Introduction
Cancer is one of the most life threatening diseases
in humans worldwide. Breast cancer is the most frequent type of
cancer affecting western females, accounting for 7–10% cases of
systemic cancer (1,2). Breast cancer is predominantly a
result of abnormal division and malignant proliferation of the
breast duct or acinar cells (3,4). The
incidence of breast cancer has increased in the majority of
countries (5) and its metastasis
to distant sites is the predominant cause of mortality (6). Currently, there remains no effective
means to treat breast cancer. Inducing the apoptosis of tumor cells
is an important strategy in cancer treatment (7–9).
There are at least two pathways, which lead to cell apoptosis, an
‘extrinsic’ and an ‘intrinsic’ pathway, which are mediated by death
receptors on the cell surface and by mitochondria, respectively
(10–12). The intrinsic apoptotic pathway is
characterized by the permeabilization of the mitochondria and the
release of cytochrome c into the cytoplasm (13,14).
Notably, the expression levels of the anti-apoptotic factor, B-cell
lymphoma 2 (Bcl-2) and pro-apoptotic protein, Bcl-2-associated X
protein (Bax), increase during the process of apoptosis, occurring
in a mitochondria-mediated apoptotic signaling pathway (15,16).
The prognosis of patients with breast cancer is
closely associated with the malignant invasion and early metastasis
of the cancer cells (17,18). The involvement of matrix
metalloproteinases (MMPs) in tumor invasion and metastases is an
area of interest. The activation of MMP in the extracellular matrix
degrade collagen and release growth factors and peptides (19,20).
Under pathological conditions, the expression of MMPs increase
significantly faster compared with that of the tissue inhibitors of
metalloproteinase (TIMPs), which induce the activation of MMPs,
alters the tumor microenvironment and activates cell surface
receptors and downstream signaling pathways (21). MMP-9 and its specific inhibitor,
TIMP-1, are closely correlated with tumor physiological and
pathological processes (22).
Activated MMP-9 and pro-MMP-9 bind to TIMP-1, and the activation of
MMP-9 is often characterized by the degradation of TIMP-1 and the
accumulation of extracellular matrix (23,24).
Thus, MMP-9 is closely associated with the occurrence and
development of breast cancer. Therefore, it is important to
identify potential effective therapeutic agents, which can
effectively activate apoptosis-mediated cell death and inhibit the
invasion and metastasis of breast cancer.
Safflower (Carthamus tinctorius L) is a
traditional Chinese medicine. It is a herbaceous plant of the
Asteraceae family and contains a variety of chemical constituents,
including flavonoids, organic acids and polysaccharides (25). It has been reported that safflower
polysaccharide (SPS) has an immune regulatory function, antitumor
effect (25–27), may activate the
phosphatidylinositide 3-kinase/Akt signaling pathways and regulate
the cell cycle of cancer cells (28). However, the role of SPS on the
proliferation and metastasis of breast cancer remains to be
elucidated. The present study investigated the effects of SPS on
the proliferation and metastasis of breast cancer cells to clarify
the mechanisms underlying breast cancer and provide novel
strategies for breast cancer therapy.
Materials and methods
Cells and reagents
The MCF-7 human breast cancer cell line (HTB-22™;
American Type Culture Collection, Manassas, VA, USA) was cultured
in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal
calf serum (HyClone Laboratories, Inc., Logan, UT, USA). The MTT
reagent was purchased from Sigma-Aldrich (St. Louis, MO, USA).
Rabbit polyclonal Bcl-2 (cat. no. ab7973), rabbit polyclonal MMP9
(cat. no. ab38898) and mouse monoclonal TIMP1 (cat. no. ab1827)
antibodies were purchased from Abcam (Cambridge, UK) and rabbit
monoclonal Bax antibody (cat. no. AJ1079a) was obtained from Abgent
(Suzhou, China).
SPS preparation
The dried red flower and leaves of the safflower
(Tongrentang Co., Ltd., Beijing, China) were used to extract the
polysaccharide. The dried red flower was fully impregnated into
water, and was boiled four times for 1 h each time. The filtrate
was combined and concentrated to a volume of 1/4 prior to adding
95% ethanol (Jinhua Chemical Reagent Co., Ltd., Guangzhou, China)
to a volume of four-five times and incubated overnight at 4°C. The
SPS was precipitated by alcohol precipitation and collected by
centrifugation at 200 × g/min for 5 min. The SPS was dissolved in
deionized water and the SPS was precipitated twice using ethanol.
The SPS content was determined using a sulfuric acid-phenol method,
as described previously (29–32),
and the polysaccharide purity was up to 76.05%.
MTT assay
Cell proliferation analysis was performed using an
MTT assay. Briefly, the MCF-7 breast cancer cells were seeded into
96-well plates (5×104 cells/well), and following
adherence, the cells were treated with SPS at concentrations of
0.04, 0.08, 0.17, 0.34, 0.68 or 1.36 mg/ml for 24, 48 and 72 h at
37°C. A total of 20 μl MTT (5 mg/ml) was then added to the
medium in each well for 4 h and 150 μl dimethylsulfoxide was
added. The absorbance of the 96-well plates was read using a
microplate reader (Ultraviolet Spectrophotometer AquaMate-Plus;
Thermo Fisher Scientific, Waltham, MA, USA) at a test wavelength of
490 nm and a reference wavelength of 570 nm.
Western blot analysis
The breast cancer cells were seeded into 48-well
plates (5×105 cells/well) for 8 h, prior to treatment
with different concentrations of SPS (0.04, 0.20 and 0.60 mg/ml)
for 24, 48 and 72 h. The cell lysates were prepared using sodium
dodecyl sulfate (SDS) loading buffer. The lysates were separated
using 10% SDS-PAGE gels and the proteins were transferred from the
gel onto a nitrocellulose membrane (Biodee Corporation, Beijing,
China) at 35 V for 3 h (constant voltage). The membrane was then
blocked with TBST supplemented with 5% bovine serum albumin for 30
min prior to incubating the membrane with the specific antibodies
in Tris-buffered saline with Tween-20 (TBST) containing 5% bovine
serum albumin at 4°C overnight. The membrane was washed three times
with TBST and then incubated with the goat anti-mouse IgG (SN133)
and goat anti-rabbit (BA1054) secondary antibodies [Sunshine
Biotechnology (Nanjing) Co., Ltd., Nanjing, China] for 1 h at room
temperature. The bands were detected in a dark room using an
Immobilon Western Chemiluminescent kit (EMD Millipore, Billerica,
MA, USA).
Flow cytometric analysis
The apoptotic rates of the MCF-7 breast cancer cells
were determined using annexin V-propidium iodide (PI) staining,
according to the manufacturer’s instructions (Santa Cruz
Biotechnolgy, Inc., Dallas, TX, USA). Briefly, the cells
(5×105 cells/well) were seeded into 6-well plates for 6
h prior to treatment with SPS at the concentrations of 0.04 and
0.68 mg/ml for 48 h. The cells were then washed and resuspended in
phosphate-buffered saline (HyClone Laboratories, Inc.). Annexin V
(0.1 μg/μl) and PI (0.05 μg/μl) were added to
the cells and incubated in the dark for 30 min on ice. The
apoptotic rate was determined using fluorescent activated cell
sorting (FACS) analysis (FACSCalibur Cell Sorting system; BD
Biosciences, Franklin Lakes, NJ, USA).
Statistical analysis
The data were analyzed using SPSS 11.5 statistical
software (SPSS, Inc., Chicago, IL, USA) and the data are expressed
as the mean ± standard error of the mean. One way analysis of
variance and Student’s t-test were used to analyze the results.
P<0.01 was considered to indicate a statistically significant
difference.
Results
SPS has an increasing antitumor effect on
the MCF-7 breast cancer cell line in a time- and dose-dependent
manner
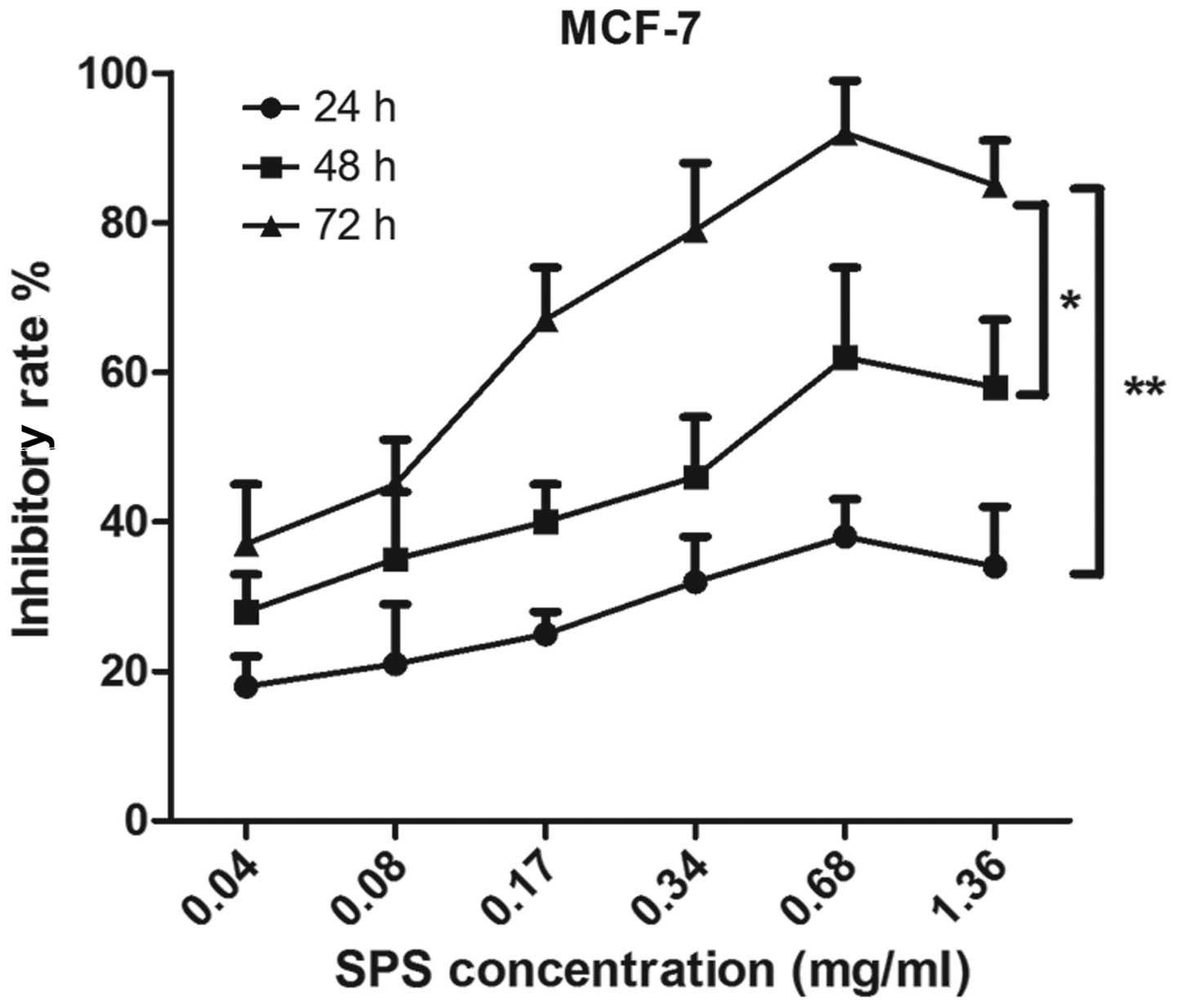
In order to investigate the antitumor activity of
SPS on breast cancer cells, the MCF-7 human breast cancer cell line
was used and the antitumor effects of SPS were detected using an
MTT assay. As shown in Fig. 1, the
MCF-7 cells were treated by SPS at concentrations of 0.04, 0.08,
0.17, 0.34, 0.68 or 1.36 mg/ml. The results demonstrated that the
inhibitory rates of SPS on the MCF-7 cells increased significantly
as the concentration of SPS increased.
The MCF-7 cells were also treated with various
concentrations of SPS for 24, 48 and 72 h, which revealed that the
antitumor effects of SPS occurred in a time-dependent manner. As
shown in Fig. 1, the inhibitory
rate on the breast cancer cells treated with SPS for 72 h was
significantly higher compared with those treated for 24 h
(P<0.01) and 48 h (P<0.05). SPS had an effective antitumor
effect at the concentration of 0.68 mg/ml at various time points.
The inhibitory rate at the concentration of 1.36 mg/ml was lower
compared with that at 0.68 mg/ml. These data revealed that SPS has
an antitumor effect and that these inhibitory effects increased in
a time- and dose-dependent manner.
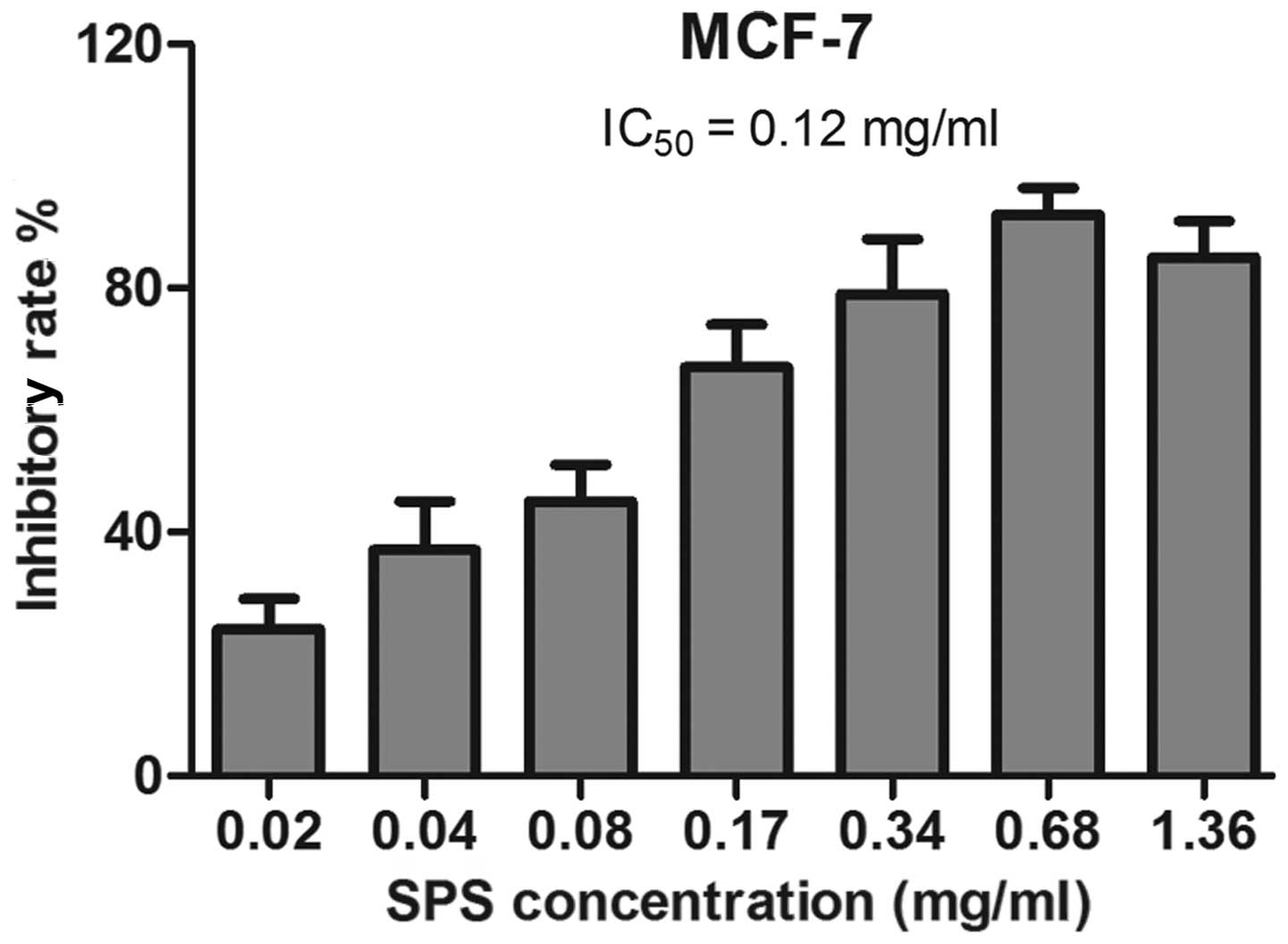
Detection of the IC50 of SPS
in breast cancer cells treated with SPS for 72 h
The duration of 72 h was selected as an appropriate
time-point to treat the MCF-7 cells with SPS. This was repeated
more than three times and all the samples were detected in
duplicates. Untreated breast cancer cells were used as a negative
control. As shown in Fig. 2, the
IC50 value for 72 h treatment was calculated as 0.12
mg/ml.
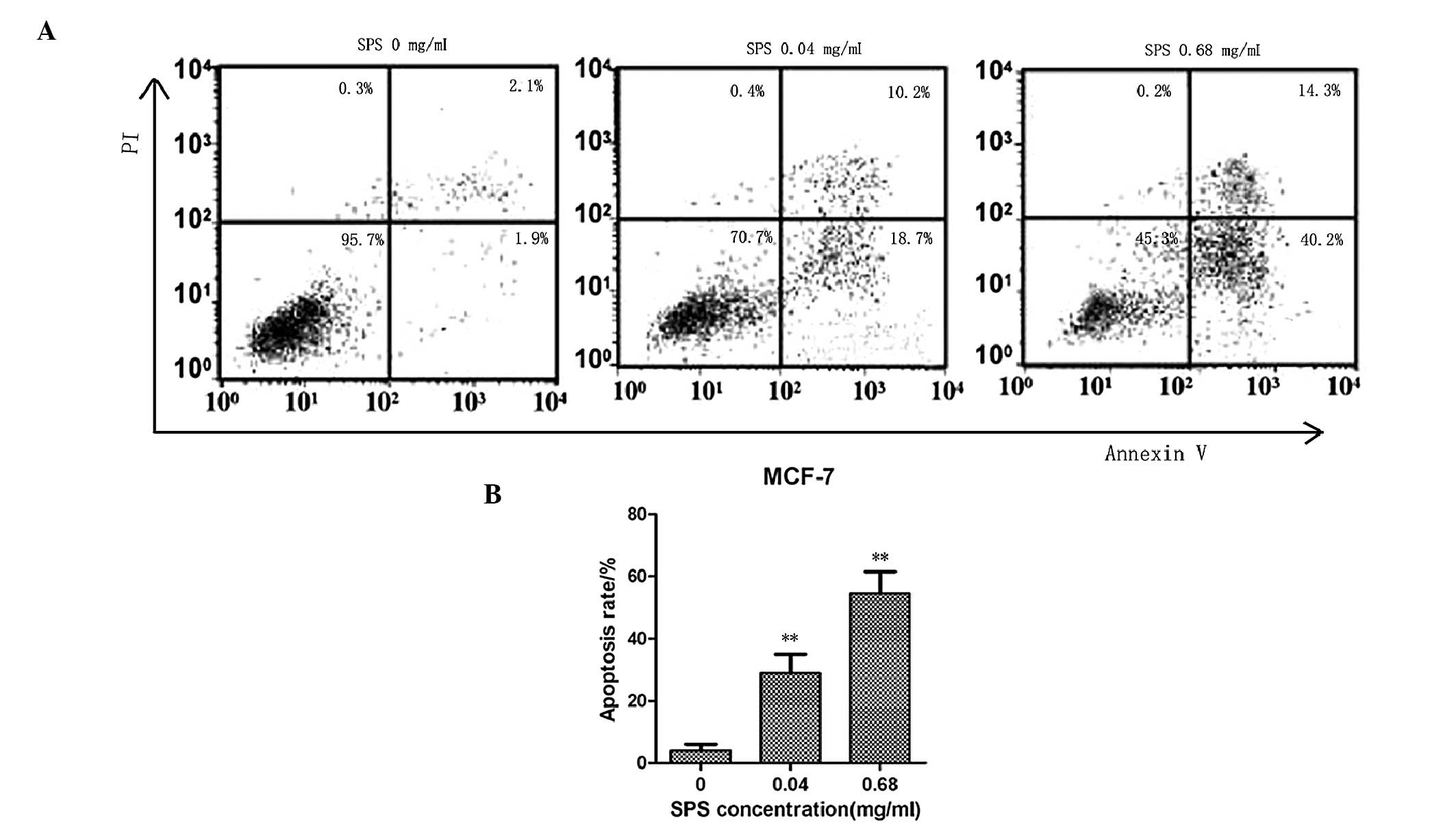
MCF-7 apoptotic rate increases with
increasing concentrations of SPS
The MCF-7 cell apoptotic rates were determined by
FACS analysis. The MCF-7 breast cancer cell line was treated with
various concentrations of SPS for 48 h. The lowest concentration of
SPS was 0.04 mg/ml and the highest concentration of SPS was 0.68
mg/ml. As shown in Fig. 3, the
apoptotic rates of the MCF-7 cells treated with SPS at the
concentrations of 0.68 and 0.04 mg/ml were 54.5 and 28.9%,
respectively. As expected, the apoptotic rates of the MCF-7 breast
cancer cells treated with SPS were significantly higher compared
with the untreated cells. The apoptotic rates also increased in a
dose-dependent manner.
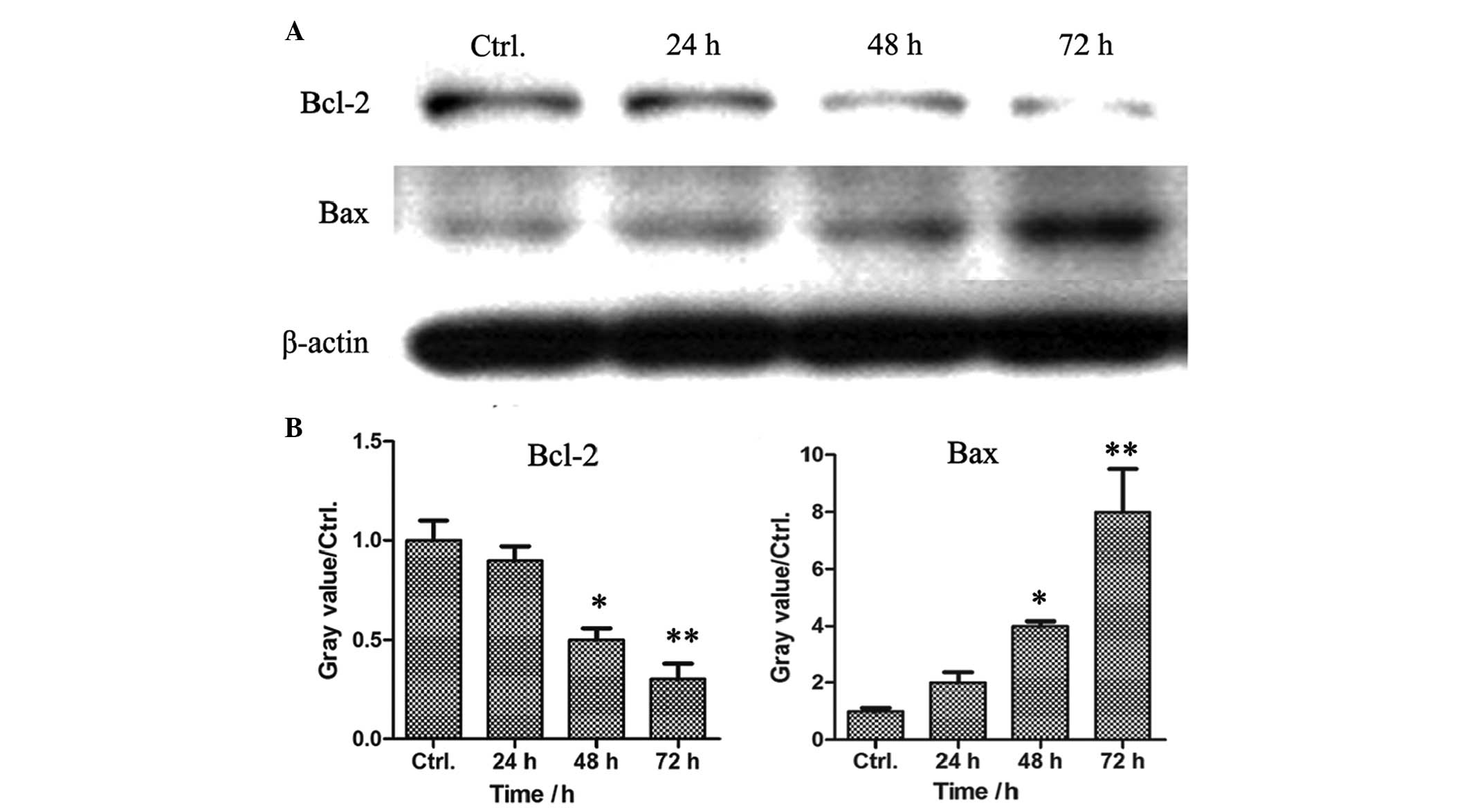
SPS downregulates the expression of Bcl-2
and upregulates the protein expression of Bax in MCF-7 breast
cancer cells
Cell apoptosis is induced via the extrinsic pathway
and the intrinsic pathway, which are mediated by death receptors
and mitochondria, respectively. In the present study, FACS analysis
demonstrated that SPS induced the apoptosis of the MCF-7 cells.
Subsequent investigation aimed to determine whether the SPS-induced
cell apoptosis was induced by the death receptor pathway or by the
mitochondria-mediated signaling pathway. The MCF-7 breast cancer
cells were treated with SPS at a concentration of 0.68 mg/ml for
various time-periods. The cell lysates were prepared and the
protein expression levels of Bcl-2 and Bax were detected by western
blot analysis. As shown in Fig. 4,
the expression of the apoptosis inhibitor, Bcl-2, reduced in a
time-dependent manner, however, the expression of the apoptosis
promoter, Bax, increased in the SPS-treated MCF-7 cells, suggesting
that the SPS-induced cell apoptosis was dependent on the
mitochondria-mediated signaling pathway.
SPS inhibits the expression of MMP-9 and
increases the expression of TIMP-1
In order to investigate the role of SPS on tumor
metastasis in breast cancer, the expression levels of MMP-9 and
TIMP-1 were detected by western blot analysis. The MCF-7 breast
cancer cells were treated with various concentrations of SPS for 72
h and the results demonstrated that the expression of MMP-9 was
downregulated and the expression of TIMP-1 was upregulated in the
SPS-treated cells at the concentration of 0.6 mg/ml (Fig. 5). All these results suggested that
SPS inhibited breast cancer cell metastases.
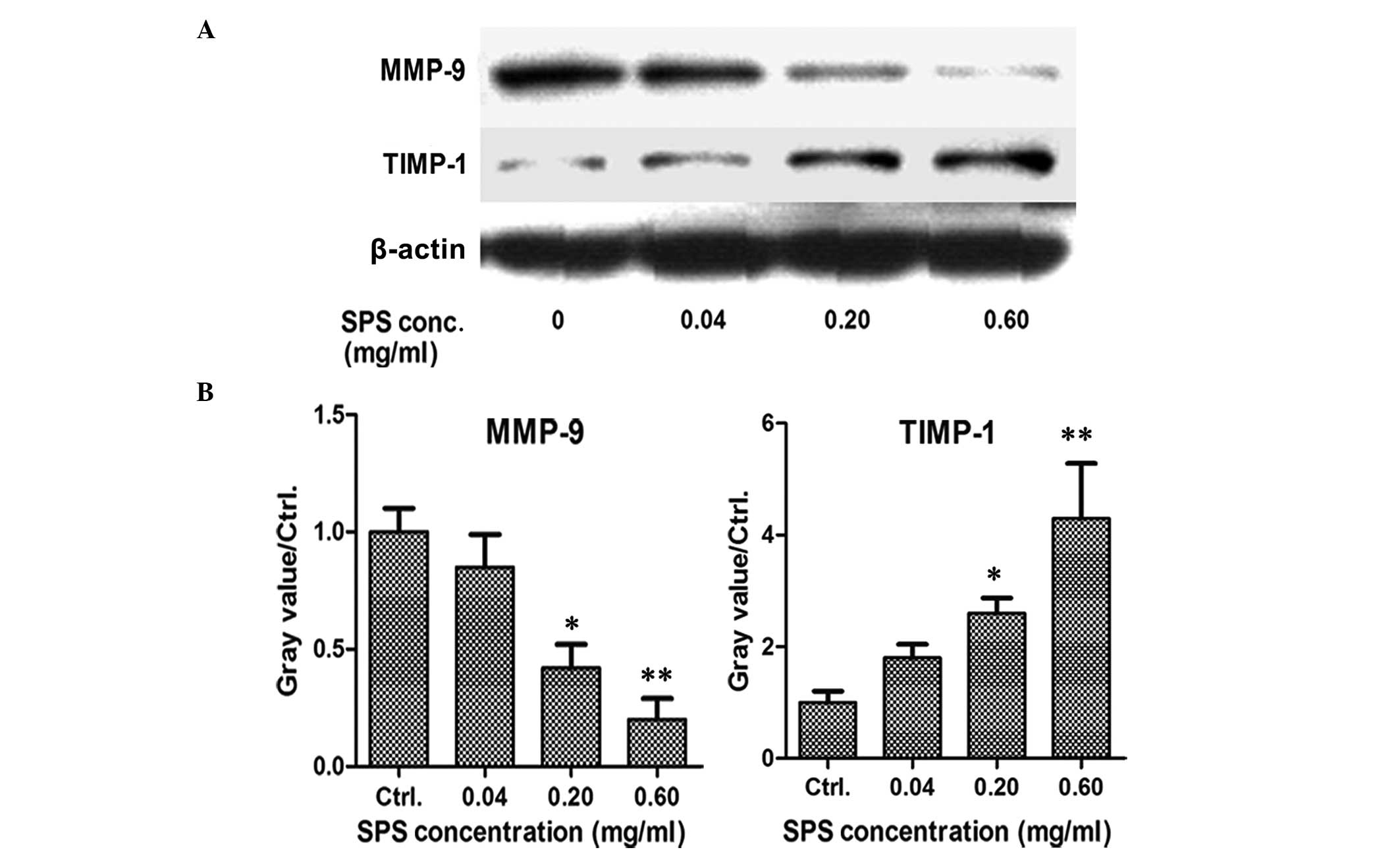 | Figure 5SPS inhibits the expression of MMP-9
and increases the expression of TIMP-1. (A) MCF-7 breast cancer
cells were seeded into 48-well plates. Following adherence, the
cells were treated with SPS for 48 h at concentrations of 0.04,
0.20 or 0.60 mg/ml. The expression levels of MMP-9 and its
inhibitor, TIMP-1, were determined by western blot analysis.
β-actin was used as an internal reference. (B) Data represent the
expression levels of MMP-9 and TIMP-1 following treatment with
various concentrations of SPS for at least two independent
experiments, performed in duplicate (*P<0.05 and
**P<0.01, as compared with the control group). MMP,
matrix metalloproteinase; TIMP, tissue inhibitor of
metalloproteinases; SPS, safflower polysaccharide; Ctrl,
control. |
Discussion
Breast cancer is the most common type of malignancy
affecting females worldwide, with high incidence and mortality
rates. Although the comprehensive treatment approach, combining
surgery, radiotherapy and chemotherapy, can significantly prolong
the survival rate of patients with breast cancer, it remains the
most common cause of cancer-associated mortality in females
(33). The recurrence and
metastasis of breast cancer is the predominant cause of mortality
(34,35), therefore, it is important to
identify an effective therapeutic approach for the treatment of
breast cancer. SPS is an active ingredient extracted from
safflower, which is a traditional Chinese medicine that is commonly
used clinically (25). SPS has
been reported to exert a variety of pharmacological effects
(36) and previous studies have
demonstrated that SPS exhibits antitumor activity (27), although the underlying mechanism
remained to be elucidated. The present study used the MCF-7 breast
cancer cell line as a breast cancer model and investigated the
effects of SPS on the proliferation and metastasis of the MCF-7
cells.
An MTT assay was used to detect whether SPS
inhibited the proliferation of breast cancer cells. The results
demonstrated that SPS suppressed the growth and proliferation rate
of the MCF-7 cells in a dose- and time-dependent manner. SPS
exhibited a potent antitumor effect at a concentration of 0.68
mg/ml for 24, 48 and 72 h. In addition, the rate of apoptosis was
induced in the SPS-treated cells at a concentration of 0.68 mg/ml
for 48 h and the expression levels of Bax and Bcl-2 were
significantly increased and decreased, respectively, in the SPS
treated MCF-7 cells. This suggested that SPS-induced cell apoptosis
was dependent on the mitochondria-mediated signaling pathway. Tumor
metastasis in breast cancer is the major cause of morality in
patients with malignant tumors. The expression levels of MMP-9 and
its inhibitor, TIMP-1, were also detected in the SPS-treated MCF-7
cells compared with the untreated cells. SPS significantly
inhibited the expression of MMP-9 and increased the expression of
TIMP-1, suggesting that SPS may suppress the invasion and
metastasis of breast cancer cells.
The results of the present study may provide novel
strategies for developing SPS-based therapies to treat breast
cancer, which can be assisted by elucidating the mechanism
underlying breast cancer.
References
|
1
|
Willis K, Lewis S, Ng F and Wilson L: The
experience of living with metastatic breast cancer-A review of the
literature. Health Care Women Int. 29:1–29. 2014. View Article : Google Scholar
|
|
2
|
Libson S and Lippman M: A review of
clinical aspects of breast cancer. Int Rev Psychiatry. 26:4–15.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Eden JA: Human breast cancer stem cells
and sex hormones - a narrative review. Menopause. 17:801–810.
2010.PubMed/NCBI
|
|
4
|
Ginossar T, De Vargas F, Sanchez C and
Oetzel J: “That word, cancer”: breast care behavior of Hispanic
women in new Mexico background and literature review”. Health Care
Women Int. 31:68–87. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Verkooijen HM, Bouchardy C, Vinh-Hung V,
Rapiti E and Hartman M: The incidence of breast cancer and changes
in the use of hormone replacement therapy: a review of the
evidence. Maturitas. 64:80–85. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Deapen D: Breast implants and breast
cancer: a review of incidence, detection, mortality, and survival.
Plast Reconstr Surg. 120:70S–80S. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wu J: Apoptosis and angiogenesis: two
promising tumor markers in breast cancer (review). Anticancer Res.
16:2233–2239. 1996.PubMed/NCBI
|
|
8
|
Amaral C, Borges M, Melo S, da Silva ET,
Correia-da-Silva G and Teixeira N: Apoptosis and autophagy in
breast cancer cells following exemestane treatment. PLoS One.
7:e423982012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yang H and Dou QP: Targeting apoptosis
pathway with natural terpenoids: Implications for treatment of
breast and prostate cancer. Curr Drug Targets. 11:733–744. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Grimm D, Wehland M, Pietsch J, Infanger M
and Bauer J: Drugs interfering with apoptosis in breast cancer.
Curr Pharm Des. 17:272–283. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Elumalai P, Gunadharini DN, Senthilkumar
K, et al: Induction of apoptosis in human breast cancer cells by
nimbolide through extrinsic and intrinsic pathway. Toxicol Lett.
215:131–142. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Park H, Bergeron E, Senta H, et al:
Sanguinarine induces apoptosis of human osteosarcoma cells through
the extrinsic and intrinsic pathways. Biochem Biophys Res Commun.
399:446–451. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li Y, He K, Huang Y, et al: Betulin
induces mitochondrial cytochrome c release associated apoptosis in
human cancer cells. Mol Carcinog. 49:630–640. 2010.PubMed/NCBI
|
|
14
|
Chen FP and Chien MH: Phytoestrogens
induce apoptosis through a mitochondria/caspase pathway in human
breast cancer cells. Climacteric. 17:385–389. 2014. View Article : Google Scholar
|
|
15
|
Aiyar SE, Park H, Aldo PB, et al: TMS, a
chemically modified herbal derivative of resveratrol, induces cell
death by targeting Bax. Breast Cancer Res Treat. 124:265–277. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Boohaker RJ, Zhang G, Lee MW, et al:
Rational development of a cytotoxic peptide to trigger cell death.
Mol Pharm. 9:2080–2093. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang XX, Cheng Q, Zhang SN, et al:
PAK5-Egr1-MMP2 signaling controls the migration and invasion in
breast cancer cell. Tumour Biol. 34:2721–2729. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zu X, Zhang Q, Cao R, et al: Transforming
growth factor-beta signaling in tumor initiation, progression and
therapy in breast cancer: an update. Cell Tissue Res. 347:73–84.
2012. View Article : Google Scholar
|
|
19
|
Folgueira MA, Maistro S, Katayama ML, et
al: Markers of breast cancer stromal fibroblasts in the primary
tumour site associated with lymph node metastasis: a systematic
review including our case series. Biosci Rep. 33:e000852013.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jezierska A and Motyl T: Matrix
metalloproteinase-2 involvement in breast cancer progression: a
mini-review. Med Sci Monit. 15:RA32–RA40. 2009.PubMed/NCBI
|
|
21
|
Nyormoi O, Mills L and Bar-Eli M: An
MMP-2/MMP-9 inhibitor, 5a, enhances apoptosis induced by ligands of
the TNF receptor superfamily in cancer cells. Cell Death Differ.
10:558–569. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sun Y, Lu N, Ling Y, et al: Oroxylin A
suppresses invasion through down-regulating the expression of
matrix metallopro-teinase-2/9 in MDA-MB-435 human breast cancer
cells. Eur J Pharmacol. 603:22–28. 2009. View Article : Google Scholar
|
|
23
|
Lewandowska U, Szewczyk K, Owczarek K, et
al: Flavanols from Japanese quince (Chaenomeles japonica) fruit
inhibit human prostate and breast cancer cell line invasiveness and
cause favorable changes in Bax/Bcl-2 mRNA ratio. Nutr Cancer.
65:273–285. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li F, Li C, Zhang H, et al: VI-14, a novel
flavonoid derivative, inhibits migration and invasion of human
breast cancer cells. Toxicol Appl Pharmacol. 261:217–226. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wakabayashi T, Hirokawa S, Yamauchi N,
Kataoka T, Woo JT and Nagai K: Immunomodulating activities of
polysaccharide fractions from dried safflower petals.
Cytotechnology. 25:205–211. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ando I, Tsukumo Y, Wakabayashi T, et al:
Safflower polysaccharides activate the transcription factor
NF-kappa B via Toll-like receptor 4 and induce cytokine production
by macrophages. Int Immunopharmacol. 2:1155–1162. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shi X, Ruan D, Wang Y, Ma L and Li M:
Anti-tumor activity of safflower polysaccharide (SPS) and effect on
cytotoxicity of CTL cells, NK cells of T739 lung cancer in mice.
Zhongguo Zhong Yao Za Zhi. 35:215–218. 2010.In Chinese. PubMed/NCBI
|
|
28
|
Chen L, Xiang Y, Kong L, et al:
Hydroxysafflor yellow A protects against cerebral
ischemia-reperfusion injury by anti-apoptotic effect through
PI3K/Akt/GSK3β pathway in rat. Neurochem Res. 38:2268–2275. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lin FM and Pomeranz Y: Effect of borate on
colorimetric determinations of carbohydrates by the phenol-sulfuric
acid method. Anal Biochem. 24:128–131. 1968. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Masuko T, Minami A, Iwasaki N, Majima T,
Nishimura S and Lee YC: Carbohydrate analysis by a phenol-sulfuric
acid method in microplate format. Anal Biochem. 339:69–72. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Saha SK and Brewer CF: Determination of
the concentrations of oligosaccharides, complex type carbohydrates,
and glycoproteins using the phenol-sulfuric acid method. Carbohydr
Res. 254:157–167. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Cuesta G, Suarez N, Bessio MI, Ferreira F
and Massaldi H: Quantitative determination of pneumococcal capsular
polysaccharide serotype 14 using a modification of phenol-sulfuric
acid method. J Microbiol Methods. 52:69–73. 2003. View Article : Google Scholar
|
|
33
|
Siponen ET, Joensuu H and Leidenius MH:
Local recurrence of breast cancer after mastectomy and modern
multidisciplinary treatment. Acta Oncol. 52:66–72. 2013. View Article : Google Scholar
|
|
34
|
Koscielny S and Tubiana M: The link
between local recurrence and distant metastases in human breast
cancer. Int J Radiat Oncol Biol Phys. 43:11–24. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Dian D, Straub J, Scholz C, et al:
Influencing factors for regional lymph node recurrence of breast
cancer. Arch Gynecol Obstet. 277:127–134. 2008. View Article : Google Scholar
|
|
36
|
Hristov AN, Kennington LR, McGuire MA and
Hunt CW: Effect of diets containing linoleic acid- or oleic
acid-rich oils on ruminal fermentation and nutrient digestibility,
and performance and fatty acid composition of adipose and muscle
tissues of finishing cattle. J Anim Sci. 83:1312–1321.
2005.PubMed/NCBI
|