Introduction
Breast cancer is one of the most commonly diagnosed
cancer types in women, and the second leading cause of
cancer-related deaths worldwide (1). Patients with estrogen receptor α
(ERα)-positive tumors greatly benefit from existing hormonal
therapies. Although anti-estrogens are being used to treat breast
cancer (2,3), numerous cases show acquired
resistance and irresponsiveness to endocrine therapy, which is a
major clinical problem (3,4). Despite the emergence of new promising
advances in therapeutics, options to treat hormone-resistant breast
tumors are limited, and the mortality rate continues to
increase.
It has been suggested, based on a number of
findings, that deregulation of cell-cycle components such as
cyclin-dependent kinases (CDKs) can contribute to endocrine
resistance (5). Therefore,
inhibition of CDKs by synthetic, small-molecule drugs has become an
attractive therapeutic strategy. Roscovitine is a small,
purine-like CDK inhibitor with increased selectivity towards CDK1,
CDK2, CDK7 and CDK9 (6–8). Previous studies have shown that
roscovitine promotes the accumulation of breast cancer cells at the
G2/M phase (9,10) and potentiates the antitumor effects
of other chemotherapeutic agents, by inducing apoptotic cell death
(11). Besides CDKs, the
progression of the cell cycle is related to polyamines (PAs), which
are amine-derived cationic molecules. Several studies provided
evidence for a PA-dependent G0–G1 transition
and G1 phase progression in different cell lines
(12,13).
Among PAs, natural putrescine (Put), spermidine
(Spd) and spermine (Spm) are required for cell growth and
proliferation (14). Intracellular
PA levels are tightly regulated in eukaryotes by the activity of
the ornithine decarboxylase (ODC), which catalyzes the conversion
of ornithine to Put (15).
Activation of PA biosynthesis leads to the accumulation of
intracellular PAs, which is a critical event in various diseases,
including breast cancer (16,17).
Previous studies have shown that PAs are involved in neoplastic
transformation by activating several proto-oncogenes, such as
c-Myc (18,19).
Autophagy, the process responsible for the
degradation of cytoplasmic proteins, macromolecules and damaged or
aged organelles, is considered a type of cell death. The most
significant sign of autophagy is the appearance of double-membrane
enclosed vesicles in the cytoplasm, which engulf portions of the
cytoplasm and/or organelles (20–22).
A number of studies have shown that PAs are
associated with autophagy via histone acetylation and chromatin
remodeling mechanisms. Specifically, Spd was suggested to be a
critical ‘tuning’ molecule in autophagy, through epigenetic
alterations (23–25). Spd was shown to inhibit the
enzymatic activity of histone acetyl transferase (HAT) and lead to
hypoacetylation of histone H3 (25). For this reason, it is considered
that autophagic processes can be activated by the acetylation, by
PAs, of autophagic promoter molecules. However, the molecular
mechanism involved in drug-induced apoptosis or autophagy related
to the regulation of PA biosynthesis has not yet been fully
clarified.
In the present study, we aimed to reveal the
potential role of PAs in roscovitine-induced apoptosis and/or
autophagy in MCF-7 and MDA-MB-231 breast cancer cells.
Materials and methods
Drugs and antibodies
Roscovitine was purchased from Sigma-Aldrich (St.
Louis, MO, USA), was dissolved in dimethyl sulfoxide (DMSO) to make
a 10 mM stock solution, and was stored at −20°C. Spd, Spm (each at
10 mM) and 3-aminoguanidine were purchased from Sigma-Aldrich.
3-Aminoguanidine was used as an amine oxidase blocker in the Spd
and Spm treatment experiments.
Antibodies targeting beclin-1 (dilution, 1:1,000),
Atg5 (1:1,000), Atg12 (1:1,000), LC3A/B (1:1,000), β-actin
(1:1,000), β-tubulin (1:1,000), pro-caspase-9 (1:1,000),
cleaved-caspase-9 (1:1,000), caspase-7 (1:1,000) and horseradish
peroxidase (HRP)-conjugated secondary IgG (1:3,000) were purchased
from Cell Signaling Technology (Danvers, MA, USA).
Cell cultures
The breast cancer cell lines MCF-7 (HTB 22) and
MDA-MB-231 (HTB 26) were purchased from the American Type Culture
Collection (ATCC; Manassas, VA, USA). The cells were maintained in
Gibco® Dulbecco’s modified Eagle’s medium (DMEM; Thermo
Fisher Scientific, Waltham, MA, USA) supplemented with 10% fetal
bovine serum (Pan-Biotech GmbH, Aidenbach, Germany) and 100 units
or 100 mg/ml penicillin or streptomycin, and were grown in
humidified air with 5% CO2 at 37°C, in a
Heracell® 150i incubator (Thermo Fisher Scientific).
Cell viability assay
The effect of roscovitine on cell viability in the
presence or absence of PAs was determined by the colorimetric assay
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT;
Roche Diagnostics, Indianapolis, IN, USA). Cells were plated in
96-well plates at a density of 1×105 cells/well, were
allowed to attach overnight, and were treated for 24 h with various
concentrations of roscovitine in the presence or absence of PAs.
After 24 h of treatment, 10 μl of the MTT reagent (5 mg/ml)
were added to the cell culture medium, and cells were incubated for
4 h. Following medium removal, 200 μl of DMSO were added to
dissolve the formazan crystals, which are produced by the activated
mitochondria. The absorbance of the suspensions was measured at 595
nm on a microplate reader (Bio-Rad, Hercules, CA, USA).
Fluorescence staining
Cells (5×104) were seeded into 12-well
plates, allowed to attach overnight and then treated with
appropriate concentrations of drugs for 24 h. In order to assess
the mitochondrial membrane potential (MMP), cells were washed once
with 1X phosphate-buffered saline (PBS) and stained with 0,4 mM
3,3′-dihexyloxacarbocyanine iodide (DiOC6). The
absorbance of samples (Abs 488/525) was measured on a Fluoroskan
Ascent Microplate fluorometer (Thermo Fisher Scientific, Beverly,
MA, USA), with excitation and emission settings of 488 and 525 nm,
respectively; the Abs 488/525 of the samples was compared to that
of the control.
For monodansylcadaverine (MDC) staining, cells
(6×104) were seeded into 6-well plates on coverslips,
allowed to attach overnight and then treated with the appropriate
drug concentrations for 24 h. Cells were washed once with 1X PBS
and stained with 50 μM MDC in order to visualize the
autophagic vesicles. Next, they were observed under a fluorescence
microscope (Olympus, Tokyo, Japan).
Cell death enzyme-linked immunosorbent
assay (ELISA) assay
The cytoplasmic histone-associated DNA-fragments
(mono- and oligonucleosomes) were measured with the Cell Death
Detection ELISA PLUS kit (Roche Diagnostics) according to the
manufacturer’s instructions. Cells (1×104) were seeded
into 96-well plates and treated with the desired drug
concentrations for 24 h. The cell lysates were placed in a
streptavidin-coated microplate. A mixture of anti-histone biotin
and anti-DNA peroxidase (POD) was added, and samples were kept at
room temperature for 2 h. Following washing of the unbound
antibodies, the colorimetric assay was performed with
2,2′-azino-bis(3-ethylbenzthiazoline-6-sulphonic acid as a
substrate, and the absorbance was measured at 405 nm.
Immunoblot analysis
Cells were treated with the appropriate
concentrations of each drug for 24 h. The MCF-7 and MDA-MB-231
cells were lysed with ProteoJET Mammalian cell lysis reagent
(Fermentas, Thermo Fisher Scientific, Waltham, MA, USA) containing
total protease inhibitor cocktail (Roche Diagnostics GmbH,
Mannheim, Germany).
Following lysis, cell debris was removed by
centrifugation for 15 min at 18,500 × g, and protein concentration
was determined with the Bradford method (Quick Start™ Bradford
Protein Assay kit; Bio-Rad Laboratories, Hercules, CA, USA). Total
protein lysates (30 μg) were separated by 15%
SDS-polyacrylamide gel electrophoresis (PAGE) and transferred onto
polyvinylidene difluoride membranes (Roche Diagnostics). The
membranes were then blocked with 5% non-fat milk, prepared in a 1%
Tris-buffered saline and Tween-20 (TBST) solution. Following
incubation of the membranes with the appropriate primary antibody
at 4°C overnight, the membranes were washed with TBST. The
membranes were then incubated with the appropriate HRP-conjugated
secondary antibody overnight at 4°C, an enhanced chemiluminescence
(ECL) reagent (Lumi-Light Western Blotting substrate; Roche
Diagnostics GmbH) was used to visualize the antigens. Finally, the
membranes were exposed to Kodak X-ray film (Kodak, Rochester, NY,
USA) in a dark room.
Statistical analysis
Differences between samples were statistically
evaluated using an Office Excel calculation file (Microsoft, New
York, NY, USA). The results from the MTT, cell death ELISA and MMP
assay were expressed as mean ± standard deviation. Student’s
t-tests were applied toassess the significance of comparisons.
Differences were regarded as statistically significant at
P<0.05.
Results
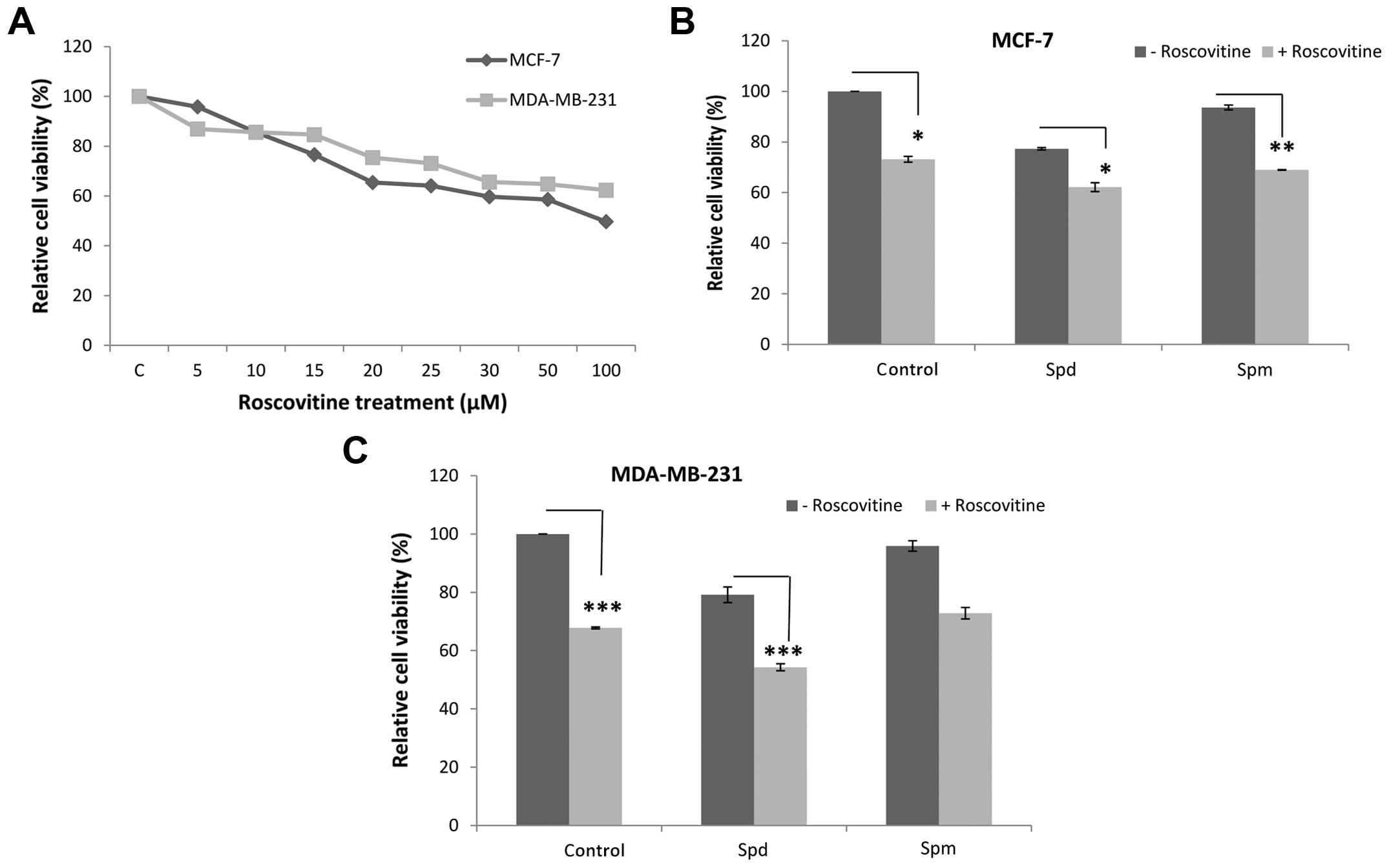
Roscovitine-induced cytotoxicity is
altered by polyamine treatment
In order to understand the effect of roscovitine on
MCF-7 and MDA-MB-231 breast cancer cells, the MTT cell viability
assay was performed following treatment with various concentrations
of the drug (0–100 μM) for 24 h. The cell viability was
decreased by 35 and 25% following treatment with 20 μM
roscovitine in MCF-7 and MDA-MB-231 breast cancer cell lines,
respectively (Fig. 1A). This
concentration was selected for the following experiments.
To evaluate the combined effect of Spd or Spm (each
10 μM) with roscovitine, each cell line was exposed to drugs
for 24 h. Although Spd treatment caused moderate cytotoxicity (23%
reduction in cell viability in MCF-7 and 21% in MDA-MB-231 cells
vs. control, respectively), Spm treatment was less effective (7% in
MCF-7 and 4% in MDA-MB-231 cells) (Fig. 1B and C). Co-treatment with Spd or
Spm and roscovitine enhanced the roscovitine-induced cytotoxicity
in both breast cancer cell lines. In addition, the promoting
effects of Spd on cytotoxicity were significant in both cell lines,
particularly in the MDA-MB-231 cells (P<0.0002).
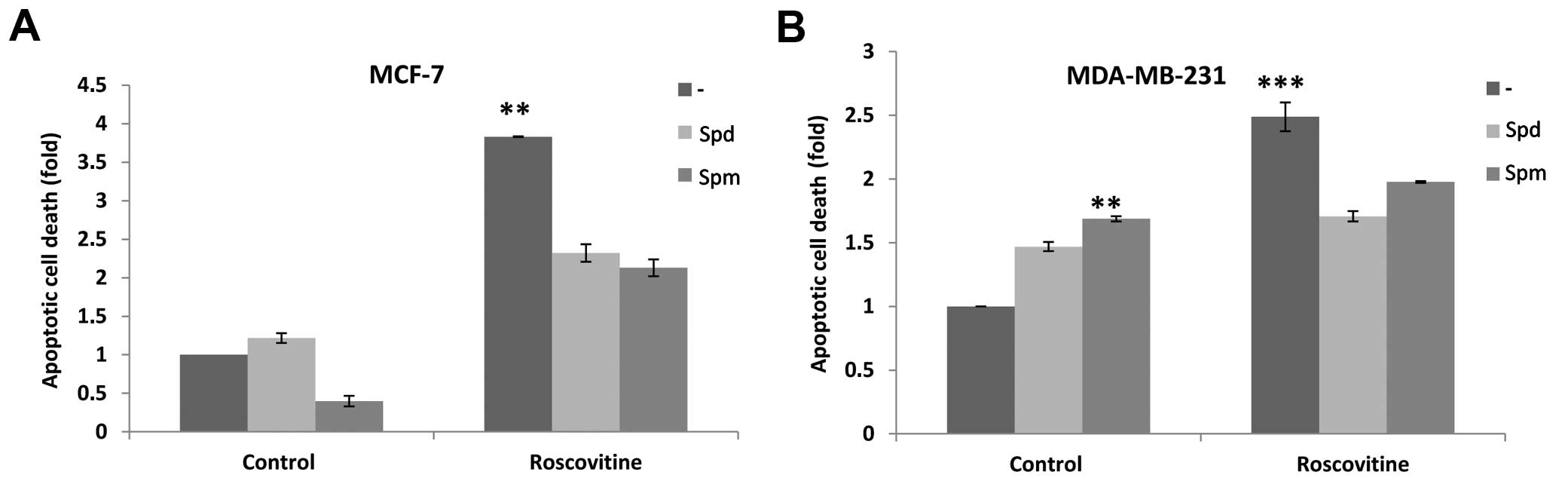
Roscovitine-induced mitochondria-mediated
apoptosis via caspase activation
We determined the apoptotic potential of roscovitine
in the presence or absence of Spd/Spm in the cells. Although
neither Spd nor Spm exerted significant apoptotic effects,
roscovitine induced apoptosis by 4- and 2.5-fold in MCF-7 and
MDA-MB-231 breast cancer cells, respectively, as compared to
untreated control cells. Spm alone slightly induced apoptosis in
MDA-MB-231 cells by 1.5-fold compared to control cells.
Co-treatment with Spd or Spm and roscovitine enhanced the cell
viability reduction in both cell lines; it also prevented
drug-induced apoptosis by decreasing the DNA fragmentation ratio
(Fig. 2).
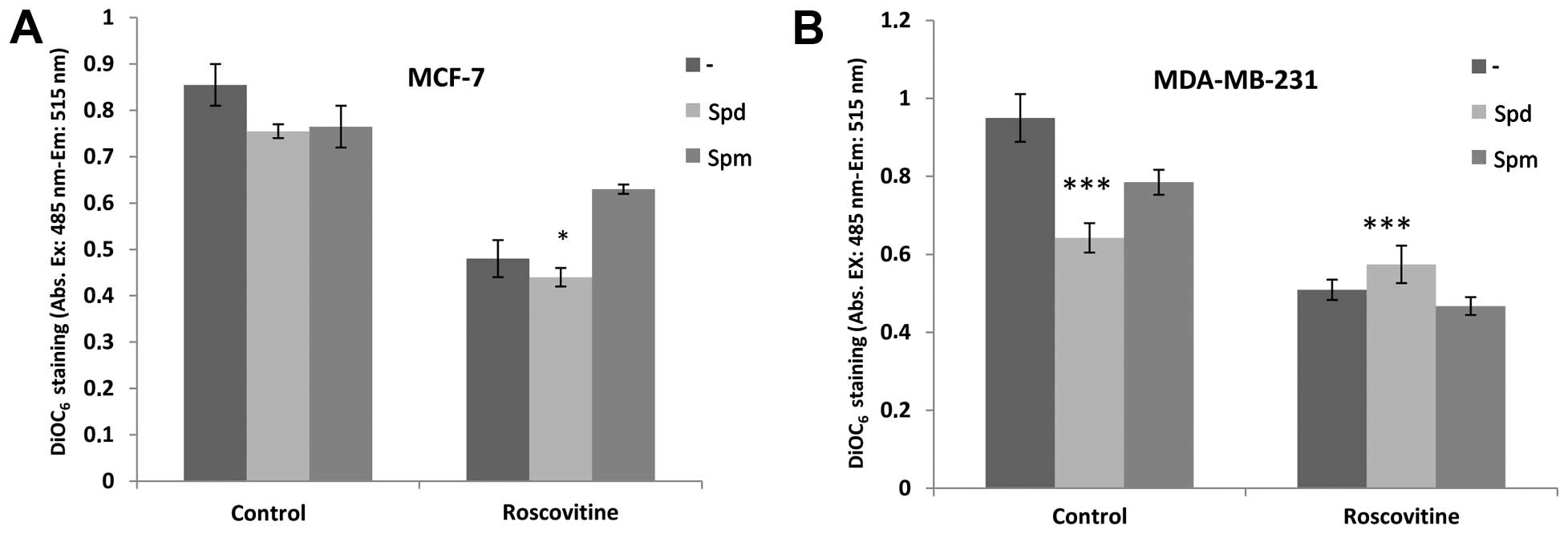
We performed DiOC6 staining to visualize
the MMP loss on a fluorometer and thus, investigate the role of PAs
in roscovitine-induced apoptosis. Although roscovitine decreased
MMP in both cell lines, co-treatment with Spd did not affect the
roscovitine-induced MMP reduction (Fig. 3). By contrast, Spm protected the
MCF-7, but not the MDA-MB-231 cells, from roscovitine-induced
mitochondria-mediated apoptosis, although theses changes were not
significant.
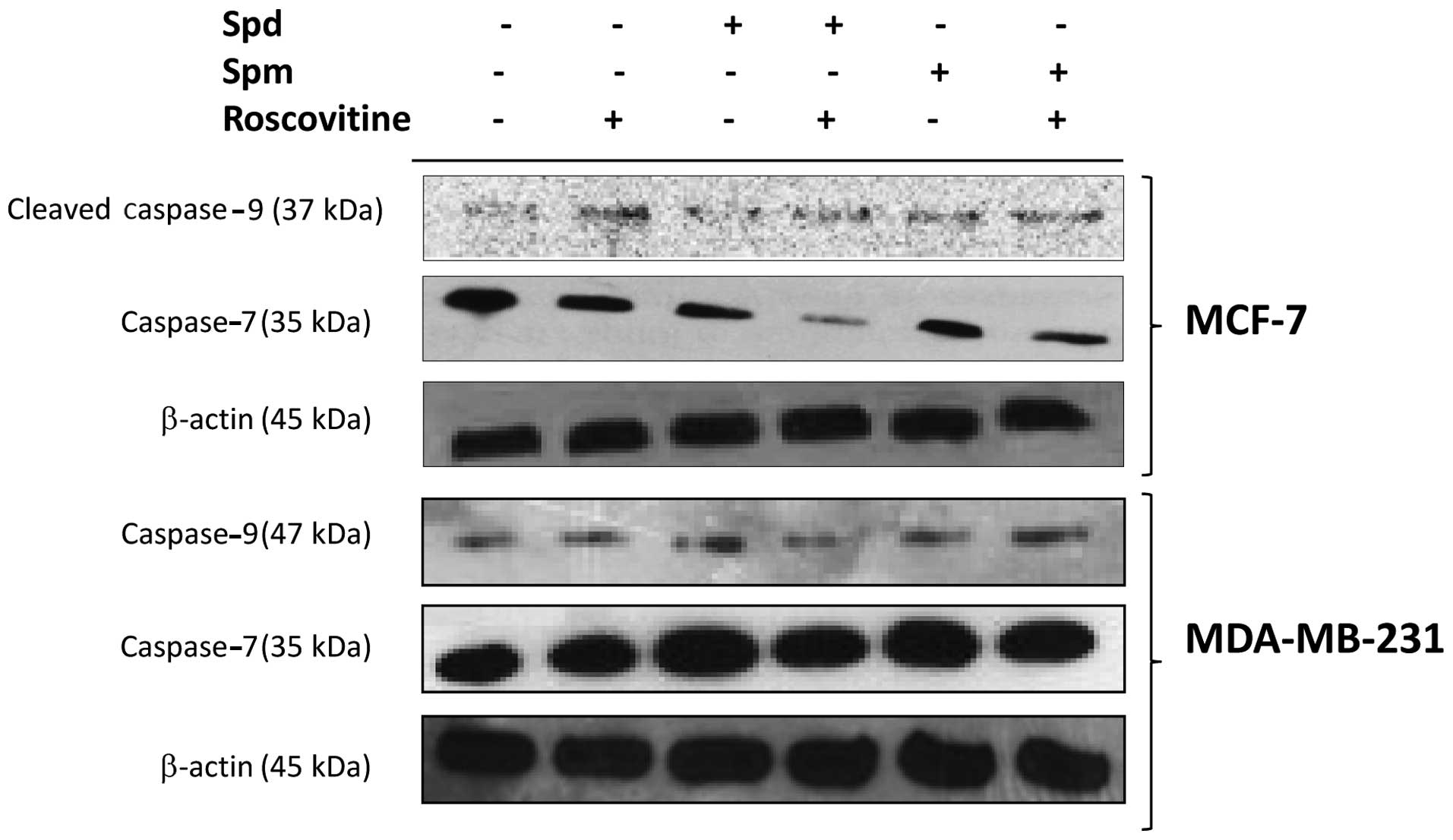
To further investigate drug-induced caspase
activation, we determined the level of cleaved fragments of
caspase-9 and -7 by immunoblotting. While caspase-9 cleavage, which
is the initial step for caspase activation in mitochondria-mediated
apoptosis, appeared increased, the level of the full-length
caspase-7, the executioner caspase for apoptosis, was decreased
after roscovitine treatment for 24 h in breast cancer cells.
Although exposure of MCF-7 cells to Spd or Spm for 24 h did not
appear to activate caspase-9, treatment with each of these PAs led
to a decrease in the caspase-7 level. In addition, combined
treatment with Spd or Spm and roscovitine further decreased the
expression level of the full-length caspase-7 in MCF-7 cells.
Roscovitine induced the cleavage of caspase-9 and -7 in MDA-MB-231
breast cancer cell lines. In addition, PAs enhanced the
roscovitine-induced caspase-9 and -7 activation by decreasing the
level of the full-length fragments of these caspases in MDA-MB-231
cells (Fig. 4).
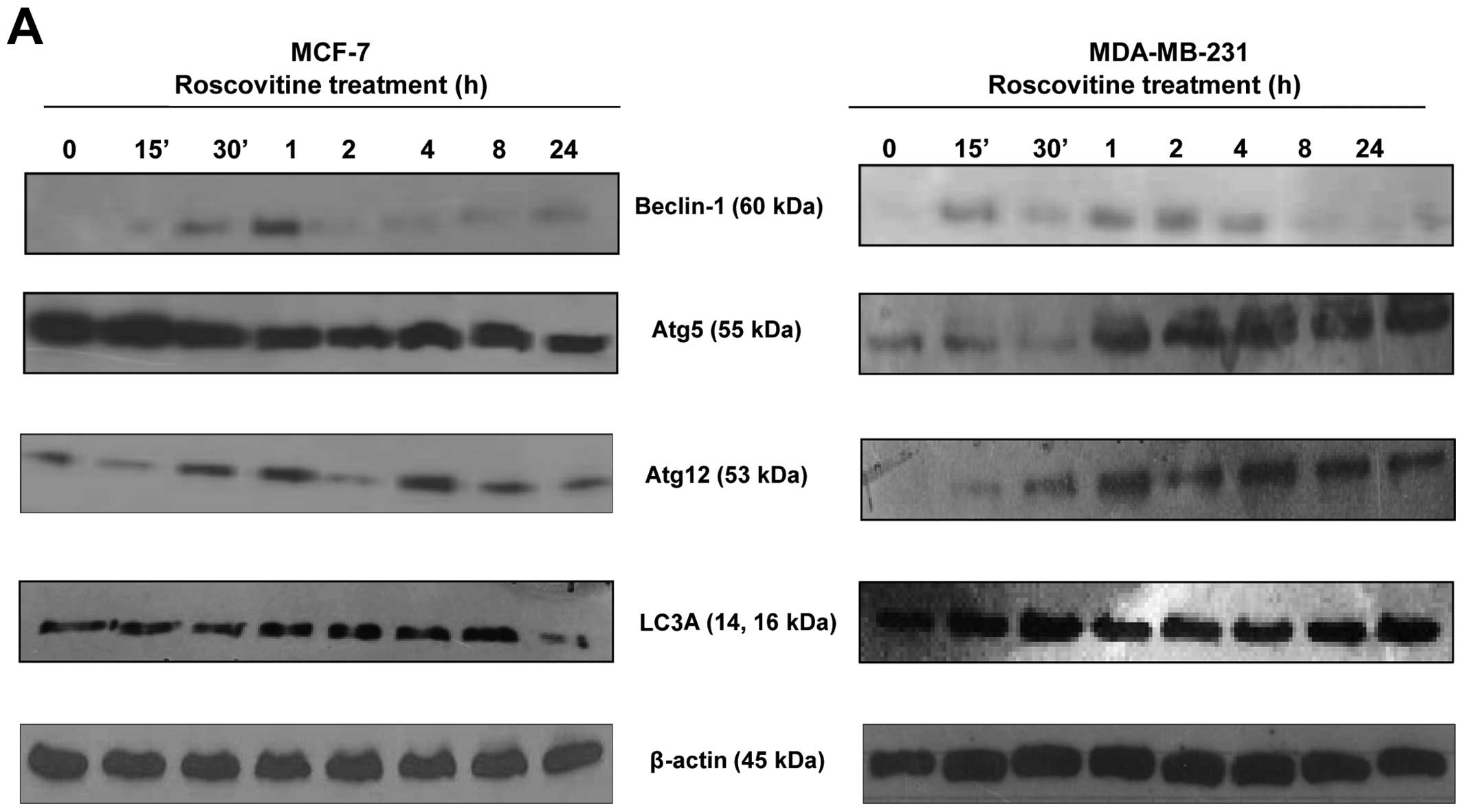
Roscovitine induces autophagic
modulation
In order to evaluate the role of roscovitine on
autophagic cell death in the MCF-7 and MDA-MB-231 breast cancer
cell lines, we performed immunoblotting assays at different
time-points. We examined the expression profile of beclin-1, which
is referred to as the initial key molecule for autophagy, following
roscovitine treatment within 24 h. Interestingly, while beclin-1
appeared to be time-dependently upregulated from 0 to 1 h in MCF-7
cells, its expression was stable in MDA-MB-231 cells for up to 4
h.
To indirectly assess the autophagosome complex
formation at different time periods of drug treatment, the
expression profile of Atg5 and Atg12, which are critical molecules
for the elongation of the autophagosomal membrane, were also
determined by immunoblotting. The basal expression levels of Atg5
and Atg12 were found to be higher in MCF-7 compared to MDA-MB-231
cells. In general, while roscovitine decreased the expression of
Atg5 within 24 h, the Atg5 expression level was increased after 1 h
of drug treatment in the MDA-MB-231 breast cancer cell line
(Fig. 5A). Another key marker of
autophagosomal formation is LC3A/B, which integrates to double
membranes; the level of this protein was increased after 8 h of
roscovitine treatment in MCF-7 cells. However, the expression level
of LC3A/B was decreased after 24 h of drug treatment. When we
examined the autophagic effect of roscovitine on LC3A/B expression
in MDA-MB-231 cells, we observed a rapid upregulation within 30 min
and an overall higher basal level compared to MCF-7 cells. In
addition, LC3A/B expression was higher in MDA-MB-231 cells after 24
h of drug treatment compared to MCF-7 cells. When the MCF-7 cells
were treated with roscovitine for 72 h, the expression of the
autophagic key markers LC3A/B, Atg5 and Atg12 was decreased from
the first 24 h. However, the protein levels of these markers
appeared increased again after 72 h of drug treatment in MCF-7
cells. By contrast, the expression levels of LC3A/B, Atg5 and Atg12
appeared increased after 48 h of drug treatment in the MDA-MB-231
cell line (Fig. 5B). Based on
these results, we conclude that MCF-7 cells are more sensitive to
roscovitine-induced autophagy than MDA-MB-231 cells.
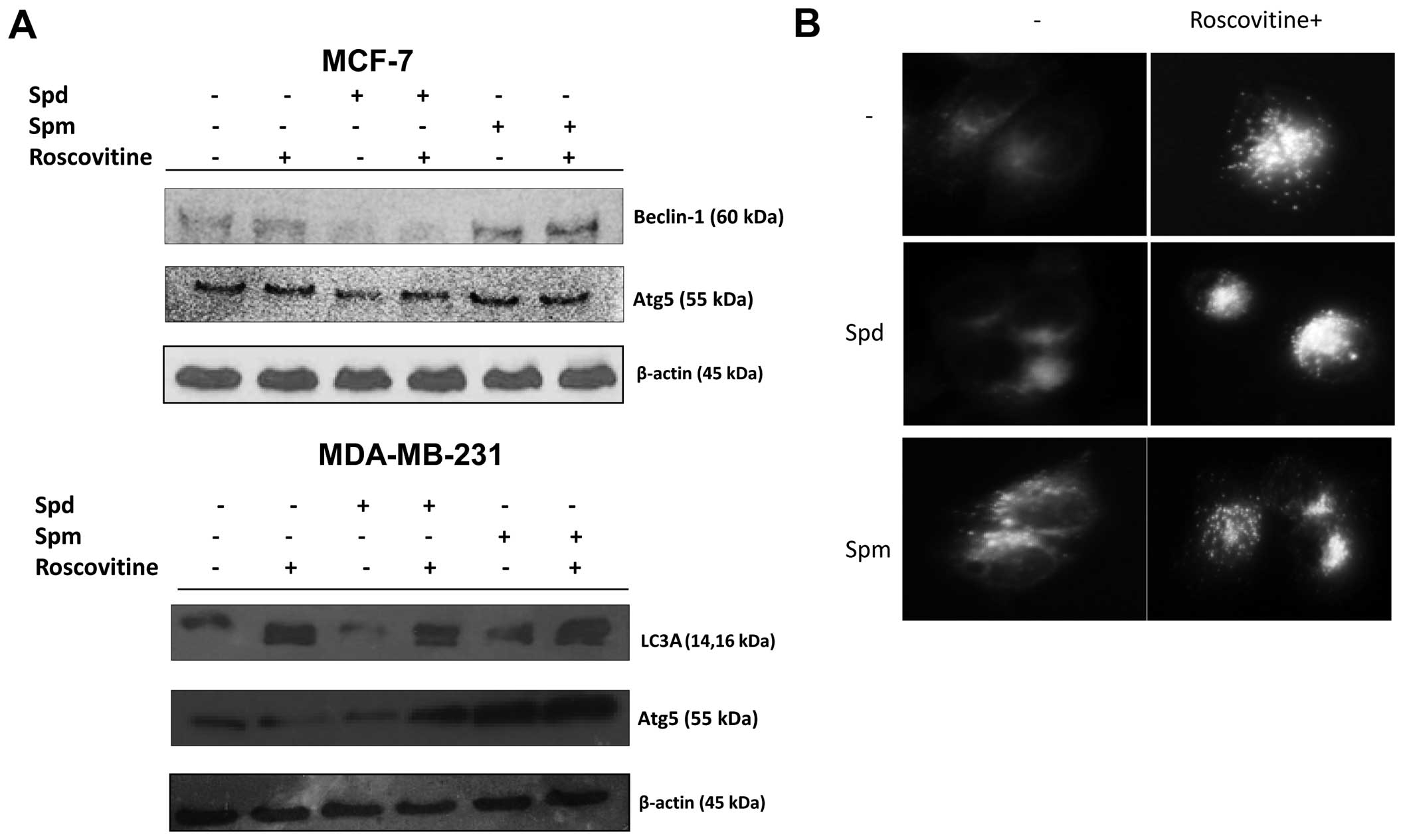
Polyamines modulate roscovitine induced
autophagy
In order to further explore the role of polyamines
in drug-induced autophagy, cells were treated with roscovitine in
the presence of Spd or Spm for 24 h. Spd was not an autophagy
inducer but Spm was a good candidate to induce autophagy by
upregu-lating beclin-1 and Atg5 in MCF-7 and MDA-MB-231 cells
(Fig. 6A). Moreover, cleaved
fragments of LC3A/B were observed following Spm treatment in
MDA-MB-231 cells. After treatment with Spd alone, beclin-1 and Atg5
expression levels decreased. By contrast, after treatment with Spm
alone, the beclin-1 and Atg5 expression levels increased.
Co-treatment with roscovitine and Spm showed opposite effects on
autophagic marker expression in the two breast cancer cell lines
compared to co-treatment with roscovitine and Spd (Fig. 6A). These results were confirmed in
MCF-7 cells by MDC staining, which allows to detect the autophagic
vacuoles (Fig. 6B).
Discussion
The majority of malignancies are associated with the
loss of functional cell-cycle control, which results in impaired
apoptosis and unlimited growth. An emerging anticancer approach is
to control the aberrant cell cycle machinery by evaluating key
molecules for drug design. As shown in previous studies, CDK
inhibitors exert their apoptotic effect by causing cell-cycle
arrest (10,26,27).
Roscovitine is a promising CDK inhibitor with high apoptotic
potential in malignant cells. It competitively binds to the ATP
binding site of CDKs and prevents cyclin-CDK complex formation
(28–31). Furthermore, roscovitine is the
first orally bioavailable CDK inhibitor in clinical trials for
B-cell malignancies and lung cancer (31,32).
A previous study indicated that roscovitine induces apoptosis in
breast cancer cells by causing cell-cycle arrest at the
G2/M phase (9). PAs are
key regulators of cellular processes such as transcription,
translation and proliferation (33). PA metabolic enzymes have been
proposed as targets for antineoplastic therapy in breast cancer,
since their high intracellular level was found associated with
rapid cel-cycle turnover in these cells compared to healthy breast
tissue cells (14,34–37).
In the present study, we demonstrated that roscovitine decreases
cell viability in a dose-dependent manner in the MCF-7 and
MDA-MB-231 breast cancer cell lines (Fig. 1A). We also determined that the
combination of Spd or Spm with roscovitine can enhance drug-induced
cytotoxicity in both breast cancer cell lines (Fig. 1B and C). MCF-7 and MDA-MB-231 cells
have a different expression status for ERα, which regulates the
transcription of genes such as CDK2, a target of
roscovitine. CDK2 has been also shown to enhance the
ligand-independent ERα activation (38–40),
which indicates that this protein can play a critical role in the
responsiveness against the hormone ablation therapy (5,41,42).
Similar to previous findings (9,10),
roscovitine inhibited the proliferation rates to different degrees
in ERα-positive and -negative breast cancer cell lines in our
study.
Exposure of cancer cells to PAs may affect the
modulation of cell responses to drug treatment in a cell-dependent
manner. While Spd treatment protected Erhlich ascite tumor cells
against apoptosis triggered by acetoxychavicol acetate (43), Spm was shown to synergistically act
with bovine serum oxidase in docetaxel-induced apoptosis in MCF-7
cells (44). According to a
previous study by our group, roscovitine-induced apoptotic cell
death may be altered when PA biosynthesis is inhibited in HCT116
colon carcinoma cells (45).
Although increased accumulation of intracellular PAs
is associated with disease progression and rapid cell-cycle
turnover, due to high PA catabolic activity, Spm may induce
apoptosis by activating cellular caspases (46–48).
Cell death in vertebrates mostly proceeds via the
mitochondrial pathway in a caspase-dependent manner (49,50).
CDK inhibitors were shown to exert their apoptotic effect through
inducing MMP loss and activating caspases in cancer cells (51,52).
Similar to these observations, we determined that exposure of cells
to roscovitine for 24 h induces the modulation of MMP in the MCF-7
and MDA-MB-231 breast cancer cell lines. However, combined
treatment of Spd or Spm with roscovitine caused different effects
in both cell lines. Spm protected cells against roscovitine-induced
mitochondria-mediated apoptosis in MCF-7, but not in MDA-MB-231
breast cancer cells (Fig. 3).
According to the results of the present study, there was a
difference in the two breast cancer cell lines treated with
roscovitine and PAs, with regards to cell death response. The MCF-7
and MDA-MB-231 cell lines have different genetic backgrounds,
particularly with regards to ER status, which is associated with
cellular growth and the fate of cells. Therefore, it may be
hypothesized that the difference in cell death response between
these cell lines is due to key targets within the ER. Furthermore,
hormone signaling may promote different cell signaling pathways to
induce either apoptosis or autophagy (2,16).
In addition, PAs also have a role in cell growth, and the treatment
of the cells with PAs resulted in an altered cell response to
roscovitine treatment. Therefore, MCF-7 and MDA-MB-231 cells may
act differently upon drug exposure. In association with these data,
we showed that in the MCF-7 breast cancer cell line, roscovitine
treatment for 24 h results in the cleavage of pro-caspase-9 and -7,
which is referred to in the literature as the initial eevent during
induction of apoptosis. Upon treatment with roscovitine, additional
Spm exposure affected the activation of both caspases in the MCF-7
and MDA-MB-231 breast cancer cell lines. However, treatment with
roscovitine only did not exert the same effect on the MDA-MB-231
cells (Fig. 4).
In the second part of the present study, we
investigated the role of the CDK inhibitor on autophagy and the
potential role of PAs on autophagic regulation in MCF-7 and
MDA-MB-231 breast cancer cell lines. The therapeutic efficiencies
of drug candidates for cancer treatment were investigated in recent
studies by examination of their potential to activate both
apoptosis and autophagy, and by studying their interactions
(53,54). Therefore, elucidation of the
molecular mechanism common to apoptosis and autophagy, as well as
of the crosstalk between these two processes is of high importance.
Inhibition of autophagy has been shown to enhance the induction of
apoptosis (55,56). Under cellular stress conditions,
such as in the presence of DNA-damaging agents, autophagy is
inhibited and the intrinsic pathway of apoptosis is triggered in
MCF-7 cells, but the induction of autophagy can delay apoptosis
(57).
In association with these findings, we found that
24-h treatment with roscovitine modulates the mechanism underlying
autophagy in MDA-MB-231, but not in MCF-7 cells (Fig. 5A). Longer exposure of both cell
lines to roscovitine confirmed that the autophagic process is more
prominent in MDA-MB-231 cells compared to MCF-7 cells. Therefore,
we conclude that MCF-7 cells are more sensitive to
roscovitine-induced autophagy than MDA-MB-231 cells (Fig. 5B).
In general, autophagy delays cell death and prolongs
the lifespan in various experimental aging models (58–60).
Recent studies showed that PAs, and in particular Spd, induce
autophagy and cause increased lifespan. For instance,
naphthalimide-PA conjugates trigerred autophagy by modulating the
mTOR signaling cascade. Exposure of HepG2 cells to the
naphthalimide-PA conjugates induced autophagic vesicle formation
(61,62). In a similar way, Spd treatment can
induce LC3 formation in HeLa cells (63). Therefore, Spd-induced autophagy may
be therapeutically useful for cancer treatment. Indeed, increased
levels of highly and positively-charged PAs have been found to
correlate with chromatin condensation, and to modulate HAT and HDAC
activities in murine skin tumors (24,25).
However, in yeast, Spd treatment has been shown to trigger the
global hypoacetylation of histone H3 and selectively acetylate the
promoter region of the atg7 gene, which led to the
upregulation of autophagic genes (23,63).
According to our findings, Spm may be proposed as an autophagic
agent in MCF-7 and MDA-MB-231 cells (Fig. 6).
Therefore, we conclude that roscovitine is a
mediator of apoptosis in the ERα+ MCF-7 breast cancer
cells, and that apoptosis is delayed by the induction of autophagy
in ERα− MDA-MB-231 cells. In addition, PAs play critical
roles in roscovitine-induced autophagy in a cell type-dependent
manner.
References
|
1
|
Jemal A, Siegel R, Ward E, Hao Y, Xu J and
Thun MJ: Cancer statistics, 2009. CA Cancer J Clin. 59:225–249.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lerner LJ and Jordan VC: Development of
antiestrogens and their use in breast cancer: eighth Cain memorial
award lecture. Cancer Res. 50:4177–4189. 1990.PubMed/NCBI
|
|
3
|
Jaiyesimi IA, Buzdar AU, Decker DA and
Hortobagyi GN: Use of tamoxifen for breast cancer: twenty-eight
years later. J Clin Oncol. 13:513–529. 1995.PubMed/NCBI
|
|
4
|
Buzdar AU and Hortobagyi G: Update on
endocrine therapy for breast cancer. Clin Cancer Res. 4:527–534.
1998.PubMed/NCBI
|
|
5
|
Nair BC and Vadlamudi RK: Regulation of
hormonal therapy resistance by cell cycle machinery. Gene Ther Mol
Biol. 12:3952008.PubMed/NCBI
|
|
6
|
Al-Minawi AZ, Saleh-Gohari N and Helleday
T: The ERCC1/XPF endonuclease is required for efficient
single-strand annealing and gene conversion in mammalian cells.
Nucleic Acids Res. 36:1–9. 2008. View Article : Google Scholar :
|
|
7
|
Hunt T: You never know: Cdk inhibitors as
anti-cancer drugs. Cell Cycle. 7:3789–3790. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Aldoss IT, Tashi T and Ganti AK:
Seliciclib in malignancies. Expert Opin Investig Drugs.
18:1957–1965. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wojciechowski J, Horky M, Gueorguieva M
and Wesierska-Gadek J: Rapid onset of nucleolar disintegration
preceding cell cycle arrest in roscovitine-induced apoptosis of
human MCF-7 breast cancer cells. Int J Cancer. 106:486–495. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wesierska-Gadek J, Gueorguieva M and Horky
M: Roscovitine-induced up-regulation of p53AIP1 protein precedes
the onset of apoptosis in human MCF-7 breast cancer cells. Mol
Cancer Ther. 4:113–124. 2005.PubMed/NCBI
|
|
11
|
Appleyard MV, O’Neill MA, Murray KE, et
al: Seliciclib (CYC202, R-roscovitine) enhances the antitumor
effect of doxorubicin in vivo in a breast cancer xenograft model.
Int J Cancer. 124:465–472. 2009. View Article : Google Scholar
|
|
12
|
Charollais RH and Mester J: Resumption of
cell cycle in BALB/c-3T3 fibroblasts arrested by polyamine
depletion: relation with ‘competence’ gene expression. J Cell
Physiol. 137:559–564. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Harada JJ and Morris DR: Cell cycle
parameters of Chinese hamster ovary cells during exponential,
polyamine-limited growth. Mol Cell Biol. 1:594–599. 1981.PubMed/NCBI
|
|
14
|
Pegg AE: Polyamine metabolism and its
importance in neoplastic growth and a target for chemotherapy.
Cancer Res. 48:759–774. 1988.PubMed/NCBI
|
|
15
|
Deng W, Jiang X, Mei Y, et al: Role of
ornithine decarboxylase in breast cancer. Acta Biochim Biophys Sin
(Shanghai). 40:235–243. 2008. View Article : Google Scholar
|
|
16
|
Hoggard N and Green CD: Polyamines and
growth regulation of cultured human breast cancer cells by 17
beta-oestradiol. Mol Cell Endocrinol. 46:71–78. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Manni A: Polyamine involvement in breast
cancer phenotype. In Vivo. 16:493–500. 2002.PubMed/NCBI
|
|
18
|
Bello-Fernandez C, Packham G and Cleveland
JL: The ornithine decarboxylase gene is a transcriptional target of
c-Myc. Proc Natl Acad Sci USA. 90:7804–7808. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Celano P, Baylin SB, Giardiello FM, Nelkin
BD and Casero RA Jr: Effect of polyamine depletion on c-myc
expression in human colon carcinoma cells. J Biol Chem.
263:5491–5494. 1988.PubMed/NCBI
|
|
20
|
Kondo Y, Kanzawa T, Sawaya R and Kondo S:
The role of autophagy in cancer development and response to
therapy. Nat Rev Cancer. 5:726–734. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gozuacik D and Kimchi A: Autophagy and
cell death. Curr Top Dev Biol. 78:217–245. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Maiuri MC, Zalckvar E, Kimchi A and
Kroemer G: Self-eating and self-killing: crosstalk between
autophagy and apoptosis. Nat Rev Mol Cell Biol. 8:741–752. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Madeo F, Eisenberg T, Büttner S,
Ruckenstuhl C and Kroemer G: Spermidine: a novel autophagy inducer
and longevity elixir. Autophagy. 6:160–162. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hobbs CA and Gilmour SK: High levels of
intracellular polyamines promote histone acetyltransferase activity
resulting in chromatin hyperacetylation. J Cell Biochem.
77:345–360. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hobbs CA, Paul BA and Gilmour SK:
Deregulation of polyamine biosynthesis alters intrinsic histone
acetyltransferase and deacetylase activities in murine skin and
tumors. Cancer Res. 62:67–74. 2002.PubMed/NCBI
|
|
26
|
Wesierska-Gadek J, Wandl S, Kramer MP,
Pickem C, Krystof V and Hajek SB: Roscovitine up-regulates p53
protein and induces apoptosis in human HeLaS(3) cervix carcinoma
cells. J Cell Biochem. 105:1161–1171. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wesierska-Gadek J, Gueorguieva M,
Wojciechowski J and Horky M: Cell cycle arrest induced in human
breast cancer cells by cyclin-dependent kinase inhibitors: a
comparison of the effects exerted by roscovitine and olomoucine.
Pol J Pharmacol. 56:635–641. 2004.PubMed/NCBI
|
|
28
|
Meijer L, Borgne A, Mulner O, et al:
Biochemical and cellular effects of roscovitine, a potent and
selective inhibitor of the cyclin-dependent kinases cdc2, cdk2 and
cdk5. Eur J Biochem. 243:527–536. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Fischer PM and Gianella-Borradori A: CDK
inhibitors in clinical development for the treatment of cancer.
Expert Opin Investig Drugs. 12:955–970. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hahntow IN, Schneller F, Oelsner M, et al:
Cyclin-dependent kinase inhibitor Roscovitine induces apoptosis in
chronic lymphocytic leukemia cells. Leukemia. 18:747–755. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Decker T, Hipp S, Hahntow I, Schneller F
and Peschel C: Expression of cyclin E in resting and activated
B-chronic lymphocytic leukaemia cells: cyclin E/cdk2 as a potential
therapeutic target. Br J Haematol. 125:141–148. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Benson C, White J, De Bono J, et al: A
phase I trial of the selective oral cyclin-dependent kinase
inhibitor seliciclib (CYC202; R-Roscovitine), administered twice
daily for 7 days every 21 days. Br J Cancer. 96:29–37. 2007.
View Article : Google Scholar
|
|
33
|
Zaletok S, Alexandrova N, Berdynskykh N,
et al: Role of polyamines in the function of nuclear transcription
factor NF-kappaB in breast cancer cells. Exp Oncol. 26:221–225.
2004.PubMed/NCBI
|
|
34
|
Wang Y and Casero RA Jr: Mammalian
polyamine catabolism: a therapeutic target, a pathological problem,
or both? J Biochem. 139:17–25. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Persson L and Rosengren E: Increased
formation of N1-acetylspermidine in human breast cancer. Cancer
Lett. 45:83–86. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Cañizares F, Salinas J, de las Heras M, et
al: Prognostic value of ornithine decarboxylase and polyamines in
human breast cancer: correlation with clinicopathologic parameters.
Clin Cancer Res. 5:2035–2041. 1999.PubMed/NCBI
|
|
37
|
Casero RA Jr and Marton LJ: Targeting
polyamine metabolism and function in cancer and other
hyperproliferative diseases. Nat Rev Drug Discov. 6:373–390. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ali S and Coombes RC: Endocrine-responsive
breast cancer and strategies for combating resistance. Nat Rev
Cancer. 2:101–112. 2002. View
Article : Google Scholar
|
|
39
|
Loi S, Haibe-Kains B, Desmedt C, et al:
Definition of clinically distinct molecular subtypes in estrogen
receptor-positive breast carcinomas through genomic grade. J Clin
Oncol. 25:1239–1246. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Sutherland RL and Musgrove EA: CDK
inhibitors as potential breast cancer therapeutics: new evidence
for enhanced efficacy in ER+ disease. Breast Cancer Res.
11:1122009. View Article : Google Scholar
|
|
41
|
Rogatsky I, Trowbridge JM and Garabedian
MJ: Potentiation of human estrogen receptor alpha transcriptional
activation through phosphorylation of serines 104 and 106 by the
cyclin A-CDK2 complex. J Biol Chem. 274:22296–22302. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Trowbridge JM, Rogatsky I and Garabedian
MJ: Regulation of estrogen receptor transcriptional enhancement by
the cyclin A/Cdk2 complex. Proc Natl Acad Sci USA. 94:10132–10137.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Moffatt J, Hashimoto M, Kojima A, et al:
Apoptosis induced by 1′-acetoxychavicol acetate in Ehrlich ascites
tumor cells is associated with modulation of polyamine metabolism
and caspase-3 activation. Carcinogenesis. 21:2151–2157. 2000.
View Article : Google Scholar
|
|
44
|
Marra M, Lombardi A, Agostinelli E, et al:
Bovine serum amine oxidase and spm potentiate docetaxel and
interferon-alpha effects in inducing apoptosis on human cancer
cells through the generation of oxidative stress. Biochim Biophys
Acta. 1783:2269–2278. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Arisan ED, Coker A and Palavan-Ünsal N:
Polyamine depletion enhances the roscovitine-induced apoptosis
through the activation of mitochondria in HCT116 colon carcinoma
cells. Amino Acids. 42:655–665. 2012. View Article : Google Scholar
|
|
46
|
Xie X, Tome ME and Gerner EW: Loss of
intracellular putrescine pool-size regulation induces apoptosis.
Exp Cell Res. 230:386–392. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Stefanelli C, Bonavita F, Stanic I, et al:
Spermine causes caspase activation in leukaemia cells. FEBS Lett.
437:233–236. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Stefanelli C, Stanic I, Zini M, et al:
Polyamines directly induce release of cytochrome c from heart
mitochondria. Biochem J. 347:875–880. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Ouyang L, Shi Z, Zhao S, et al: Programmed
cell death pathways in cancer: a review of apoptosis, autophagy and
programmed necrosis. Cell Prolif. 45:487–498. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Green DR and Kroemer G: The
pathophysiology of mitochondrial cell death. Science. 305:626–629.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Yenugonda VM, Deb TB, Grindrod SC, et al:
Fluorescent cyclin-dependent kinase inhibitors block the
proliferation of human breast cancer cells. Bioorg Med Chem.
19:2714–2725. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Ringer L, Sirajuddin P, Yenugonda VM, et
al: VMY-1-103, a dansylated analog of purvalanol B, induces
caspase-3-dependent apoptosis in LNCaP prostate cancer cells.
Cancer Biol Ther. 10:320–325. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Liu B, Wu JM, Li J, et al: Polygonatum
cyrtonema lectin induces murine fibrosarcoma L929 cell apoptosis
and autophagy via blocking Ras-Raf and PI3K-Akt signaling pathways.
Biochimie. 92:1934–1938. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Cheng Y, Qiu F, Huang J, Tashiro S,
Onodera S and Ikejima T: Apoptosis-suppressing and
autophagy-promoting effects of calpain on oridonin-induced L929
cell death. Arch Biochem Biophys. 475:148–155. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Lambert LA, Qiao N, Hunt KK, et al:
Autophagy: a novel mechanism of synergistic cytotoxicity between
doxorubicin and roscovitine in a sarcoma model. Cancer Res.
68:7966–7974. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Amaravadi RK, Yu D, Lum JJ, et al:
Autophagy inhibition enhances therapy-induced apoptosis in a
Myc-induced model of lymphoma. J Clin Invest. 117:326–336. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Boya P, González-Polo RA, Casares N, et
al: Inhibition of macroautophagy triggers apoptosis. Mol Cell Biol.
25:1025–1040. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Abedin MJ, Wang D, McDonnell MA, Lehmann U
and Kelekar A: Autophagy delays apoptotic death in breast cancer
cells following DNA damage. Cell Death Differ. 14:500–510. 2007.
View Article : Google Scholar
|
|
59
|
Jia K and Levine B: Autophagy is required
for dietary restriction-mediated life span extension in C. elegans.
Autophagy. 3:597–599. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Meléndez A, Tallóczy Z, Seaman M,
Eskelinen EL, Hall DH and Levine B: Autophagy genes are essential
for dauer development and life-span extension in C. elegans.
Science. 301:1387–1391. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Tavernarakis N, Pasparaki A, Tasdemir E,
Maiuri MC and Kroemer G: The effects of p53 on whole organism
longevity are mediated by autophagy. Autophagy. 4:870–873. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Tian ZY, Xie SQ, Mei ZH, Zhao J, Gao WY
and Wang CJ: Conjugation of substituted naphthalimides to
polyamines as cytotoxic agents targeting the Akt/mTOR signal
pathway. Org Biomol Chem. 7:4651–4660. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Eisenberg T, Knauer H, Schauer A, et al:
Induction of autophagy by spermidine promotes longevity. Nat Cell
Biol. 11:1305–1314. 2009. View Article : Google Scholar : PubMed/NCBI
|