Ltd). Colonies containing inserts (white
colonies) were assayed by colony PCR using the following procedure:
Denature at 94°C for 5 min, followed with 30 cycles (94°C for 30
sec, 58°C for 30 sec, and 72°C for 30 sec). The primer pair was as
follows: Nested PCR primer 1, 5′-TCGAGCGGCCGCCCGGGCAGGT-3′ and
nested PCR primer 2R, 5′-AGCGTGGTCGCGGCCGAGGT-3′ (Clontech). The
PCR products were electrophoresed on agarose gels to confirm the
presence and size of the inserts prior to sequencing.
Sequencing analysis
Differentially expressed cDNA fragments from
subtracted cDNA libraries were sequenced by Invitrogen Life
Technologies (Shanghai, China) and the sequences were evaluated
using Chromas software. Contig Express (New York, NY, USA)
performed contig assembly and the obtained sequences were analyzed
using the basic local alignment search tool for nucleotides
(BLASTn; www.blast.ncbi.nlm.nih.gov) to search for homologous
sequences. Kyoto Encyclopedia of Genes and Genomes (KEGG;
http://www.genome.jp/kegg/pathway
analysis was performed in order to identify the function of the
relevant genes. Only genes which were present in the results of all
three independent SSH experiments were defined as DEGs and used for
subsequent experiments.
Validation of mRNA expression
Total RNA samples were reverse-transcribed using the
PrimeScript RT reagent kit (Takara) and cDNA samples were used as
templates for reverse transrciption-quantitative PCR (RT-qPCR)
using the primers listed in Table
II. RT-qPCR was performed using a CFX Connect™ Cycler with
SsoAdvanced™ SYBR Green supermix (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA) according to the manufacturer’s instructions.
The expression levels of each DEG were normalized to those of
β-actin mRNA and calculated using the 2−ΔΔCT method with
SPSS version 13.0 (SPSS, Inc., Chicago, IL, USA) as previously
described (18).
 | Table IIPrimers used for reverse
transcription-quantitative polymerase chain reaction. |
Table II
Primers used for reverse
transcription-quantitative polymerase chain reaction.
| Gene | Primer sequence
| Product size
(bp) |
|---|
| Forward
(5′→3′) | Reverse
(5′→3′) |
|---|
| TBRI |
AATGGGCTTAGTATTCTGGG |
TTTCTTCAACTGATGGGTCA | 113 |
| TBRIII |
TTTTGGTGTCTGAGGGTTCT |
TGCTATCTTGAGTTCGGTGA | 161 |
| VEGF |
TGCCCACTGAGGAGTCCAAC |
ACAAATGCTTTCTCCGCTCT | 177 |
| MMP-3 |
GGCAGTTTGCTCAGCCTATC |
TCCAGAGTGTCGGAGTCCAG | 219 |
| Cbf-α1 |
CTCTTCCCAAAGCCAGAGTG |
ATCAGCGTCAACACCATCAT | 208 |
| BMP-2 |
CCGCTGTCTTCTAGCGTTGC |
CTCGTCAGAGGGCTGGGATG | 130 |
| β-actin |
GAGACCTTCAACACCCCAGC |
ATGTCACGCACGATTTCCC | 263 |
Western blot analysis
Total proteins were extracted from frozen tissue
samples using IP Cell Lysis Reagent (Beyotime, Haimen, Jiangsu,
China). Proteins were separated by 10% SDS-PAGE and transferred to
polyvinylidene difluoride membranes (EMD Millipore, Billerica, MA,
USA). The membranes were blocked at 37°C with 5% non-fat milk
(Beyotime) and incubated overnight with 1:1,000 dilutions
(Beyotime) of polyclonal rabbit-derived antibodies against human
β-actin and vascular endothelial growth factor (VEGF; both from
Beyotime), human bone morphogenetic protein 2 (BMP-2) and matrix
metalloproteinase-3 (MMP-3; both from Santa Cruz Biotechnology,
Inc., Dallas, TX, USA) and core binding factor-α1 (Cbf-α1), human
transforming growth factor-β type I (TBRI) and TBRIII (all from
Cell Signaling Technology, Inc., Danvers, MA, USA). The blots were
washed with PBS containing 0.5% Tween-20 (Sangon Biotech Co., Ltd)
and incubated with secondary goat anti-rabbit or anti-mouse
immunoglobulin G antibodies (Beyotime; 1:10,000). Protein bands
were visualized with the SuperSignal West Pico chemiluminescence
substrate (Thermo Pierce, Rockford, IL, USA) using a ChemiDoc XRS
Gel Imaging System (Bio-Rad Laboratories, Inc.).
Statistical analysis
The levels of mRNA expression are presented as the
mean ± standard deviation and were compared using Student’s t tests
using SPSS version 13.0 (SPSS, Inc.). P<0.05 was considered to
indicate a statistically significant difference.
Results
Pathological changes in supraspinal
ligaments
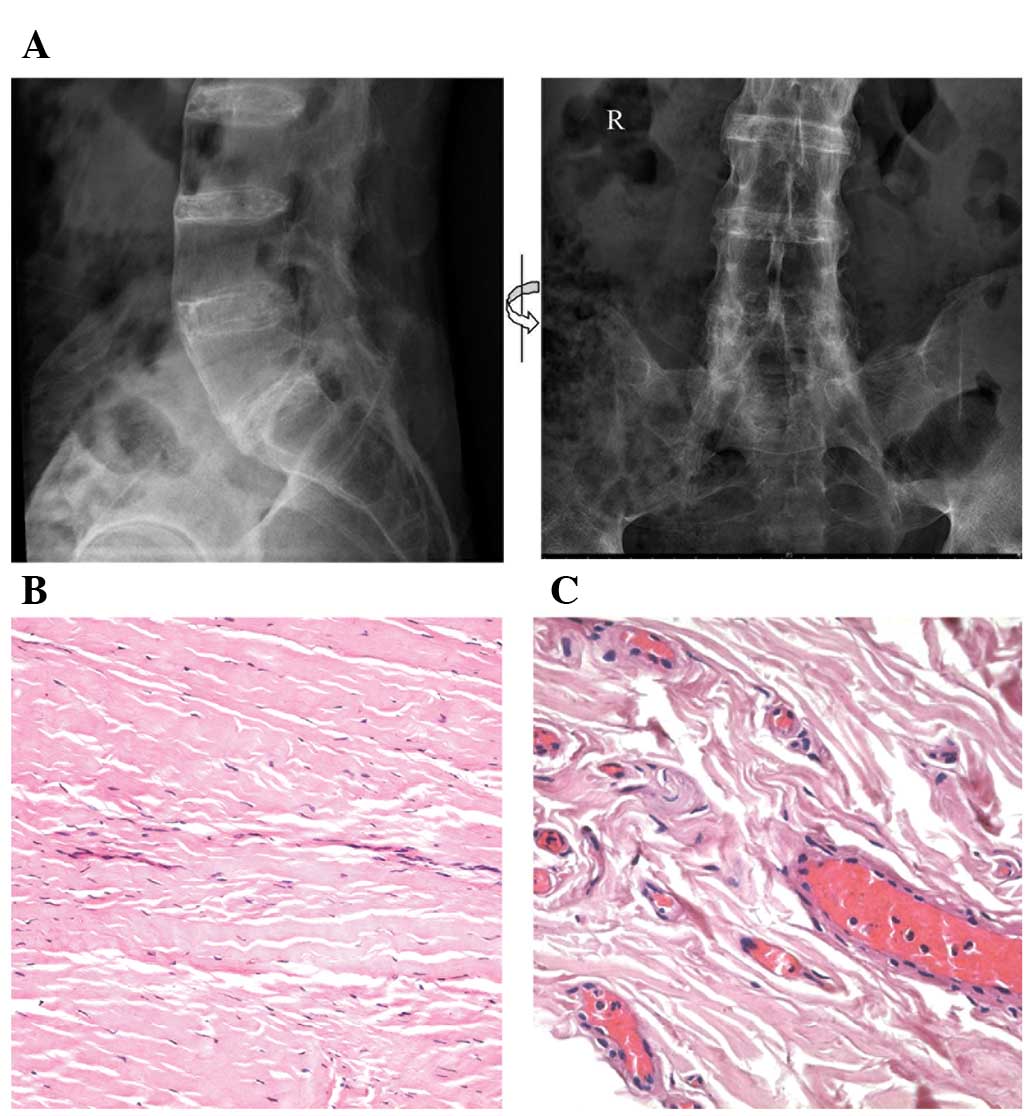
Pelvic X-ray images of a patient with AS
demonstrated typical spinal fusion (Fig. 1A). The pathological changes in the
supraspinal ligaments of patients with AS, which were subsequently
used in the SSH experiments, and in control patients with spinal
fractures were examined by light microscopy. Histological
examination demonstrated that the collagen fibrils of control
ligaments were regularly arranged and filled with diffuse
extracellular matrix, with spindle-like fibroblasts running
parallel to these fibrils (Fig.
1B). By contrast, the collagen fibrils of ligaments from
patients with AS were irregularly organized and did not contain
extracellular matrix. Furthermore, fewer fibrils were observed in
these samples, in which there was also evidence of hyaline
degeneration (Fig. 1C).
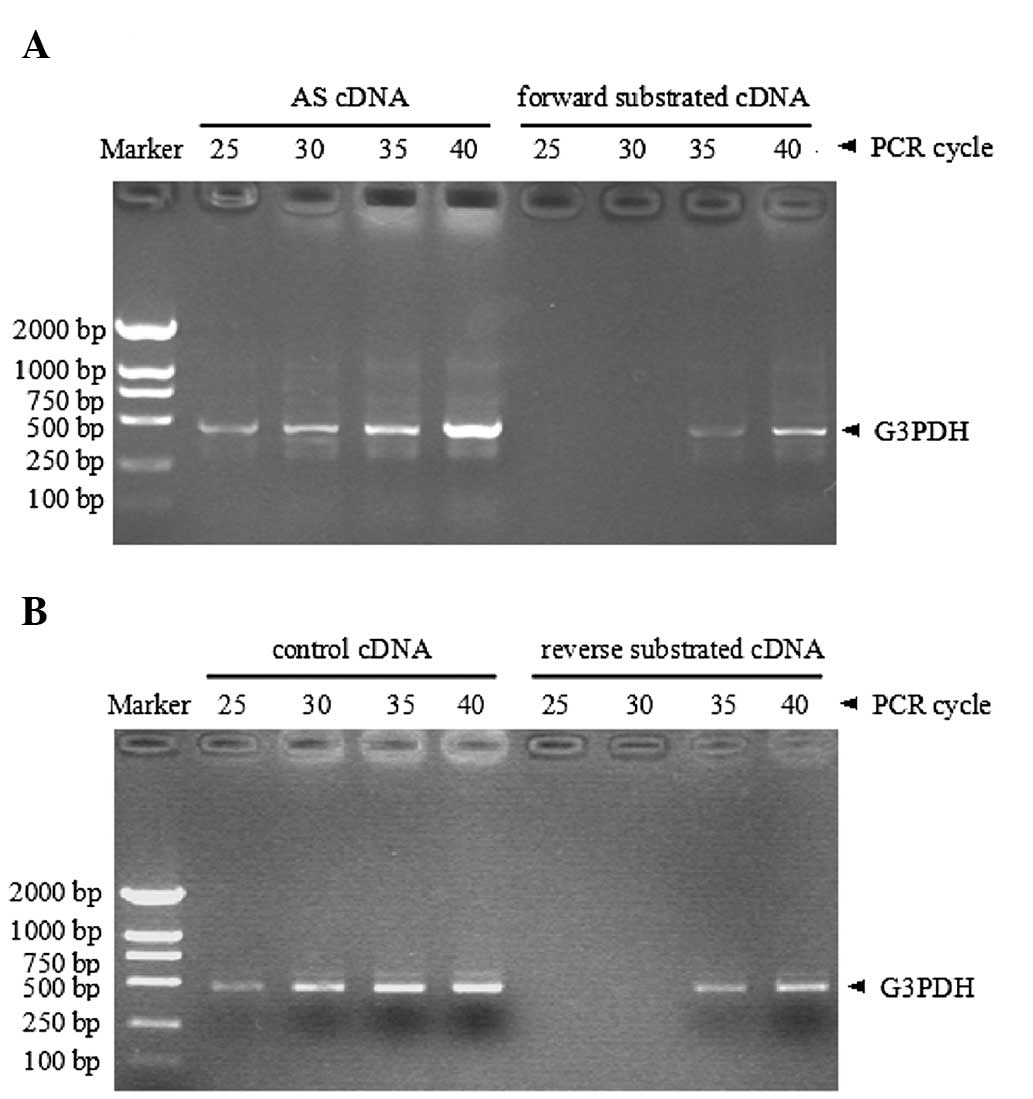
Efficiency of SSH
The efficiency of each SSH experiment involving
glyceraldehyde 3-phosphate dehydrogenase (G3PDH) cDNA, was
tested by quantitative comparison of subtracted and unsubtracted
cDNA. PCR products were detectable following 25 cycles in the AS
unsubtracted sample, compared with >10 cycles in the forward
subtracted sample (Fig. 2A).
Similarly, G3PDH PCR products were detected following 25
cycles in the unsubtracted control sample, whereas >10 cycles
were required in the reverse subtracted sample (Fig. 2B).
Characterization of the subtracted cDNA
libraries
Two cDNA libraries, forward and reverse, were
constructed by PCR-based SSH from the supraspinal ligaments of
patients with AS and control patients in each independent SSH
experiment. On average, ~200 differentially expressed cDNA
fragments from the forward subtracted cDNA library and ~150 from
the reverse library were identified in each SSH experiment. Prior
to sequencing, all clones were isolated and assayed by colony PCR
for the presence of cDNA inserts and in order to estimate the size
of each product. In the SSH experiments under optimized conditions,
a total of 212 clones, 153 from the forward and 59 from the reverse
libraries, were isolated and sequenced, with insert sizes ranging
from 67 to 839 bp. Following removal of the vector sequences and
low-quality expressed sequence tags (ESTs), the average available
sequence was ~319 bp in length.
The cleaned ESTs of each SSH experiment were
subsequently assembled. Using the BLASTn tool, all sequences from
forward or reverse libraries demonstrated significant similarity
with genes from the GenBank database (http://www.ncbi.nlm.nih.gov/genbank/). A total of 27
DEGs were presented in all of the three forward libraries. All of
these were functionally annotated in the KEGG; http://www.genome.jp/kegg/ online server. These genes
are thus candidate DEGs associated with AS, and are listed in
Table I.
 | Table IDEGs in AS supraspinous ligaments. |
Table I
DEGs in AS supraspinous ligaments.
| DEG ID | Length (bp) | No. of ESTs | Best hits | Identity | Annotation |
|---|
| FL01 | 224 | 3 | NM_000576.2 | 100% | Interleukin 1,
beta |
| FL02 | 218 | 14 | NM_005517.3 | 100% | High mobility group
nucleosomal binding domain 2 |
| FL03 | 529 | 8 | XM_005260402.1 | 100% | G protein, alpha
stimulating activity polypeptide 1 |
| FL04 | 213 | 22 | XM_005264576.1 | 99% | Calmodulin 2
(phosphorylase kinase delta) |
| FL05 | 234 | 2 | XM_005249363.1 | 100% | Vascular endothelial
growth factor A |
| FL06 | 399 | 1 | NM_001568.2 | 100% | Eukaryotic
translation initiation factor 3, subunit E |
| FL07 | 706 | 8 | NM_002298.4 | 99% | Lymphocyte cytosolic
protein 1 |
| FL08 | 494 | 1 | XM_005274125.1 | 100% | Carnosine synthase
1 |
| FL09 | 725 | 6 | NM_001731.2 | 99% | B-cell translocation
gene 1 |
| FL10 | 833 | 5 | NM_000090.3 | 100% | Collagen, type III,
alpha 1 |
| FL11 | 552 | 15 | NM_001161727.1 | 98% | Phospholipase A2,
group IIA |
| FL12 | 839 | 3 | NM_003380.3 | 99% | Vimentin |
| FL13 | 803 | 5 | NM_001663.3 | 100% | ADP-ribosylation
factor 6 |
| FL14 | 211 | 1 | NM_002966.2 | 100% | S100 calcium
binding protein A10 |
| FL15 | 556 | 2 | NM_000840.2 | 93% | Glutamate receptor,
metabotropic 3 |
| FL16 | 396 | 1 | XM_005252150.1 | 99% | Transforming growth
factor, beta receptor I |
| FL17 | 731 | 2 | XM_005248668.1 | 100% | Eukaryotic
translation elongation factor 1 alpha 1 |
| FL18 | 367 | 1 | XM_005270519.1 | 99% | Interleukin 23
receptor |
| FL19 | 180 | 3 | NM_000594.3 | 100% | Tumor necrosis
factor alpha |
| FL20 | 578 | 1 | XM_005274246.1 | 99% | AHNAK
nucleoprotein |
| FL21 | 118 | 1 | NM_001200.2 | 100% | Bone morphogenetic
protein 2 |
| FL22 | 176 | 3 | NM_002422.3 | 99% | Matrix
metalloproteinase 3 |
| FL23 | 429 | 1 | NM_012232.5 | 99% | Polymerase I and
transcript release factor |
| FL24 | 214 | 1 | HUMCBFA | 99% | Core-binding
factor, runt domain, alpha subunit 1 |
| FL25 | 67 | 1 | XM_005252909.1 | 98% | Interferon
regulatory factor 7 |
| FL26 | 459 | 1 | NM_002933.4 | 99% | Ribonuclease, RNase
A family, 1 |
| FL27 | 729 | 1 | NM_001195683.1 | 99% | Transforming growth
factor, beta receptor III |
Confirmation of differentially expressed
mRNA
In order to confirm the differential gene expression
pattern in supraspinous ligaments from patients with AS, six
upregulated DEGs were selected based on functional annotations.
Although inflammation-associated genes, including tumor necrosis
factor α (TNF-α), interleukin-1β (IL-1β) and interleukin 23
receptor (IL-23R) are hypothesized to be involved in the
pathogenesis of AS (19,20), the present study focused on spinal
fusion rather than inflammation. Primers for these genes were
designed using Primer Premier 5 software (Table II). Reverse
transcription-quantitative PCR (RT-qPCR) analyses of the same AS
and control samples as those used for SSH experiments were
performed in order to quantify the expression of mRNAs encoded by
the candidate genes. Theoretically, the expression levels of
expression of each candidate gene would be higher in the samples
from patients with AS than those in the samples from control
patients.
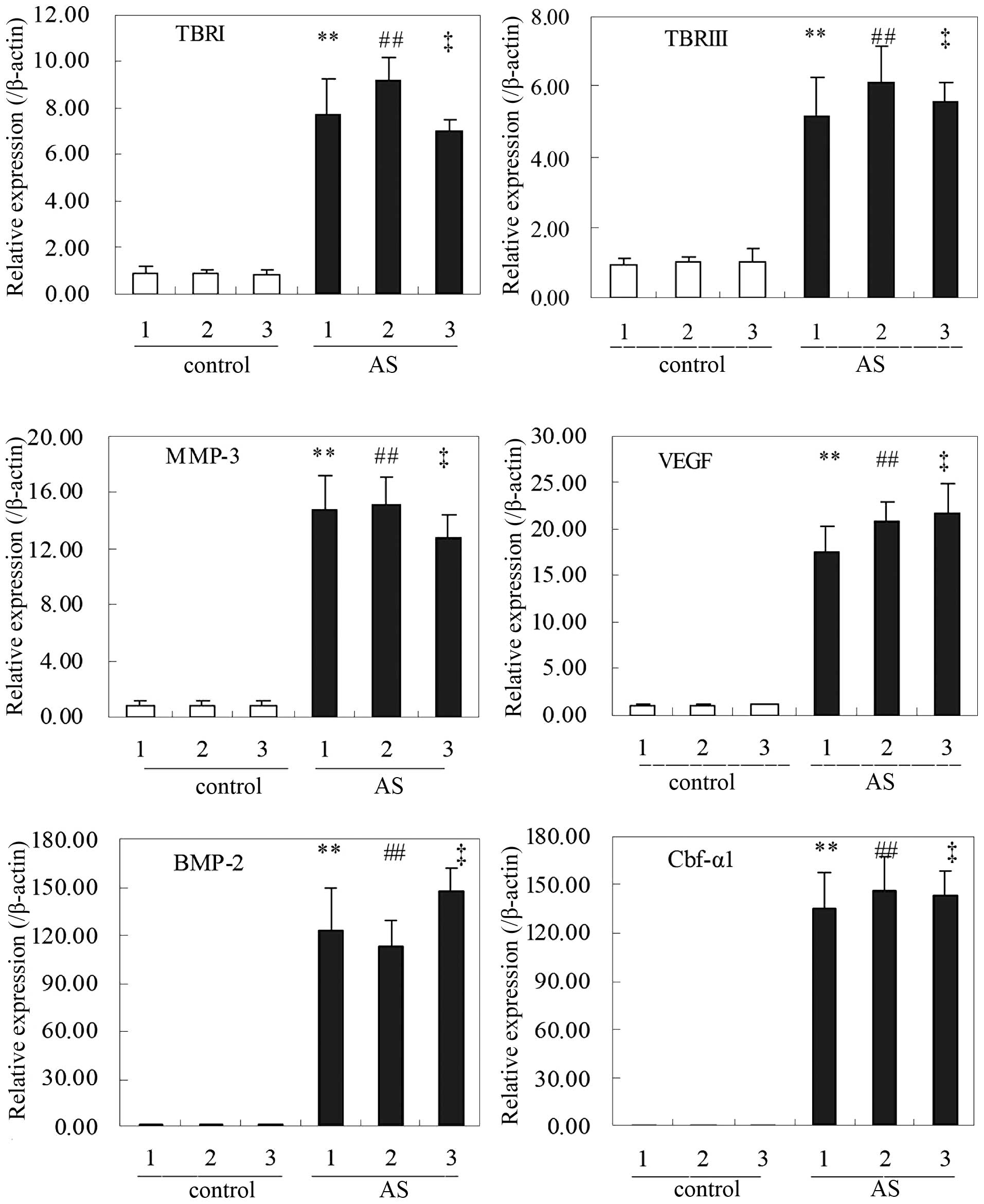
RT-qPCR analyses showed that, in accordance with the
results of the SSH experiments, the levels of mRNAs encoding the
six proteins TBRI/III, VEGF, MMP-3, Cbf-α1 and BMP-2 were
significantly increased in AS samples as compared with those in the
control samples (Fig. 3).
Confirmation that the encoded proteins
are differentially expressed
In order to further investigate the expression of
these six DEGs, western blot analysis was conducted using total
protein extracted from the supraspinal ligaments of the six
patients with AS and three control subjects. An increased
expression of all six proteins was detected in the samples from
patients with AS as compared with those in the three controls
(Fig. 4). Furthermore, these
proteins were not detected at all in the control samples, although
their mRNA was present at low levels. These results indicated that
TBRI, TBRIII, VEGF, Cbf-α1, BMP-2 and MMP-3 proteins are abundantly
expressed in the supraspinal ligaments of patients with AS.
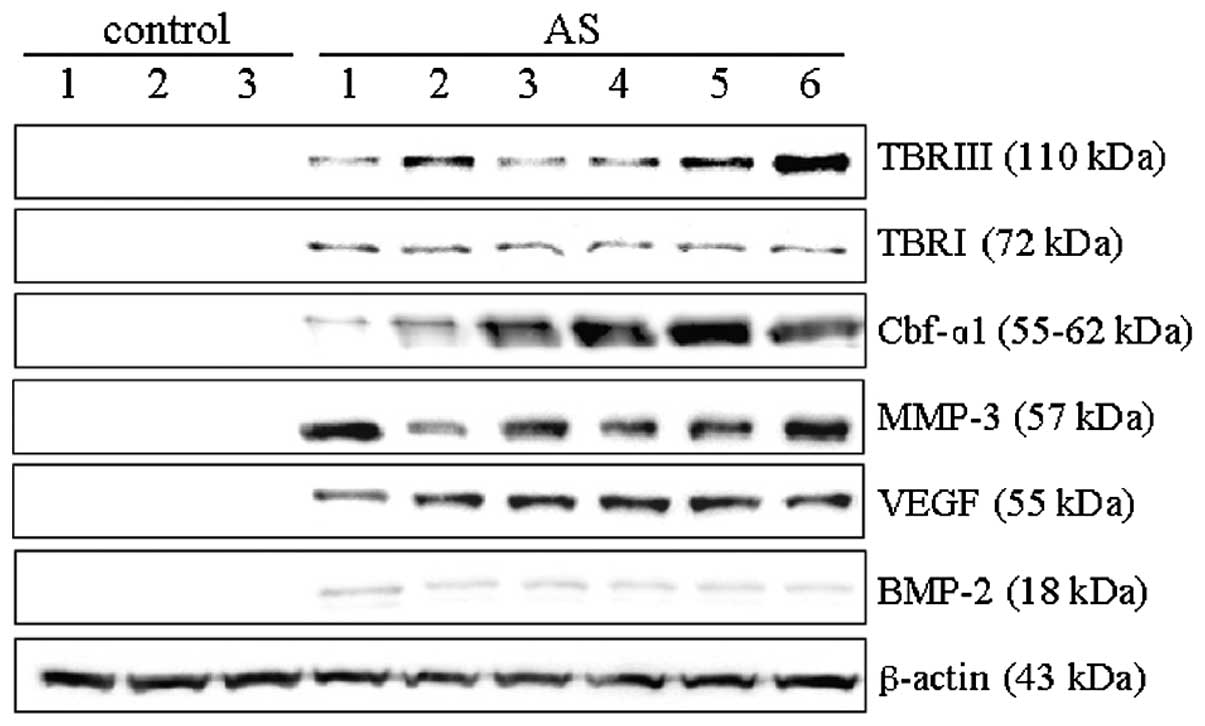 | Figure 4Quantitative western blot analyses of
identified differentially expressed genes in supraspinal ligaments
from patients with AS and control subjects. Total protein was
extracted from supraspinal ligaments of six patients with AS and
three patients with spinal fractures. Equal quantities of total
protein were loaded onto each lane and the subsequent blots probed
with antibodies to TBRI, TBRIII, Cbf-α1, MMP-3, VEGF and BMP-2, and
normalized relative to the expression of β-actin. A blot
representative of three independent experiments is shown. TBRI/III,
transforming growth factor β type I/III; VEGF, vascular endothelial
growth factor; MMP-3, matrix metal-loproteinase-3; Cbf-α1, core
binding factor-α1; BMP-2, bone morphogenetic protein 2; AS,
ankylosing spondylitis. |
Discussion
Chronic inflammation, back pain, bone erosion and
syndesmophyte formation are characteristic features of AS (6,21),
with bone formation and subsequent spinal fusion being fundamental
causes of AS-associated disability. Whilst histopathological
changes have been observed in the spinal tissues of patients with
AS (1,5,17),
the molecular mechanisms underlying spinal fusion remain elusive.
There are currently no available methods of reversing the process
of spinal fusion, with the exception of orthopedic spinal
surgery.
PCR-based SSH, which is distinct from microarray
technology, is a rapid and sensitive method for the identification
of DEGs. SSH directly provides partial sequences of DEGs, including
scarce or novel genes. Genes that were differentially expressed in
the supraspinous ligaments of AS compared with those of control
patients were identified using SSH. The three forward libraries
yielded 27 genes defined as DEGs in the AS samples. While the
association between HLA-B27 and AS was identified in patients aged
>30 years (7), it was not
detected in AS supraspinous ligaments via SSH. However,
differential expression of inflammation-associated genes, including
IL-1β, TNF-α and IL-23R, which are hypothesized to be associated
with the development of AS (20,22),
was detected. RT-qPCR and western blot analyses identified six
candidate proteins, TBRI, TBRIII, VEGF, MMP-3, Cbf-α1 and BMP-2, as
being associated with AS, but not inflammation, and contributing to
spinal fusion.
Members of the TGF-β superfamily are known to be
important regulators of cell proliferation, differentiation,
morphogenesis, apoptosis cancer and heritable disorders (23). TGF-β was shown to promote
downstream signaling pathways through three cell surface receptors:
TBRI, TBRII and TBRIII. TBRI and TBRII were identified as
serine/threonine kinases. Following ligand binding, TBRI recruits
TBRII and the two receptors form a stable heteromeric complex on
the cell surface. TBRII phosphorylates and activates TBRI (24). TBRIII is an abundant cell surface
proteoglycan, characterized as a ubiquitously-expressed
transmembrane receptor that acts as a TGF-β and inhibin receptor
(25). TBRIII has a large
extracellular domain, which tightly binds TGF-β with a high
affinity (26). TBRIII regulates
TGF-β signaling by presenting it to the activated TBRI/TBRII
complex. The present study demonstrated that TBRI and TBRIII were
highly expressed in the supraspinal ligaments of patients with AS,
compared with those from control patients, suggesting that there is
excessive activation of TGF-β signaling within AS tissues. This
increase in activation may contribute to uncontrolled bone
formation and spinal fusion.
TBRIII also acts as a BMP-2 co-receptor, increasing
the quantity of BMP-2 that binds to the BMP signaling receptors,
activin-like receptor kinases 3 (ALK3) and ALK6 (26). BMP-2 is an essential regulator of
bone development and facilitates osteoblastic differentiation and
bone formation (26). Of note,
BMP-2 and TBRIII were highly expressed in all of the AS samples
examined, indicating that excess activation of the BMP-2/TBRIII
pathway may be a bone morphogenetic mechanism that contributes to
spinal fusion in patients with AS.
Cbf-α1 is an important osteoblast-specific
transcription factor that may differentially activate osteoblasts
during embryonic development (27). In the absence of Cbf-α1, osteoblast
differentiation and bone development have been shown to be limited
(28). The present study
demonstrated that the levels of Cbf-α1 mRNA and protein were
markedly higher in AS than those in control samples, suggesting
that Cbf-α1 may contribute to spinal fusion in patients with
AS.
During skeletal development, bone formation is known
to be coupled to angiogenesis (29). VEGF is an endothelial cell growth
factor, which is an early marker of angiogenesis and also a marker
of bone formation (30). The
present study showed that VEGF expression was higher in the samples
from patients with AS than that in control samples, indicating that
VEGF may also contribute to spinal fusion.
The present study demonstrated that the expression
of MMP-3 mRNA and protein was higher in AS samples than that in
control samples. MMP-3 has been shown to be an important mediator
of bone matrix and cartilage damage (31). Recent reports have suggested that
MMP-3 may also serve as a biomarker with which to assess disease
activity in patients with AS (32), and for monitoring the response of
patients to drug treatment in clinical practice (33). Damage to articular surfaces and
cartilage that occurs in spinal joints are considered to be the
significant degenerative changes during the early pathogenesis of
spinal fusion (3). Although, to
the best of our knowledge, there is no evidence to suggest that
MMP-3 primes bone formation, it may participate in the process of
spinal joint damage, thereby contributing to spinal fusion during
the development of AS.
In conclusion, the present study isolated six DEGs,
namely TBRI, TBRIII, VEGF, MMP-3, Cbf-α1 and BMP-2, in the
supraspinal ligaments of patients with AS. These six DEGs were
hypothesized to be potential osteogenic factors associated with
excessive bone formation during AS. Further investigations are
required in order to determine whether any of these genes
participate in bone formation and subsequent spinal fusion during
the pathogenesis of AS.
Acknowledgments
This study was supported by the National Natural
Science Foundation of China (grant no. 81071523) and the Natural
Science Foundation of Chongqing (grant no. CSTC 2011BA5009). The
authors would like to thank the staff and patients at the
Department of Orthopaedics of Xinqiao Hospital (Chongqing, China)
who participated in this study.
References
|
1
|
Appel H, Kuhne M, Spiekermann S, et al:
Immunohistochemical analysis of hip arthritis in ankylosing
spondylitis: evaluation of the bone-cartilage interface and
subchondral bone marrow. Arthritis Rheum. 54:1805–1813. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chou CH, Lin MC, Peng CL, et al: A
nationwide population-based retrospective cohort study: increased
risk of acute coronary syndrome in patients with ankylosing
spondylitis. Scand J Rheumatol. 43:132–136. 2014. View Article : Google Scholar
|
|
3
|
Braun J and Sieper J: Ankylosing
spondylitis. Lancet. 369:1379–1390. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Thomas GP and Brown MA: Genomics of
ankylosing spondylitis. Discov Med. 10:263–271. 2010.PubMed/NCBI
|
|
5
|
Lories RJ and Baeten DL: Differences in
pathophysiology between rheumatoid arthritis and ankylosing
spondylitis. Clin Exp Rheumatol. 27(Suppl 55): S10–S14. 2009.
|
|
6
|
Tam LS, Gu J and Yu D: Pathogenesis of
ankylosing spondylitis. Nat Rev Rheumatol. 6:399–405. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Khan MA, Mathieu A, Sorrentino R and Akkoc
N: The pathogenetic role of HLA-B27 and its subtypes. Autoimmun
Rev. 6:183–189. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wei JC, Tsai WC, Lin HS, Tsai CY and Chou
CT: HLA-B60 and B61 are strongly associated with ankylosing
spondylitis in HLA-B27-negative Taiwan Chinese patients.
Rheumatology (Oxford). 43:839–842. 2004. View Article : Google Scholar
|
|
9
|
Diaz-Peña R, Blanco-Gelaz MA,
Suárez-Alvarez B, et al: Activating KIR genes are associated with
ankylosing spondylitis in Asian populations. Hum Immunol.
69:437–442. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kim TH, Stone MA, Rahman P, et al:
Interleukin 1 and nuclear factor-kappaB polymorphisms in ankylosing
spondylitis in Canada and Korea. J Rheumatol. 32:1907–1910.
2005.PubMed/NCBI
|
|
11
|
McGarry F, Neilly J, Anderson N, Sturrock
R and Field M: A polymorphism within the interleukin 1 receptor
antagonist (IL-1Ra) gene is associated with ankylosing spondylitis.
Rheumatology (Oxford). 40:1359–1364. 2001. View Article : Google Scholar
|
|
12
|
van Sijl AM, van Eijk IC, Peters MJ, et
al: Tumour necrosis factor blocking agents and progression of
subclinical atherosclerosis in patients with ankylosing
spondylitis. Ann Rheum Dis. 2013.Epub ahead of print. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Feldtkeller E and Braun J: Impact of sex
on inheritance of ankylosing spondylitis. Lancet. 355:1096–1097;
author reply 1098. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Davey-Ranasinghe N and Deodhar A:
Osteoporosis and vertebral fractures in ankylosing spondylitis.
Curr Opin Rheumatol. 25:509–516. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xu HF, Zhang L, Zhang Y and Chu TW:
Histopathologicai and ultra-structural observation of the
supraspinal ligaments in ankylosing spondylitis. Chinese Journal of
Rheumatology. 17:152–154. 2013.In Chinese.
|
|
16
|
Arnett FC, Edworthy SM, Bloch DA, et al:
The American Rheumatism Association 1987 revised criteria for the
classification of rheumatoid arthritis. Arthritis Rheum.
31:315–324. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Appel H, Loddenkemper C, Grozdanovic Z, et
al: Correlation of histopathological findings and magnetic
resonance imaging in the spine of patients with ankylosing
spondylitis. Arthritis Res Ther. 8:R1432006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pan X, Yue J, Ding G, et al: Leucine-rich
repeat 11 of Toll-like receptor 9 can tightly bind to
CpG-containing oligodeoxy-nucleotides, and the positively charged
residues are critical for the high affinity. J Biol Chem.
287:30596–30609. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Stone MA, Inman RD, Wright JG and Maetzel
A: Validation exercise of the Ankylosing Spondylitis Assessment
Study (ASAS) group response criteria in ankylosing spondylitis
patients treated with biologics. Arthritis Rheum. 51:316–320. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hreggvidsdottir HS, Noordenbos T and
Baeten DL: Inflammatory pathways in spondyloarthritis. Molecular
immunology. 57:28–37. 2014. View Article : Google Scholar
|
|
21
|
Gratacós J, Collado A, Filella X, et al:
Serum cytokines (IL-6, TNF-alpha, IL-1 beta and IFN-gamma) in
ankylosing spondylitis: a close correlation between serum IL-6 and
disease activity and severity. Br J Rheumatol. 33:927–931. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Stone MA and Inman RD: The genetics of
cytokines in ankylosing spondylitis. J Rheumatol. 28:1203–1206.
2001.PubMed/NCBI
|
|
23
|
Massagué J, Blain SW and Lo RS: TGFbeta
signaling in growth control, cancer, and heritable disorders. Cell.
103:295–309. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Derynck R, Akhurst RJ and Balmain A:
TGF-beta signaling in tumor suppression and cancer progression. Nat
Genet. 29:117–129. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Blobe GC, Schiemann WP, Pepin MC, et al:
Functional roles for the cytoplasmic domain of the type III
transforming growth factor beta receptor in regulating transforming
growth factor beta signaling. J Biol Chem. 276:24627–24637. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kirkbride KC, Townsend TA, Bruinsma MW,
Barnett JV and Blobe GC: Bone morphogenetic proteins signal through
the transforming growth factor-beta type III receptor. J Biol Chem.
283:7628–7637. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ducy P, Starbuck M, Priemel M, et al: A
Cbfa1-dependent genetic pathway controls bone formation beyond
embryonic development. Genes Dev. 13:1025–1036. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Otto F, Thornell AP, Crompton T, et al:
Cbfa1, a candidate gene for cleidocranial dysplasia syndrome, is
essential for osteoblast differentiation and bone development.
Cell. 89:765–771. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Portal-Núñez S, Lozano D and Esbrit P:
Role of angiogenesis on bone formation. Histol Histopathol.
27:559–566. 2012.PubMed/NCBI
|
|
30
|
Clarkin CE and Gerstenfeld LC: VEGF and
bone cell signalling: an essential vessel for communication? Cell
Biochem Funct. 31:1–11. 2013. View
Article : Google Scholar
|
|
31
|
Maksymowych WP: Biomarkers in
spondyloarthritis. Curr Rheumatol Rep. 12:318–324. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Soliman E, Labib W, el-Tantawi G, Hamimy
A, Alhadidy A and Aldawoudy A: Role of matrix metalloproteinase-3
(MMP-3) and magnetic resonance imaging of sacroiliitis in assessing
disease activity in ankylosing spondylitis. Rheumatol Int.
32:1711–1720. 2012. View Article : Google Scholar
|
|
33
|
Arends S, van der Veer E, Groen H, et al:
Serum MMP-3 level as a biomarker for monitoring and predicting
response to etanercept treatment in ankylosing spondylitis. J
Rheumatol. 38:1644–1650. 2011. View Article : Google Scholar : PubMed/NCBI
|