Introduction
Prostate cancer (PCa) is the second most common
cause of cancer-associated mortality, and the most frequently
diagnosed malignancy in males in the USA (1,2).
Androgen receptors (AR) have the most significant functions in PCa,
in disease initiation and progression (3,4).
Targeting the expression of AR and inhibiting AR activity are
important in androgen-dependent and androgen-independent diseases.
Initially, AR is expressed by the PCa cells, which are dependent on
androgens to facilitate growth (5). Common treatments involving androgen
ablation have been demonstrated to temporarily alleviate the
disease, however, they lead to the recurrence of highly aggressive
and androgen-independent types of metastatic cancer (6,7).
Previous evidence has suggested that the progression of PCa to the
androgen-independent stage does not involve the loss of AR, but is
induced by the restoration of AR signaling in the PCa cells
(6). During the transformation
from the androgen-dependent stage to the androgen-independent
stage, PCa cells develop several mechanisms to facilitate the
activation of AR in the androgen-depleted environment, including
amplification or mutation of the AR gene, overexpression of AR
co-activators and the ligand-independent activation of AR (7–9). As
the development of PCa progresses, AR activity may evade regulation
by ligand binding, resulting in tumor overgrowth (7). Following the activation of molecular
pathways to restore AR signaling by PCa cells during the
androgen-independent phase, no single therapeutic agent is able to
eliminate PCa, resulting in poor patient prognosis (10). As AR is important in the
development and progression of PCa, a strategy to downregulate the
expression of AR and the inhibition of AR activity, in combination
with anti-androgen therapy may prevent or delay the development of
androgen-independent PCa (11–16).
Micro (mi)RNAs are short, endogenous, non-coding RNA
molecules, which bind to the 3′-untranslated regions (UTRs) of
target mRNAs, resulting in translational repression or message
degradation (17–18). miRNAs are important in
physiological cellular processes, including differentiation,
proliferation and apoptosis (19–22),
and have also been implicated in types of cancer (23,24).
Several studies have demonstrated that miRNA (miR)-185 is able to
suppress tumor growth and progression in non-small cell lung cancer
(25), ovarian, pediatric renal
and breast cancer cell lines (26,28),
and it has been suggested that miR-185 may be important in cell
proliferation.
The present study aimed to investigate whether
miR-185 affected the expression of AR, cell proliferation or
apoptosis of PCa LNCaP cells. The results may provide evidence for
the utilization of miRNAs as novel therapeutics to target AR in
PCa.
Materials and methods
Cell culture
The human LNCaP-AD (androgen-dependent LNCaP) PCa
cell line was obtained from American Type Culture Collection (ATCC;
Manassas, VA, USA) and cultured in RPMI-1640 medium (HyClone, GE
Healthcare Life Sciences, Little Chalfont, UK) supplemented with
10% charcoal-treated fetal bovine serum (FBS; Sigma-Aldrich, St.
Louis, MO, USA), 2.05 mM L-glutamine, 100 U/ml penicillin, 100
μg/ml streptomycin and 10−8 mol/l R1881
(Sigma-Aldrich).
Androgen-independent LNCaP cells (LNCaP-AI) were
generated from LNCaP-AD cells under androgen-depleted conditions.
LNCAP-AI cells were produced from LNCaP-AD cells following
continuous culture with phenol red-free RPMI-1640 medium (HyClone,
GE Healthcare Life Sciences, Logan, UT, USA) supplemented with 10%
charcoal-stripped fetal bovine serum (Serana, Bunbury, Australia)
for 50 passages. The human PC-3 PCa cell line was obtained from
ATCC and cultured in RPMI-1640 medium (HyClone) supplemented with
10% FBS (TBD Science, Tianjin, China), 2.05 mM L-glutamine, 100
U/ml penicillin and 100 μg/ml streptomycin. All the cell
lines were maintained at 37°C in a humidified atmosphere containing
5% CO2.
Plasmid construction
The wild-type (WT) 3′UTR of the human AR gene was
amplified from human AR complementary (c)DNA by polymerase chain
reaction (PCR) using a PCR kit purchased from Takara (Dailan,
China), and the fragments produced by PCR were cut using
MluI and HindIII (Thermo Fisher Scientific, Waltham,
MA, USA) before being cloned into the 3′ end of the luciferase
(LUC) reporter gene in a pMIR-REPORT Luciferase vector (Ambion Life
Technologies, Carlsbad, CA, USA), and named pMIR-AR-3′UTRw. The PCR
primers used to amplify the AR-3′UTR were as follows: AR forward
(ARF), 5′-CGACGCGTAGTCAAGCCCATCTAT-3′ and reverse (ARR),
5′-CCAAGCTTGTTTGCTTGTTTTTGTT-3′ (BGI, Shenzhen, China). The PCR
cycling conditions were as follows: Initial denaturation at 94°C
for 45 sec; 30 cycles of 60°C for 30 sec and 72°C for 1 min and
final elongation at 72°C for 5 min.
Deletion mutagenesis of the putative target site for
miR-185 in the WT-3′UTR of AR was performed using a two-step PCR
method. Using pMIR-AR-3′UTRw as the template, a 5′ flanking
fragment of the deletion region was PCR amplified with the primers,
ARF and deletion reverse (5′-GAAAAAGAAAAAAAGCCCAGCAAAT-3′), and the
3′ lanking fragment was PCR amplified with the primers, ARR and
deletion forward (5′-GCTTTTTTTCTTTTTCTTCTTCCCTC-3′) (BGI). The two
fragments were linked to the primers by ligation with PCR. The
ligated fragments were cloned into the MluI and
HindIII sites of pMIR-REPORT Luciferase, and the plasmid was
named pMIR-AR-3′UTRm.
For construction of the pGL4-androgen response
element (ARE) reporter plasmid, oligonucleotides of the putative
ARE were synthesized. The double-stranded ARE was generated by
annealing equal quantities of forward 5′-TCGAGTGGAGGAACATATTGT
ATTTATTTGGAGGAACATATTGTATTTATTA-3′ and reverse
5′-AGCTTAATAAATACAATATGTTCCTCCA AATAAATACAATATGTTCCTCCAC-3′
oligonucleotides at 95°C for 10 min, followed by cooling to room
temperature. Subsequently, the double-stranded ARE was inserted
upstream of the TATA box in the pGL4.23 [luc2/minp] vector (Promega
Corporation, Madison, WI, USA) to generate the recombinant plasmid,
ARE-TATA box-luciferase reporter, which was named pGL4-ARE.
Nucleotide sequences of all the constructs were confirmed by DNA
sequencing.
Cell transfection
The LNCaP cells were transfected with the miR-185
mimic (160 nM), normal control (NC) mimic (160 nM), miR-185
inhibitor (160 nM) or NC inhibitor (160 nM) at 40% confluency using
siPORT™ NeoFX™ transfection agent (Ambion Life Technologies)
at room temperature, according to the manufacturer’s instructions.
The pMIR-AR-3′UTRw, pMIR-AR-3′UTRm and pGL4-ARE plasmids were
transfected into the cells at 90% confluency using FuGENE HD (Roche
Diagnostics, Basel, Switzerland) at room temperature, according to
the manufacturer’s instructions.
Cell viability assay
The LNCaP-AD, LNCaP-AI and PC-3 cells were seeded
into 96-well plates at 40% confluency and transfected with either
the miR-185 mimic, miR-185 inhibitor, NC mimic or the NC inhibitor
using the siPORT™ NeoFX™ transfection agent at room
temperature. The cell viabilities were then determined 24, 48, 72
and 96 h after transfection using an MTT assay (Sangon Biotech Co.,
Ltd, Shanghai, China), according to the manufacturer’s
instructions. Three independent experiments were performed.
Nuclear staining with Hoechst 33342
The LNCaP-AD or LNCaP-AI cells were seeded into
24-well plates at 40% confluency and transfected with either
miR-185 mimic, miR-185 inhibitor, NC mimic or NC inhibitor using
siPORT™ NeoFX™ transfection agent at room temperature.
Apoptotic cells were detected 48, 72 and 96 h after transfection
using Hoechest 33258 (Beyotime Institute of Biotechnology,
Shanghai, China), according to the manufacturer’s instructions.
Images of the stained cells were captured under a fluorescent
microscope (Eclipse TE2000-U; Nikon Corp., Tokyo, Japan) at 350 nm
excitation and 460 nm emission.
Reverse transcription quantitative
polymerase chain reaction (RT-qPCR)
The LNCaP-AD or LNCaP-AI cells were seeded into
6-well plates and transfected with either the miR-185 mimic,
miR-185 inhibitor, NC mimic or NC inhibitor using siPORT™
NeoFX™ transfection agent. The total RNA was extracted 72 h
after transfection using TRIzol reagent (Invitrogen Life
Technologies, Carlsbad, CA, USA). First strand cDNA was synthesized
from 10 ng total RNA using gene specific primers. RT-qPCR was
performed using MonsterScript™ Reverse Transcriptase (Epicentre,
Illumina, Inc., Madison, WI, USA) and SYBR® Green I
nucleic acid gel stain (Molecular Probes Life Technologies,
Carlsbad, CA, USA).
The primers used were as follows: miR-185, forward
5′- GGTGGAGAGAAAGGCAGT-3′ and reverse 5′-CAGTGCGTGTCGTGGAG-3′; U6
snRNA, forward 5′-GCTTCGGCAGCACATATACTAAAAT-3′ and reverse
5′-CGCTTCACGAATTTGCGTGTCAT-3′. U6 snRNA was used as a control gene
for normalization. The relative quantity of miR-185 was determined
using the 2−ΔΔCt method (29).
The primers used for AR were: For ward
5′-CTTCCCTCCCT ATCTAACCCTC-3′ and reverse 5′-TCTAAAC
TTCCCGTGGCATAA-3′; for PSA were: Forward
5′-AGGTGTGCTGACTATGTGGTGAC-3′ and reverse
5′-GGTTGAGGTTCCAGGTGCTT-3′; and for GAPDH were: Forward
5′-GGGAAACTGTGGCGTGAT-3′ and reverse 5′-GAGTGGGTGTCGCTGTTGA-3′. PCR
conditions for AR were: initial denaturation at 95°C for 5 min; 35
cycles of 95°C for 10 sec, 59°C for 15 sec, 72°C for 20 sec and
82°C (fluorescence collection) for 5 sec. PCR conditions for PSA
and GAPDH were: initial denaturation at 95°C for 5 min; 35 cycles
of 95°C for 10 sec, 59°C for 15 sec, 72°C for 20 sec and 80°C
(fluorescence collection) for 15 sec. GAPDH was used as a control
for normalization, and the data were analyzed with Rotor-Gene
Real-Time Analysis software 6.0 (Corbett Research, Mortlake,
Australia) according to the standard curve.
Dual-luciferase assay
The LNCaP-AD cells were seeded in 24-well plates and
transfected for 24 h with either the miR-185 mimic, miR-185
inhibitor, NC mimic or NC inhibitor using siPORT™ NeoFX™
transfection agent. Subsequently, the LNCaP-AD cells were
co-transfected with either pMIR-AR-3′UTRw (0.3 μg),
pMIR-AR-3′UTRm (0.3 μg) or pMIR-REPORT (0.3 μg), or
co-transfected with pGL4-ARE (0.3 μg) or pGL4.23[luc2/minp]
using FuGENE HD transfection reagent (Roche Diagnostics, Mannheim,
Germany) for 48 h. The activities of firefly (M1) and Renilla (M2)
luciferase were measured using a Dual Luciferase Assay system
(Promega Corporation), according to the manufacturer’s
instructions. The transfection efficiency was normalized to the
pRL-TK control vector (0.04 μg; Promega Corporation). Three
independent experiments were performed in duplicates.
Western blot analysis
The LNCaP-AD or LNCaP-AI cells were seeded into
6-well plates and transfected with either the miR-185 mimic,
miR-185 inhibitor, NC mimic or NC inhibitor using siPORT™
NeoFX™ transfection agent. The cells were harvested 48 and
72 h after transfection for protein extraction. The protein (20
μg) was resolved on an 10% SDS-PAGE gel and
electrotransferred onto nitrocellulose membranes (Bio-Rad
Laboratories, Inc., Hercules, CA, USA) using a DYCZ-40D (Beijing
Liuyi Instrument Factory, Beijing, China). Following blocking with
5% milk in Tris-buffered saline with 0.05% Tween-20 (TBST) buffer
(Sangon Biotech Co., Ltd) and washing with TBST buffer, the
membrane was incubated with mouse monoclonal anti-human AR
(1:1,000; BD Biosciences, Franklin Lakes, NJ, USA) or mouse
anti-β-actin (1:1,000; Santa Cruz Biotechnology, Inc., Dallas, TX,
USA) at 4°C for 12 h, followed by incubation with horseradish
peroxidase-labeled goat anti-mouse polyclonal secondary antibody
(1:2,000; Zhongshan Golden Bridge Biological Technology, Beijing,
China) for 1 h at room temperature. The immunoreactive bands were
visualized by enhanced chemiluminescence (Santa Cruz Biotechnology,
Inc.). The protein expression levels of AR in each sample were
determined by normalizing AR band intensity to that of β-actin.
Sequence Analysis
TargetScan predicts the biological targets of
miRNAs. This is via searching a database for conserved 8mer and
7mer sites, which match seed regions of the miRNA being screened,
nonconserved sites are also predicted. In addition sites with
mismatches in the seed region that are compensated by conserved 3′
pairing are also identified. In mammals, the predictions are ranked
based on the predicted targeting efficacy as calculated using
context+ scores of the sites. The predictions are also ranked by
their probability of conserved targeting. Conserved targeting has
also been detected within open reading frames.
Results
miR-185 downregulates protein expression
levels of AR in LNCaP cells
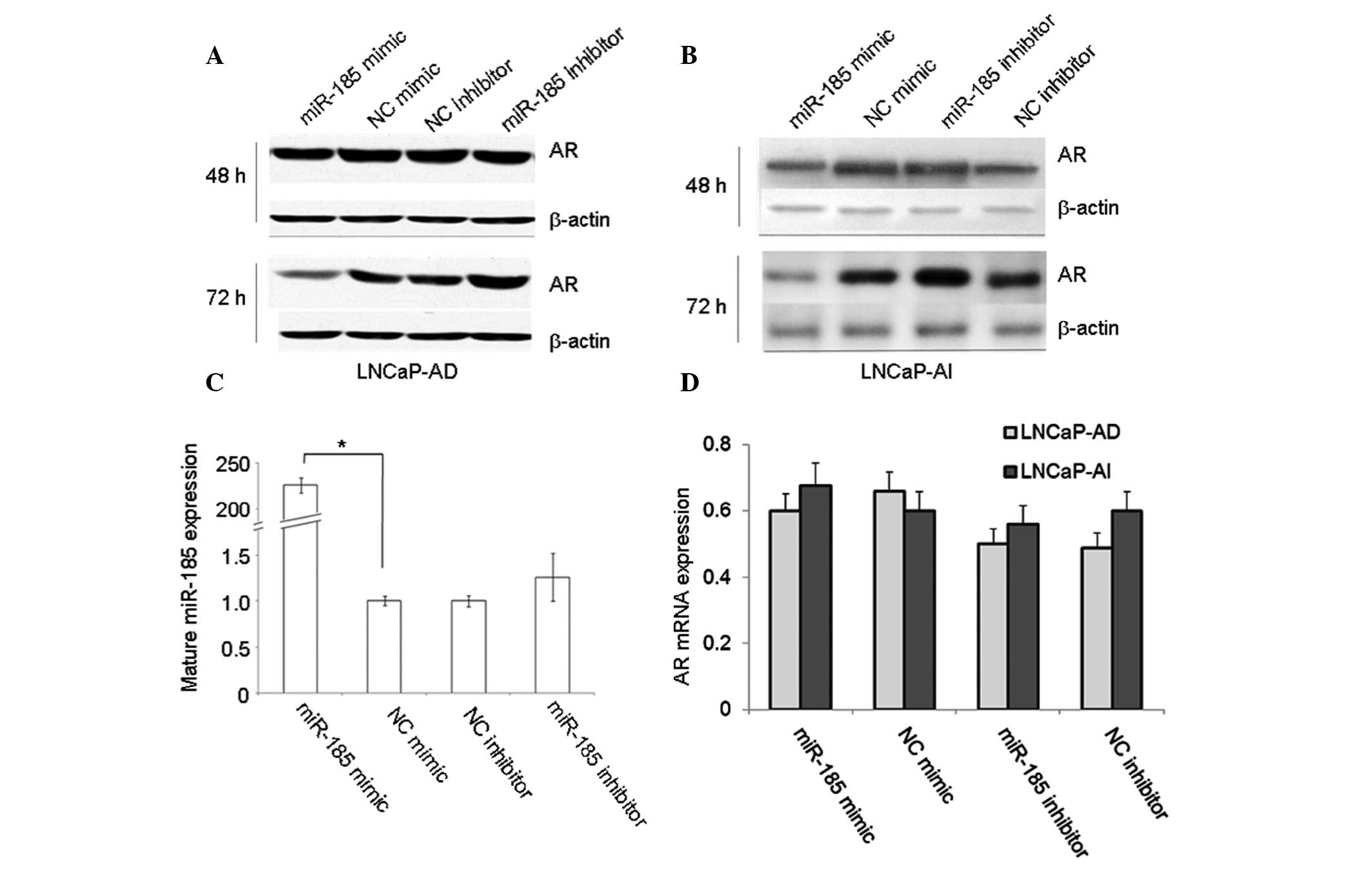
To investigate the potential effects of miR-185 on
the regulation of AR expression, LNCaP-AD and LNCaP-AI cells were
transfected with an miR-185 mimic or inhibitor for 48 and 72 h, and
the expression levels of AR were detected using western blot and
qPCR analyses. The results indicated that transfection with the
miR-185 mimic markedly reduced the protein levels of AR, while
transfection with the miR-185 inhibitor increased the protein
levels of AR in the LNCaP-AD (Fig.
1A) and LNCaP-AI (Fig. 1B)
cells.
The mature miR-185 levels in the miR-185-transfected
LNCaP cells were measured by qPCR. The mature levels of miR-185 in
the LNCaP cells transfected with the miR-185 mimic were
significantly higher compared with those in the NC mimic-treated
cells (Fig. 1C), which indicated
that the synthetic miR-185 mimic was effectively transfected and
matured in the LNCaP cells. However, differences in the mRNA
expression levels of AR were not detected in all types of
transfected LNCaP cells (Fig. 1D).
The results demonstrated that miR-185 reduced the protein levels of
AR by inducing translational repression, but not AR mRNA
degradation.
miR-185 interacts with the putative
target site in the AR-3′UTR
Sequence analysis, using the TargetScan tool
(version 5.2; http://www.targetscan.org/vert_50/), indicated that a
putative binding site for miR-185 was located at 173–179 bp of the
AR-3′ UTR. To assess whether miR-185 interacted with this
predicted binding site in the AR-3′ UTR to reduce the expression of
AR, wild-type 3′UTR (3′UTRw) and the 3′UTR of the AR gene,
containing deletion mutations (3′UTRm), were cloned and inserted
downstream of the luciferase gene in a reporter plasmid
(pMIR-REPORT Luciferase), and named pMIR-AR-3′UTRw and
pMIR-AR-3′UTRm. Each of these constructs were cotransfected into
the LNCaP-AD cells with either the miR-185 mimic, miR-185
inhibitor, NC mimic or NC inhibitor. Luciferase activity was
measured 48 h after transfection. The results (Fig. 2) indicated that transfection with
the miR-185 mimic markedly reduced luciferase activity, while the
miR-185 inhibitor enhanced luciferase activity of the
pMIR-AR-3′UTRw. However, neither the miR-185 mimic nor miR-185
inhibitor altered the luciferase activities of the pMIR-AR-3′UTRm
or pMIR-Report plasmids. Therefore, these data indicated that the
predicted target site in the AR 3′UTR was a specific functional
binding site for miR-185, and that AR was a direct target of
miR-185.
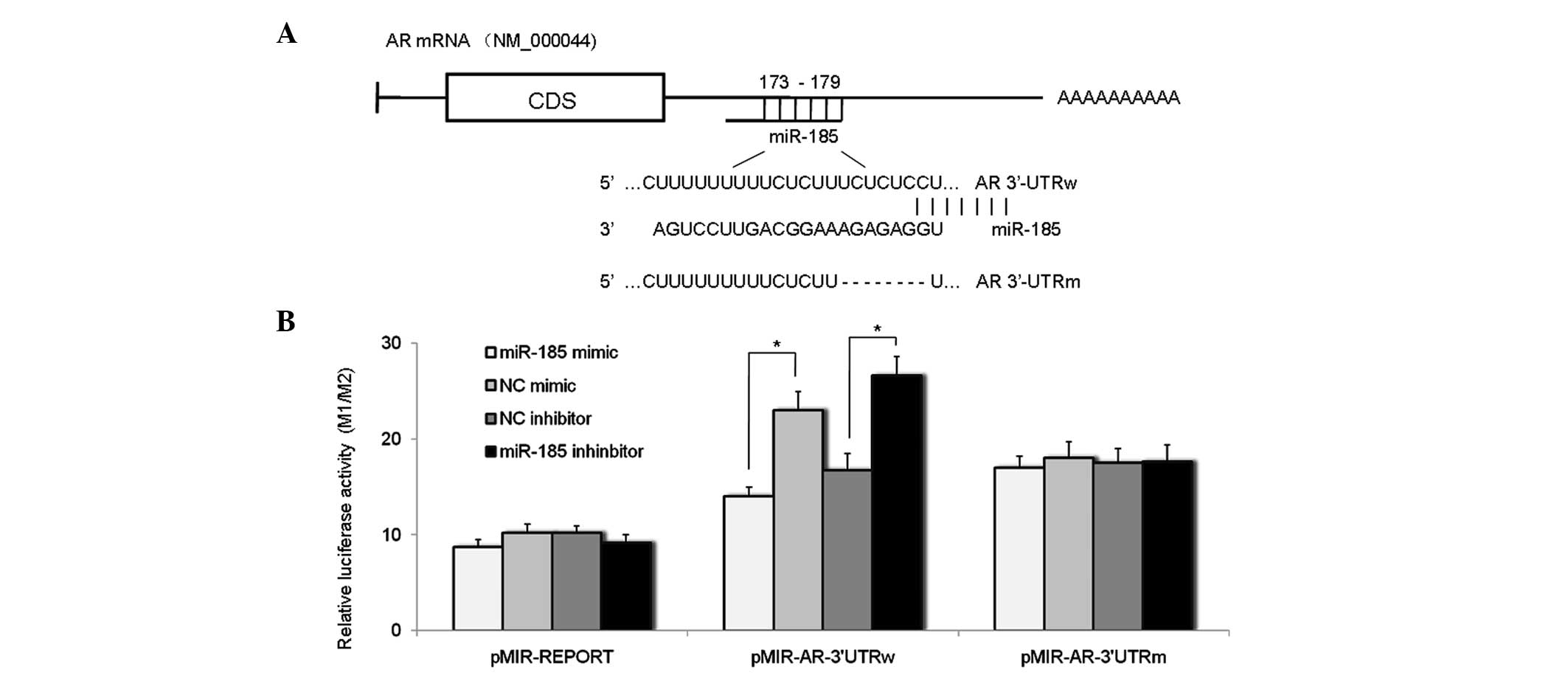 | Figure 2miR-185 directly targets the
AR-3′UTR. (A) Schematic representation of AR mRNA, indicating the
positions and sequences of the predicted miR-185 binding sites
located in the AR-3′UTR. (B) LNCaP cells were transfected with 160
nM miR-185 mimic, miR-185 inhibitor, NC mimic or NC inhibitor for
48 h, and subsequently cotransfected with pMIR-AR-3′UTRw or
pMIR-AR-3′UTRm for another 48 h. Luciferase activity was detected
using a Dual Luciferase Assay system and was plotted as the ratio
of firefly (M1) to Renilla (M2) luciferase activity (M1/M2). Data
are expressed as the mean ± standard deviation of six individual
values. Experiments were repeated more than three times.
*P<0.05. miR, microRNA; AR, androgen receptor; 3′UTR,
3′ untranslated region; NC, normal control; pMIR-REPORT, luciferase
reporter gene plasmid; pMIR-AR-3′UTRw, plasmid containing wild-type
3′UTR of human AR gene; pMIR-AR-3′UTRm, plasmid containing mutant
3′UTR of human AR gene. |
miR-185 impairs the interaction of AR
with ARE
AR is a ligand-activated transcription factor, which
induces the transcription of genes exhibiting ARE in their
regulatory regions (3). In order
to examine whether the downregulation of AR by miR-185 impaired the
interaction between AR and ARE, an ARE sequence was synthesized and
inserted upstream of the TATA box in pGL4.23 [luc2/minp] plasmids,
named pGL4-ARE. pGL4-ARE was cotransfected into the LNCaP-AD and
LNCaP-AI cells with either the miR-185 mimic or inhibitor.
Luciferase activity was measured 72 h after transfection. The
results indicated that transfection with the miR-185 mimic reduced
luciferase activity, while miR-185 inhibitor increased the
luciferase activity of pGL4-ARE in LNCaP-AD (Fig. 3A) and LNCaP-AI (Fig. 3B) cells, demonstrating that
downregulation of AR by miR-185 may impair the interaction between
AR and ARE.
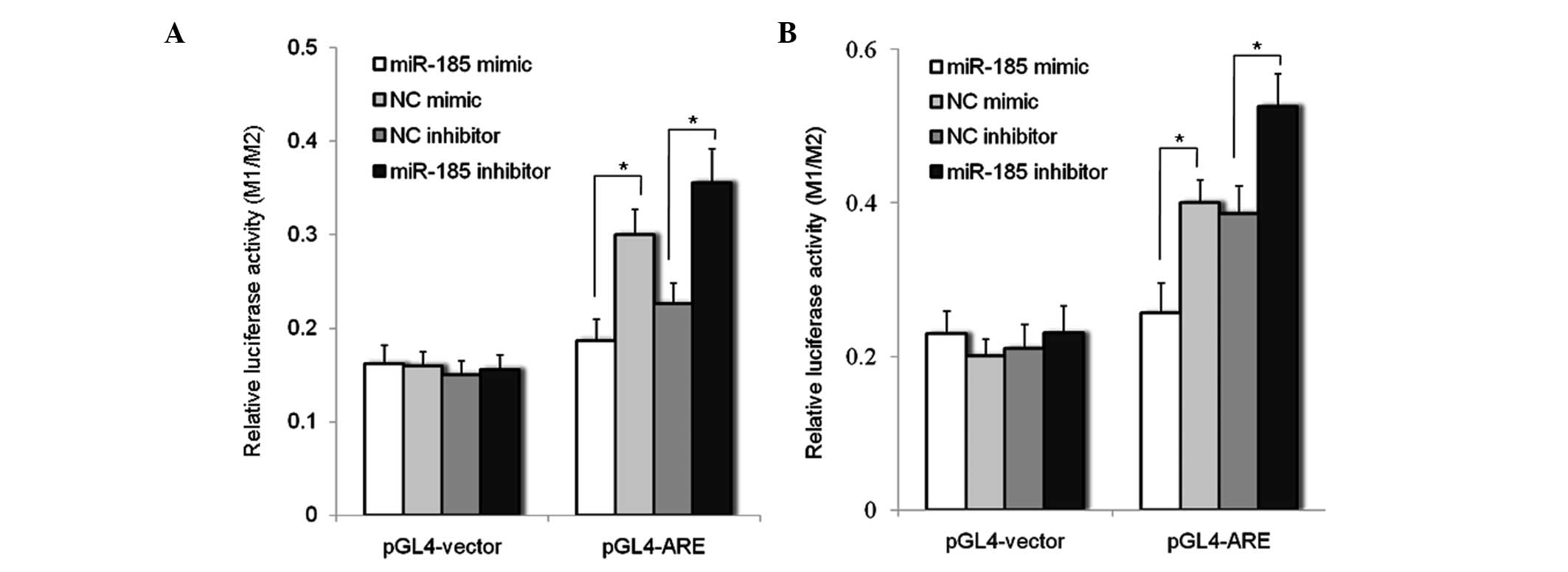 | Figure 3miR-185 impairs the interaction
between AR and ARE. (A) LNCaP-AD and (B) LNCaP-AI cells were
transfected with miR-185 mimic, miR-185 inhibitor, NC mimic or NC
inhibitor for 48 h, and subsequently cotransfected with the
pGL4-ARE or pGL4 vector for another 48 h. Luciferase activity was
evaluated using a Dual Luciferase Assay system and was plotted as
the ratio of firefly (M1) to Renilla (M2) luciferase activity
(M1/M2). Data are expressed as the mean ± standard deviation of six
individual values. Experiments were repeated more than three times.
*P<0.05. AR, androgen receptor; ARE, androgen
response element; AD, androgen dependent; AI, androgen independent;
miR, microRNA; NC, normal control; pGL4-ARE, pGL4 ARE reporter
plasmid; pGL4 vector, control plasmid. |
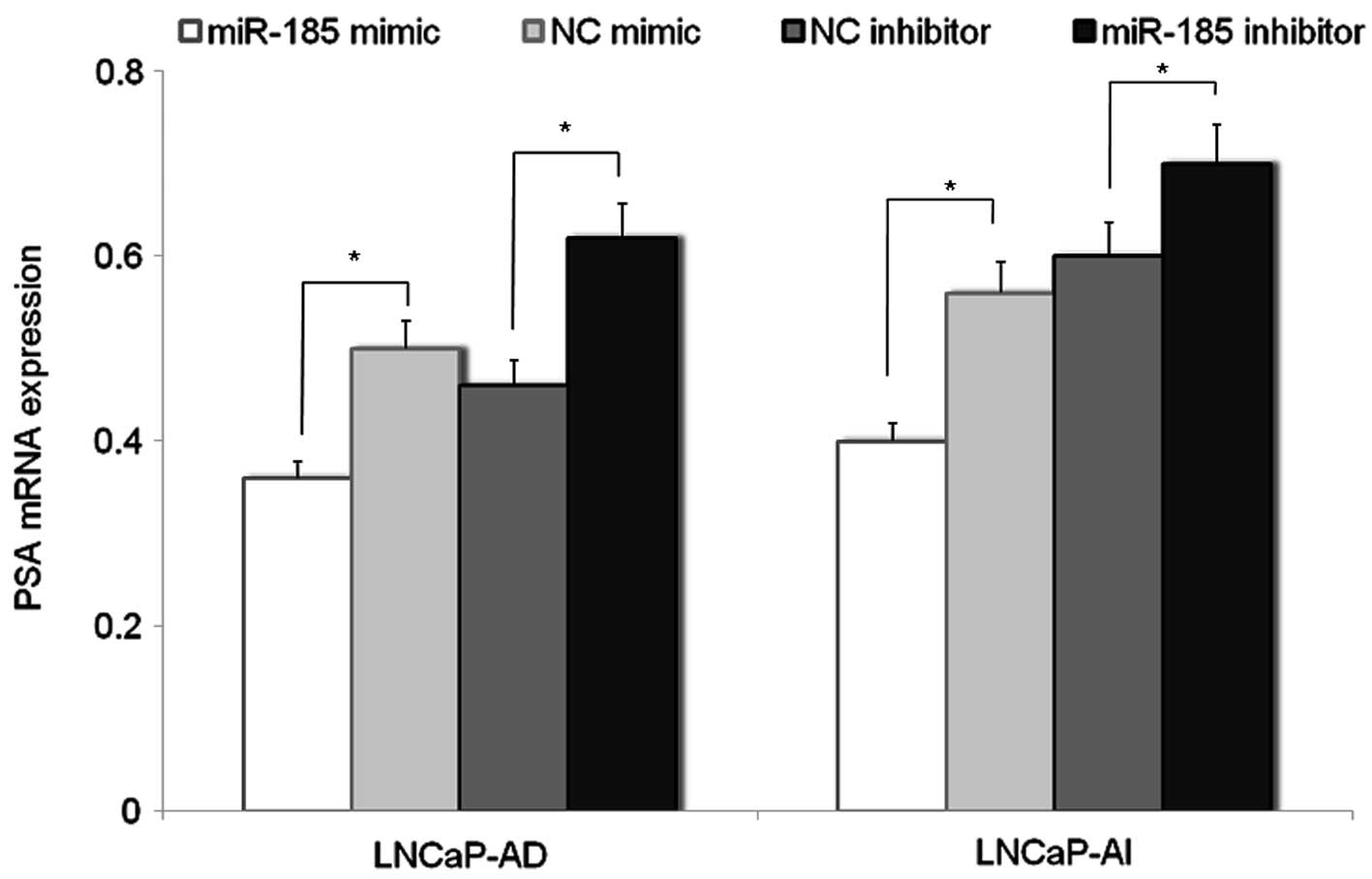
miR-185 down-regulates the expression of
AR target gene prostate specific antigen (PSA)
PSA is a prostate specific gene, regulated by AR. In
the present study, the effects of AR downregulation by miR-185 on
the expression of PSA mRNA in LNCaP cells was evaluated. The
results of PCR analysis indicated that the expression of PSA mRNA
in LNCaP-AD and LNCaP-AI cells transfected with miR-185 mimic was
reduced compared with that in cells transfected with the NC mimic,
while the mRNA expression of PSA in the miR-185
inhibitor-transfected LNCaP cells was increased compared with the
NC inhibitor-transfected cells (Fig.
4).
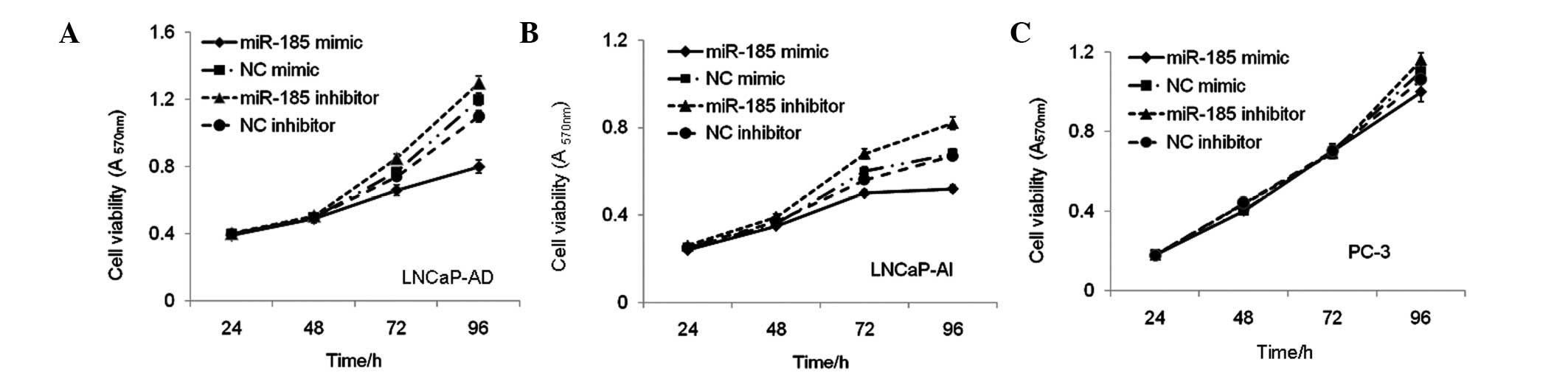
miR-185 inhibits the proliferation of
LNCaP cells
To investigate the potential of miR-185 to inhibit
the growth of LNCaP cells by downregulation the expression of AR,
the LNCaP-AD, LNCaP-AI and PC-3 cells were transfected with either
the miR-185 mimic or inhibitor, and the numbers of viable cells
were measured 24, 48, 72 and 96 h after transfection using an MTT
assay. The results indicated that the miR-185 mimic reduced, while
the miR-185 inhibitor increased the numbers of viable LNCaP-AD
(Fig. 5A) and LNCaP-AI (Fig. 5B) cells. The same transfection
procedure was performed in PC-3 cells, not expressing AR, in which
the miR-185 mimic or inhibitor had no significant effects on cell
proliferation (Fig. 5C). These
data demonstrated that the effect of miR-185 on cell growth was
associated with downregulation of AR in the LNCaP cells.
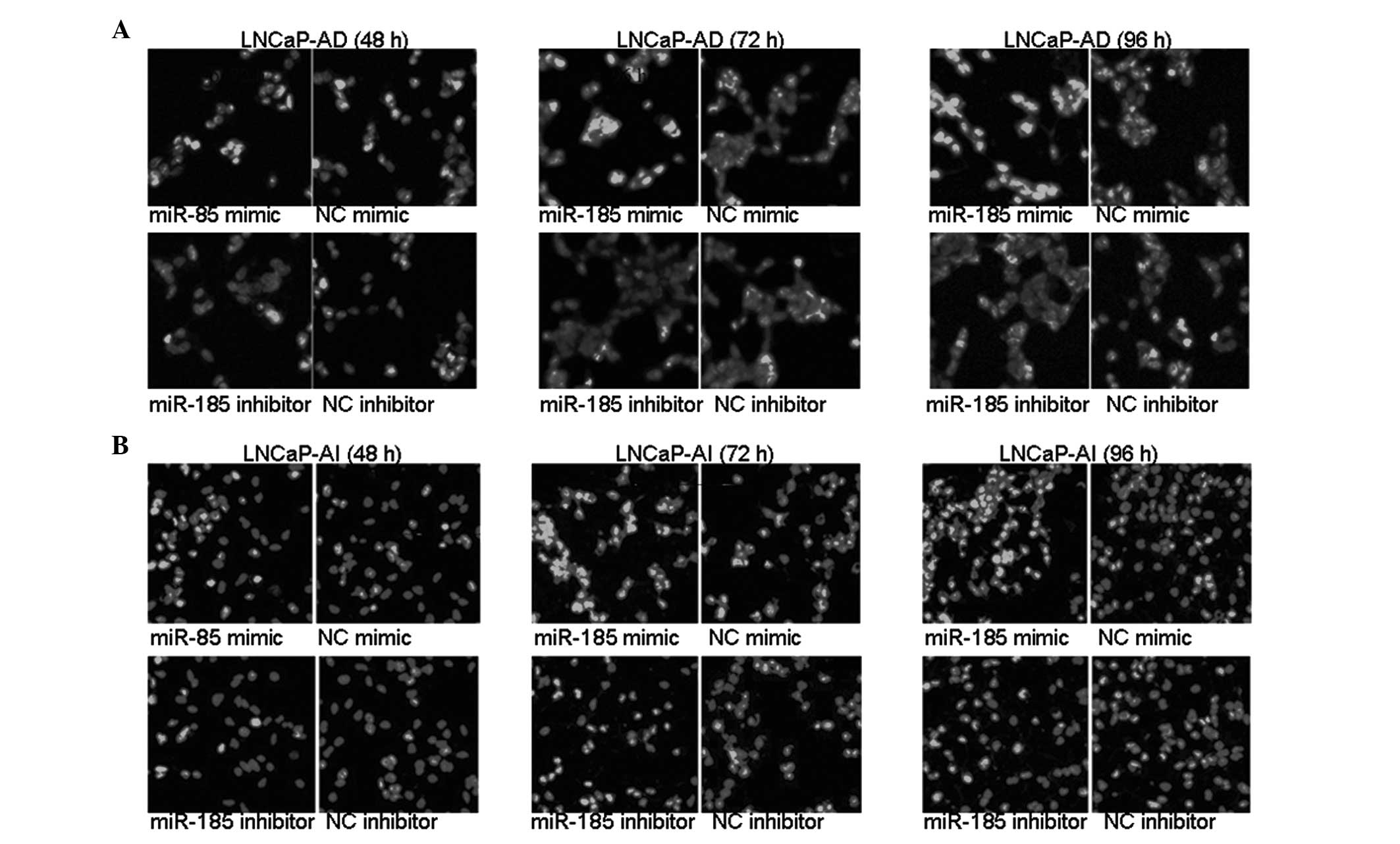
miR-185 induces the apoptosis of LNCaP
cells
The LNCaP-AD and LNCaP-AI cells were transfected
with either miR-185 mimic or inhibitor for 48, 72 and 96 h.
Apoptosis was assessed using Hoechest 33258 staining. As shown in
Fig. 6, following transfection
with miR-185 mimic, significantly more LNCaP-AD and LNCaP-AI cells
exhibited chromatin condensation and marginalization compared with
the NC mimic-transfected cells. By contrast, transfection with the
miR-185 inhibitor resulted in a decreased number of LNCaP-AD and
LNCaP-AI cells with nucleic chromatin condensation compared with
the cells transfected with the NC inhibitor. These data
demonstrated that the miR-185-mediated downregulation of AR induced
apoptosis of the LNCaP cells.
Discussion
In the past few years, numerous studies have
demonstrated that miRNAs are important in the developmental process
of cancer (30–33). They may function as oncogenes or
tumor suppressor genes (34) in
tumorigenesis, and regulate multiple cellular processes involved in
the progression of cancer. Due to each miRNA regulating numerous
potential targets, identification of the true target genes that are
involved in cancer cell behaviors is important for understanding
the mechanisms underlying the functional contributions of miRNAs in
tumor development and progression.
Several studies have demonstrated that miR-185
functions as a tumor suppressor, which is able to suppress tumor
growth and progression in non-small lung carcinoma, ovarian,
pediatric renal and breast cancer cell lines (25–28).
It has also been suggested that miR-185 may be important in cell
proliferation (25–28). However, the mechanisms by which
miR-185 inhibits cell proliferation in different cancer cells vary.
miR-185 suppresses the growth of the human non-small cell lung
cancer cell lines and induces cell cycle arrest by suppressing the
mRNA expression of cell cycle regulating genes, including CDK6 and
AKT1 (25). Additionally, miR-185
suppresses tumor growth and progression by targeting the Six1
oncogene in multiple types of human cancer, including pediatric
renal tumors, aggressive ovarian cancer and breast cancer (26). In colorectal cancer cells, miR-185
directly regulates the expression of RhoA and Cdc42 and their
associated functions, including proliferation and invasion
(35). In contrast to its role in
tumor suppression, miR-185 was also demonstrated to induce tumor
growth and progression in clear cell renal cell carcinoma (36). The expression of miR-185 was
inversely correlated with its putative target PTPN13, which
suppressed cell growth and induced apoptosis as a tumor suppressor
gene by inhibiting phosphoinositide 3-kinase (PI3K)/AKT signaling
(37). These findings suggest that
miR-185 may target differing genes in various cell types, which
contribute to different biological processes.
The results of the present study confirmed miR-185
as a tumor suppressor gene, which exerted its effect through
modulating the expression of AR, inhibiting cell proliferation and
enhancing apoptosis of the LNCaP cells. Computational analysis
revealed a potential binding site for miR-185 in the 3′UTR of AR.
Deletion mutagenesis and a luciferase-based reporter gene assay
demonstrated that the predicted miR-185 target sites in the AR
3′UTR were functional. Furthermore, data indicated that miR-185
effectively downregulated the protein expression of AR, impaired
the interaction of AR with ARE and downregulated the expression of
the AR target gene, PSA.
AR is a ligand-dependent transcription factor
belonging to the nuclear hormone receptor superfamily (38). AR functions as a ligand-dependent
transcription factor by binding to AREs in the regulatory regions
of specific androgen-regulated target genes (39). The development of PCa and growth of
prostate tissue depend on androgen signaling via the AR (14,40);
therefore, downregulation of the expression levels of AR and
androgens is a basic therapeutic strategy in preventing the
development of PCa.
Based on the significance of AR for the
androgen-dependent and androgen-independent growth of PCa cells
(6), understanding the association
between miR-185 and AR is important for investigation of prostate
carcinogenesis and therapeutics. An increasing number of studies
have focused on improving PCa treatment by developing more
effective strategies for silencing AR (41–44).
A combinatorial approach, involving the simultaneous targeting of
multiple pathways, is an accepted approach in the development of
PCa therapy and the development of numerous therapeutic options,
which can be evaluated in combinatorial therapy settings is being
emphasized at present (10).
Therefore, the results of the present study, revealing that miR-185
mediated the repression of AR expression and activity, are of
significance.
In conclusion, the present study demonstrated that
miR-185 directly targeted the AR-3′UTR, to inhibit the expression
of AR, and acted as a tumor suppressor in the PCa cells. miR-185
is, therefore, a potential negative modulator of AR-mediated
signaling with potential for use in PCa therapeutics.
Acknowledgments
This study was supported by grants from the National
Natural Science Foundation of China (nos. 81071720 and 81172045),
Shandong Provincial Programs for Science and Technology Development
(no. 2012GSF11820) and the Foundation for Outstanding Young
Scientists in Shandong Province (no. 2006BS03066).
References
|
1
|
Jemal A, Siegel R, Xu J and Ward E: Cancer
statistics, 2010. CA Cancer J Clin. 60:277–300. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jemal A, Siegel R, Ward E, Hao Y, Xu J and
Thun MJ: Cancer statistics, 2009. CA Cancer J Clin. 59:225–249.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dehm SM and Tindall DJ: Androgen receptor
structural and functional elements: role and regulation in prostate
cancer. Mol Endocrinol. 21:2855–2863. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Buchanan G, Irvine RA, Coetzee GA and
Tilley WD: Contribution of the androgen receptor to prostate cancer
predisposition and progression. Cancer Metastasis Rev. 20:207–223.
2001. View Article : Google Scholar
|
|
5
|
Agoulnik IU and Weigel NL: Androgen
receptor action in hormone-dependent and recurrent prostate cancer.
J Cell Biochem. 99:362–372. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Taplin ME and Balk SP: Androgen receptor:
a key molecule in the progression of prostate cancer to hormone
independence. J Cell Biochem. 91:483–490. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Feldman BJ and Feldman D: The development
of androgen-independent prostate cancer. Nat Rev Cancer. 1:34–45.
2001. View
Article : Google Scholar
|
|
8
|
Pienta KJ and Bradley D: Mechanisms
underlying the development of androgen-independent prostate cancer.
Clin Cancer Res. 12:1665–1671. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen Y, Sawyers CL and Scher HI: Targeting
the androgen receptor pathway in prostate cancer. Curr Opin
Pharmacol. 8:440–448. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Knudsen KE and Scher HI: Starving the
addiction: new opportunities for durable suppression of AR
signaling in prostate cancer. Clin Cancer Res. 15:4792–4798. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Eder IE, Culig Z, Ramoner R, Thurnher M,
Putz T, Nessler-Menardi C, Tiefenthaler M, Bartsch G and Klocker H:
Inhibition of LncaP prostate cancer cells by means of androgen
receptor antisense oligonucleotides. Cancer Gene Ther. 7:997–1007.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zegarra-Moro OL, Schmidt LJ, Huang H and
Tindall DJ: Disruption of androgen receptor function inhibits
proliferation of androgen-refractory prostate cancer cells. Cancer
Res. 62:1008–1013. 2002.PubMed/NCBI
|
|
13
|
Wright ME, Tsai MJ and Aebersold R:
Androgen receptor represses the neuroendocrine transdifferentiation
process in prostate cancer cells. Mol Endocrinol. 17:1726–1737.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen CD, Welsbie DS, Tran C, et al:
Molecular determinants of resistance to antiandrogen therapy. Nat
Med. 10:33–39. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hååg P, Bektic J, Bartsch G, Klocker H and
Eder IE: Androgen receptor down regulation by small interference
RNA induces cell growth inhibition in androgen sensitive as well as
in androgen independent prostate cancer cells. J Steroid Biochem
Mol Biol. 96:251–258. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Compagno D, Merle C, Morin A, Gilbert C,
Mathieu JR, Bozec A, Mauduit C, Benahmed M and Cabon F:
SIRNA-directed in vivo silencing of androgen receptor inhibits the
growth of castration-resistant prostate carcinomas. PLoS One.
2:e10062007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Di Giacomo G, Koss M, Capellini TD,
Brendolan A, Pöpperl H and Selleri L: Spatio-temporal expression of
Pbx3 during mouse organogenesis. Gene Expr Patterns. 6:747–757.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lichtenauer UD, Duchniewicz M, Kolanczyk
M, et al: Pre-B-cell transcription factor 1 and steroidogenic
factor 1 synergistically regulate adrenocortical growth and
steroidogenesis. Endocrinology. 148:693–704. 2007. View Article : Google Scholar
|
|
19
|
Carleton M, Cleary MA and Linsley PS:
MicroRNAs and cell cycle regulation. Cell Cycle. 6:2127–2132. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Schmittgen TD: Regulation of microRNA
processing in development, differentiation and cancer. J Cell Mol
Med. 12:1811–1819. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bartel DP: MicroRNAs: genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ambros V: MicroRNA pathways in flies and
worms: growth, death, fat, stress and timing. Cell. 113:673–676.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hwang HW and Mendell JT: MicroRNAs in cell
proliferation, cell death, and tumorigenesis. Br J Cancer.
96(Suppl): R40–R44. 2007.PubMed/NCBI
|
|
24
|
Wiemer EA: The role of microRNAs in
cancer: no small matter. Eur J Cancer. 43:1529–1544. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Takahashi Y, Forrest AR, Maeno E,
Hashimoto T, Daub CO and Yasuda J: Mir-107 and mir-185 can induce
cell cycle arrest in human non small cell lung cancer cell lines.
PLoS One. 4:e66772009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Imam JS, Buddavarapu K, Lee-Chang JS,
Ganapathy S, Camosy C, Chen Y and Rao MK: MicroRNA-185 suppresses
tumor growth and progression by targeting the six1 oncogene in
human cancers. Oncogene. 29:4971–4979. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liu M, Lang N, Chen X, et al: MiR-185
targets RhoA and Cdc42 expression and inhibits the proliferation
potential of human colorectal cells. Cancer Lett. 301:151–160.
2011. View Article : Google Scholar
|
|
28
|
Akçakaya P, Ekelund S, Kolosenko I, et al:
MiR-185 and miR-133b deregulation is associated with overall
survival and metastasis in colorectal cancer. Int J Oncol.
39:311–318. 2011.PubMed/NCBI
|
|
29
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
30
|
Akao Y, Nakagawa Y and Naoe T: let-7
microRNA functions as a potential growth suppressor in human colon
cancer cells. Biol Pharm Bull. 9:903–906. 2006. View Article : Google Scholar
|
|
31
|
Majid S, Dar AA, Saini S, et al: miR-23b
represses proto-oncogene Src kinase and functions as
methylation-silenced tumor suppressor with diagnostic and
prognostic significance in prostate cancer. Cancer Res.
72:6435–6446. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ni Y, Meng L, Wang L, Dong W, Shen H, Wang
G, Liu Q and Du J: MicroRNA-143 functions as a tumor suppressor in
human esophageal squamous cell carcinoma. Gene. 517:197–204. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wu N, Lin X, Zhao X, Zheng L, Xiao L, Liu
J, Ge L and Cao S: MiR-125b acts as an oncogene in glioblastoma
cells and inhibits cell apoptosis through p53 and
p38MAPK-independent pathways. Br J Cancer. 109:2853–2863. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhang B, Pan X, Cobb GP and Anderson TA:
MicroRNAs as oncogenes and tumor suppressors. Dev Biol. 302:1–12.
2007. View Article : Google Scholar
|
|
35
|
Liu H, Brannon AR, Reddy AR, et al:
Identifying mRNA targets of microRNA dysregulated in cancer: with
application to clear cell renal cell carcinoma. BMC Syst Biol.
4:512010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Abaan OD and Toretsky JA: PTPl1: a large
phosphatase with a split personality. Cancer Metastasis Rev.
27:205–214. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Mangelsdorf DJ, Thummel C, Beato M, et al:
The nuclear receptor superfamily: the second decade. Cell.
83:835–839. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Heinlein CA and Chang C: Androgen receptor
(AR) coregulators: an overview. Endocr Rev. 23:175–200. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Chmelar R, Buchanan G, Need EF, Tilley W
and Greenberg NM: Androgen receptor coregulators and their
involvement in the development and progression of prostate cancer.
Int J Cancer. 120:719–733. 2007. View Article : Google Scholar
|
|
40
|
Chen S, Song CS, Lavrovsky Y, Bi B,
Vellanoweth R, Chatterjee B and Roy AK: Catalytic cleavage of the
androgen receptor messenger RNA and functional inhibition of
androgen receptor activity by a hammerhead ribozyme. Mol
Endocrinol. 12:1558–1566. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Eder IE, Hoffmann J, Rogatsch H, Schafer
G, Zopf D, Bartsch G and Klocker H: Inhibition of LNCaP prostate
tumor growth in vivo by an antisense oligonucleotide directed
against the human androgen receptor. Cancer Gene Ther. 9:117–125.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Cheng H, Snoek R, Ghaidi F, Cox ME and
Rennie PS: Short hairpin RNA knockdown of the androgen receptor
attenuates ligand independent activation and delays tumor
progression. Cancer Res. 66:10613–10620. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Liao X, Tang S, Thrasher JB, Griebling TL
and Li B: Small-interfering RNA-induced androgen receptor silencing
leads to apoptotic cell death in prostate cancer. Mol Cancer Ther.
4:505–515. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Lim AC and Attard G: Improved therapeutic
targeting of the androgen receptor: rational drug design improves
survival in castration-resistant prostate cancer. Curr Drug
Targets. 14:408–419. 2013. View Article : Google Scholar : PubMed/NCBI
|