Introduction
Colorectal cancer (CRC) is a significant health
problem worldwide, which has become the second most common cause of
cancer-associated mortality (1,2).
Every year, there are >1,200,000 cases of CRC diagnosed
worldwide, the majority of cases occurring in developing countries
(3). Surgical resection remains
the first choice for CRC therapy; however, the patients are
subjected to significant pain during the recovery process (4). Furthermore, due to the high risk of
CRC relapse, surgery is frequently combined with chemotherapy and
radiation therapy (5). Several
anticancer drugs have been clinically applied in the treatment of
CRC, including irinotecan, Tomudex and oxaliplatin (6), of which 5-fluorouracil (5-FU) is the
most widely prescribed. However, clinical insufficiencies occur as
a result of 5-FU resistance and severe side effects (7,8).
Therefore, the identification of a safer and more effective drug,
which can inhibit the progression of colon cancer is required.
Apoptosis describes the mechanism by which cells
undergo programmed death in order to control cell proliferation or
in response to DNA damage (9).
There are two main apoptotic pathways, termed the extrinsic and
intrinsic pathway (10). The
intrinsic pathway refers to the mitochondrial pathway, in which
caspase activation is closely linked to mitochondrial changes,
mediated by members of the B-cell lymphoma 2 (Bcl-2) family
(11,12). The Bcl-2 family proteins are
divided into pro-apoptotic and anti-apoptotic proteins, which
balance the levels of apoptosis amongst cells (13–15).
A number of studies have demonstrated that various
herbal medicines have potential antitumor activities (16–18).
4-[(Tetrahydro-2H-pyran-2-yl)oxy] phenol (XG-d) is a hydroquinone
analog, which exists in Vaccinium vitis-idaea, which is an
ericaceous plant used for the treatment of dysuria, gonorrhoea and
diarrhoea (19). It has been
reported that the leaves and berries of the plant have antiviral
and anti-inflammatory effects (20), however, to the best of our
knowledge, no previous studies have investigated the antitumor
effects of XG-d in cancer.
In the present study, the anticancer activity of
XG-d was investigated in the C26 murine colon carcinoma cell line
and the effects of XG-d on a tumor-bearing mouse model were
evaluated. The aim was to demonstrate the potential antitumor
activity of XG-d in vitro and in vivo and contribute
to the development of a novel potential antitumor drug with low
toxicity.
Materials and methods
Chemicals reagents
XG-d, with a purity >99%, was purchased from
Shanghai Boyle Chemical Co., Ltd (Shanghai, China). Standard stock
solution (10 mg/ml) were prepared with dimethyl sulfoxide (DMSO;
Sigma-Aldrich, St. Louis, MO, USA) and diluted to the indicated
concentrations with basal medium [RPMI-1640 (Gibco Life
Technologies, Carlsbad, CA, USA), Dulbecco's modified Eagle’s
medium (DMEM; Invitrogen Life Technologies, Grand Island, NY, USA)
and DMEM/F-12 medium (HyClone, GE Healthcare Life Science, Little
Chalfont, UK)] prior to each experiment. 5-FU was purchased from
Sinopharm Chemical Reagent Co., Ltd (Shanghai, China). Mouse
monoclonal anti-GAPDH (1:5,000–10,000; G8795) were purchased from
Sigma-Aldrich. The following primary antibodies were purchased from
Cell Signaling Technology, Inc. (Beverly, MA, USA): Rabbit
polyclonal anti-caspase-3 (1:1,000; #9662S), mouse monoclonal
anti-caspase-9 (1:1,000; #9508S), rabbit polyclonal
anti-poly(adenosine diphosphate ribose) polymerase (PARP; 1:1,000;
#9542S), rabbit monoclonal anti-Bcl-2 (1:500; #2870S) and rabbit
polyclonal anti-Bax (1:1,000; #2772S). Horseradish
peroxide-conjugated goat anti-mouse immunoglobulin (Ig)G (1:5,000;
sc-2005) and goat anti-rabbit IgG (1:5,000; sc-2004) were purchased
from Santa Cruz Biotechnology Inc. (Santa Cruz, CA, USA).
Cell lines and culture
The A549 human lung carcinoma cell line, HK-2 human
proximal tubule epithelial cell line and HT-29 human colon
carcinoma cell line were obtained from The American Type Culture
Collection (Manassas, VA, USA). The C26 murine colon carcinoma cell
line and L02 human liver cell line were purchased from the Cell
Bank of the Shanghai Institute of Biochemistry and Cell Biology,
Chinese Academy of Sciences (Shanghai, China). The A549 and HT-29
cell lines were cultured in DMEM, the C26 and L02 cell lines were
maintained in RPMI-1640 medium and the HK-2 cell line was cultured
in DMEM/F-12 medium. All cell culture mediums were supplemented
with 10% fetal bovine serum (Gibco, Auckland, New Zealand), 100
U/ml penicillin and 100 μg/ml streptomycin (HyClone) at 37°C
in a humidified atmosphere containing 5% CO2.
Animals
Female BALB/c mice (4–6 weeks old) with body weights
ranging from 18–22 g were purchased from Chengdu Dashuo
Biotechnology Co, Ltd (Chengdu, China). The present study was
approved by the ethics committee of the Institutional Animal Care
and Treatment Committee of Sichuan University (Sichuan, China). The
animal room was controlled to maintain temperature (22±2°C), light
(12 h light/dark cycles) and humidity (50±10%).
Cell viability assay
Cell viability was measured using a cell counting
kit-8 (CCK-8) kit (Dojindo, Tokyo, Japan), according to the
manufacturer's instructions. Briefly, the cells were seeded into
96-well culture plates (Costar Corning, Inc., Corning, NY, USA) at
a density of 4,000 cells/well. Following incubation overnight, the
cells were treated with various concentrations of XG-d (2.5, 5, 10,
20 or 40 μg/ml). DMSO (<0.1%) was used as an untreated
control. Following 48 h of incubation at 37°C, 10 μl CCK-8
solution was added to each well and incubated for 3 h, prior to the
measurement of the absorbance at 450 nm using a SpectraMax M5
microplate reader (Molecular Devices, LLC, Sunnyvale, CA, USA).
Hoechst staining
The cells were washed with phosphate-buffered saline
(PBS; Solarbio Science and Technology Co., Ltd, Beijing, China) and
fixed with 4% paraformaldehyde (HyClone) for ~20 min at room
temperature. The fixed cells were subsequently stained with Hoechst
33258 (Biyuntian Biotechnology Co., Shanghai, China) at 37°C for 15
min, washed with PBS twice and the changes in the nuclei were
examined using a fluorescence microscope (uX71;Olympus Corp.,
Tokyo, Japan).
Cell cycle analysis
In order to evaluate the cell cycle distribution
following drug treatment, the DNA contents were analyzed using flow
cytometry. Following treatment with the drug for 24 h, the cells
were harvested, washed once with PBS and fixed using 70% ethanol
(Sigma-Aldrich) at 4°C overnight. Following centrifugation at 600 ×
g for 5 min, the cells were analyzed using a Cell Cycle and
Apoptosis analysis kit (Biyuntian Biotechnology Co., Shanghai,
China), according to the manufacturer's instructions. The cells
were analyzed using a fluorescence-activated cell sorting (FACS)can
flow cytometer (Navios; Beckman Coulter, Brea, CA, USA).
Transmission electron microscopy
XG-d was prefixed with a mixed solution of 3%
glutaraldehyde (Sinopharm Chemical Reagent Co., Ltd) and then
post-fixed with 1% osmium tetroxide, dehydrated in series of
acetone solutions, infiltrated in Epox 812 (Beijing Zhongjingkeyi
Technology Co., Ltd., Beijing, China) for approximately 3 h and
were subsequently embedded. The semi-thin sections (0.6~0.8
μm; used in for optical positioning) were cut with a slicer
and stained with methylene blue (Sigma-Aldrich) and ultra-thin
sections (<0.1 μm; used for observation) were cut using a
diamond knife (Beijing Zhongjingkeyi Technology Co., Ltd.), then
stained with uranyl acetate and lead citrate. Sections were then
examined using a transmission electron microscope (H-600IV;
Hitachi, Tokyo, Japan).
Annexin V/propidium iodide (PI) double
staining assay
The C26 cells were treated with XG-d for 24 h,
harvested and washed three times with PBS. The apoptotic assay was
performed using an annexin V-fluorescein isothiocyanate (FITC)/PI
apoptosis kit (KeyGEN, Nanjing, China), according to the
manufacturer's instructions. The cells were resuspended in 500
μl binding buffer and 5 μl Annexin V-FITC and PI were
added. The cells were subsequently incubated for 15 min in the dark
and the samples were measured using a fluorescence activated cell
sorting (FACS)can flow cytometer (Beckman Coulter).
Western blot analysis
The cells were washed with cold PBS and lysed in
radioimmunoprecipitation assay lysis buffer (Biyuntian Bitechnology
Co., Shanghai, China), containing 50 mM Tris-HCl (pH 7.4), 150 mM
NaCl, 1% TritonX-100, 1% sodium deoxycholate, 0.1% SDS, 1 mM sodium
orthovanadate, 1 mM EDTA, 1 mM phenylmethanesulfonylfluoride and 50
mM NaF for 20 min on ice. Subsequently, the cell lysates were
centrifuged for 10 min at 12,000 × g at 4°C. The total protein in
the supernatants was determined using a Bicinchoninic Acid Protein
Assay kit (Biyuntian Biotechnology, Co.). Equal quantities of
protein was separated by 10% SDS-PAGE and transferred onto a
polyvinylidene fluoride membrane (EMD Millipore, Billerica, MA,
USA) by electroblotting. The membrane was then blocked with 5%
non-fat milk for 1 h and was incubated with primary antibodies for
PARP (1:1,000), caspase-9 (1:1,000), caspase-3 (1:1,000), Bcl-2
(1:500), Bax (1:500) and GAPDH (1:5,000–10,000), as described
above, at 4°C overnight (>18 h). Following washing, the blots
were incubated with a horseradish peroxidase-conjugated goat
anti-mouse and goat anti-rabbit IgG secondary antibodies (1:5,000)
at room temperature for 1 h, with agitation. The reactive bands
were detected by enhanced chemiluminescence using either
SuperSignal West Pico Chemiluminescent Substrate (Thermo Fisher
Scientific, Waltham, MA, USA) or Immobilon Western Chemiluminescent
HRP Substrate (Millipore), according to the manufacturer's
instructions.
Tumor growth assay
The C26 cells (1.0×106) were
subcutaneously implanted in the right flank region of the BALB/c
mice (21). Seven days following
implantation, the mice with tumor sizes >50 mm3 were
selected and randomly divided into three groups (n=10 per group),
termed the vehicle control, XG-d and positive groups. These groups
were treated with 0.5% carboxymethyl cellulose sodium (Aladdin
Industrial, Inc., Shanghai, China), 100 mg/kg XG-d and 30 mg/kg
5-FU (Sinopharm Chemical Reagent Co., Ltd), respectively, the
concentrations of which were determined in a preliminary study.
Equal volumes of the drugs and vehicle were administered orally to
the mice every day for 3 weeks. The body weight (MP5002 Electronic
balance; Shanghai Sunny Henping Scientific Instrument Co., Ltd.,
Shanghai, China) and tumor size were measured twice weekly and the
tumor volumes were calculated using the formula 0.52 × a ×
b2, where ‘a’ represented the long diameter and ‘b’
represented the short diameter. At the end of the treatment, the
mice were sacrificed by cervical dislocation and the tumors were
excised and weighed.
Statistical analysis
All the values are expressed as the mean ± standard
error of the mean. Statistical significance was analyzed by one-way
analysis of variance, followed by Scheffe's test for multiple
comparisons. Statistical analyses were performed using GraphPad
Prism 5.0 software (GraphPad Software, Inc., La Jolla, CA, USA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
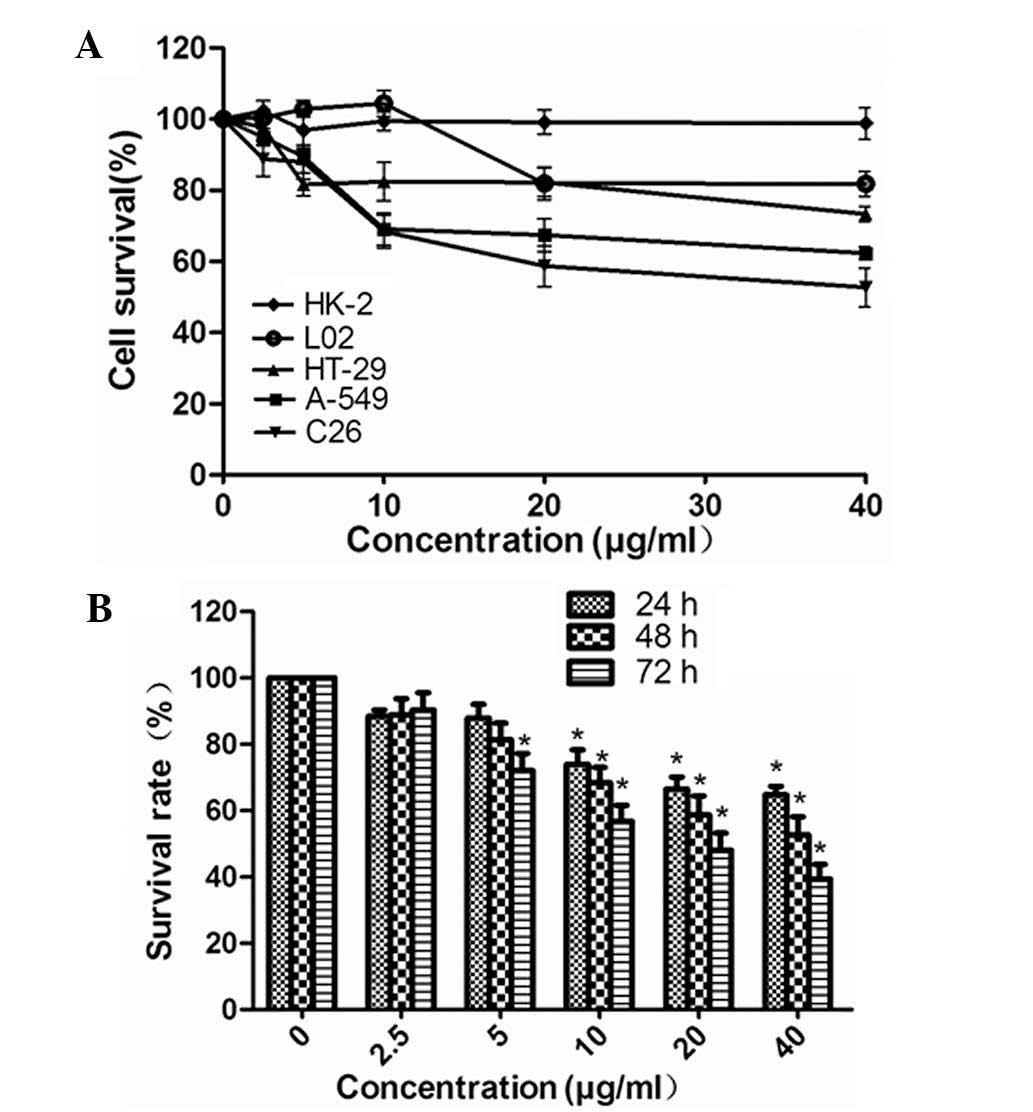
XG-d inhibits C26 cell proliferation with
low levels of toxicity on the L02 and HK-2 cells
The effects of XG-d on the proliferation of various
cell lines were determined using a CCK-8 assay. The cell viability
was examined in three carcinomatosis cell lines (A549, C26 and
HT-29) and two normal cell lines (HK-2 and L-02), which had been
treated with various concentrations of XG-d (2.5, 5, 10, 20 or 40
μg/ml) for 48 h. XG-d significantly inhibited the
proliferation of the C26 cells and had less marked effects on the
A549 and HT-29 cancer cell lines and the L02 and HK-2 normal cell
lines (Fig. 1A). The inhibitory
effect of XG-d on C26 growth at various concentrations were similar
after 24 and 48 h incubation (P>0.05); therefore, a duration of
24 h was used in the subsequent investigations (Fig. 1B).
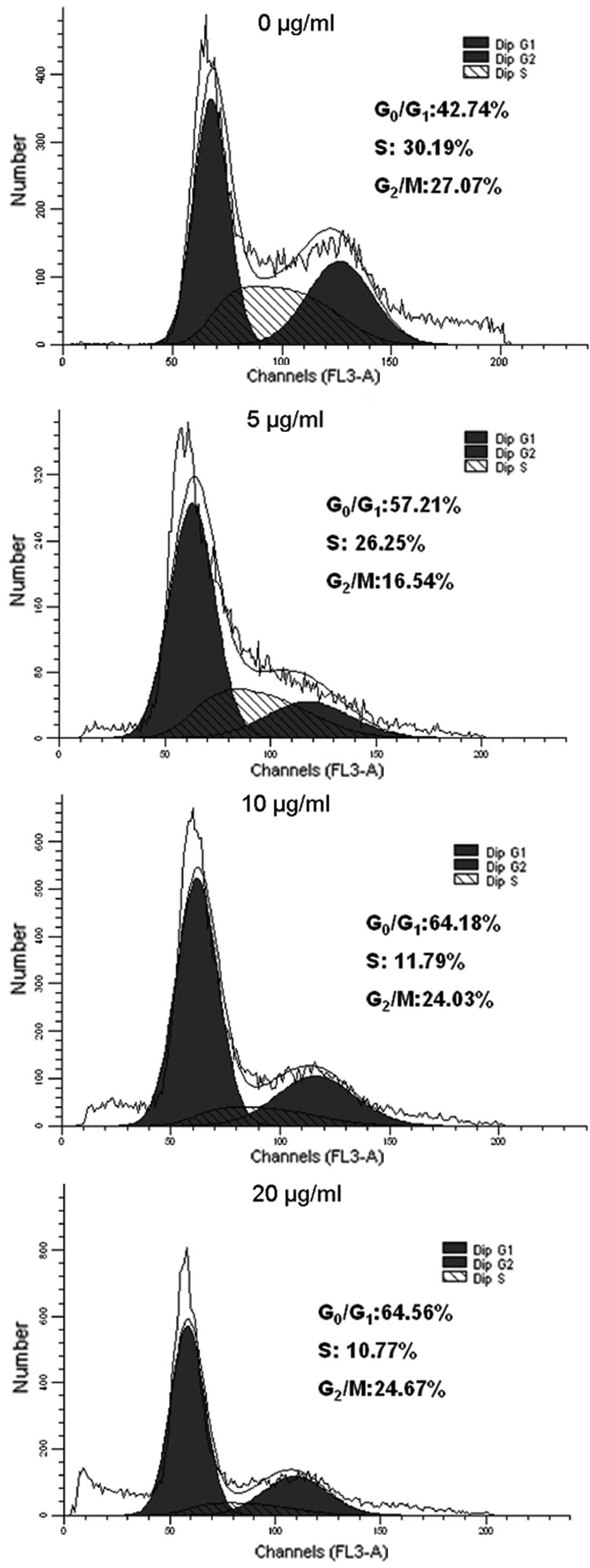
XG-d induces C26 cell cycle arrest in the
G0/G1 phase
DNA cell cycle analysis was used to examine the
effects of XG-d on the growth of the C26 cells. The C26 cells were
incubated with various concentrations of XG-d for 24 h. As shown in
Fig 2, compared with the control
group, the G0/G1 phase was significantly
increased between 42.74 and 64.56%, while the number of cells in
the S phase gradually decreased between 30.19 and 10.77% in the
XG-d-treated group with increasing XG-d concentration.
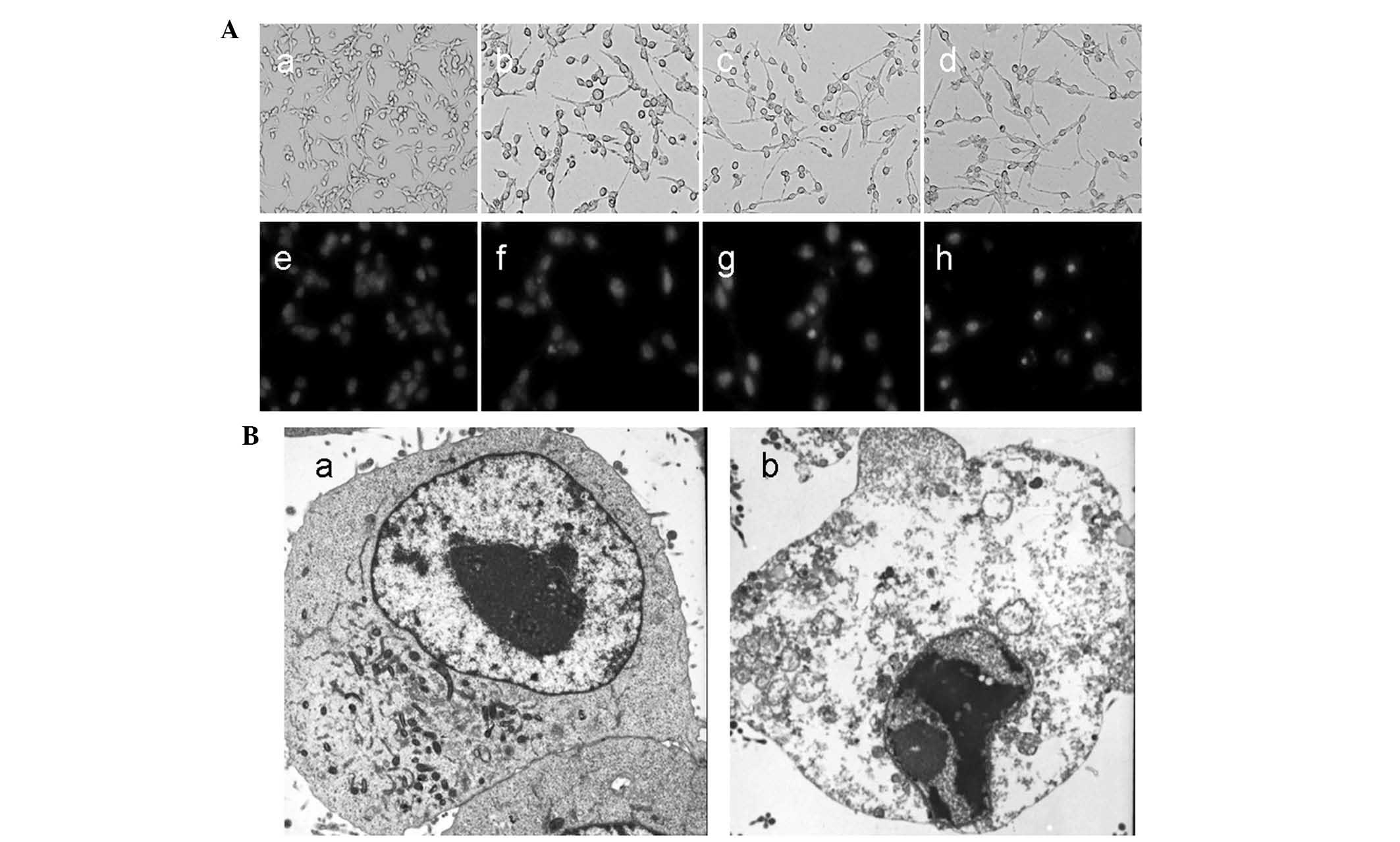
Cell morphological assessment
In order to investigate how XG-d inhibited cell
viability, cell morphology was examined. As shown in Fig. 3A, morphological changes were
observed in the C26 cells following treatment with XG-d for 24 h.
Notably, the control cells emitted light blue fluorescence and the
nuclei were round, suggesting that the chromatin was equivalently
distributed. However, the XG-d-treated cells exhibited shrinkage,
chromatin congregation and nuclear fragmentation.
Subsequently, a transmission electronic microscope
was used to identify morphological changes in the ultrastructure of
the C26 cells. No clear damage was observed in the control group
(Fig. 3Ba), however, the
XG-d-treated group exhibited chromatin congregation, nucleic
degeneration and cytoplasm shrinkage, while the nuclear envelope
and cell membrane remained intact (Fig. 3Bb). These results suggested that
XG-d was capable of inducing apoptosis in the C26 cells.
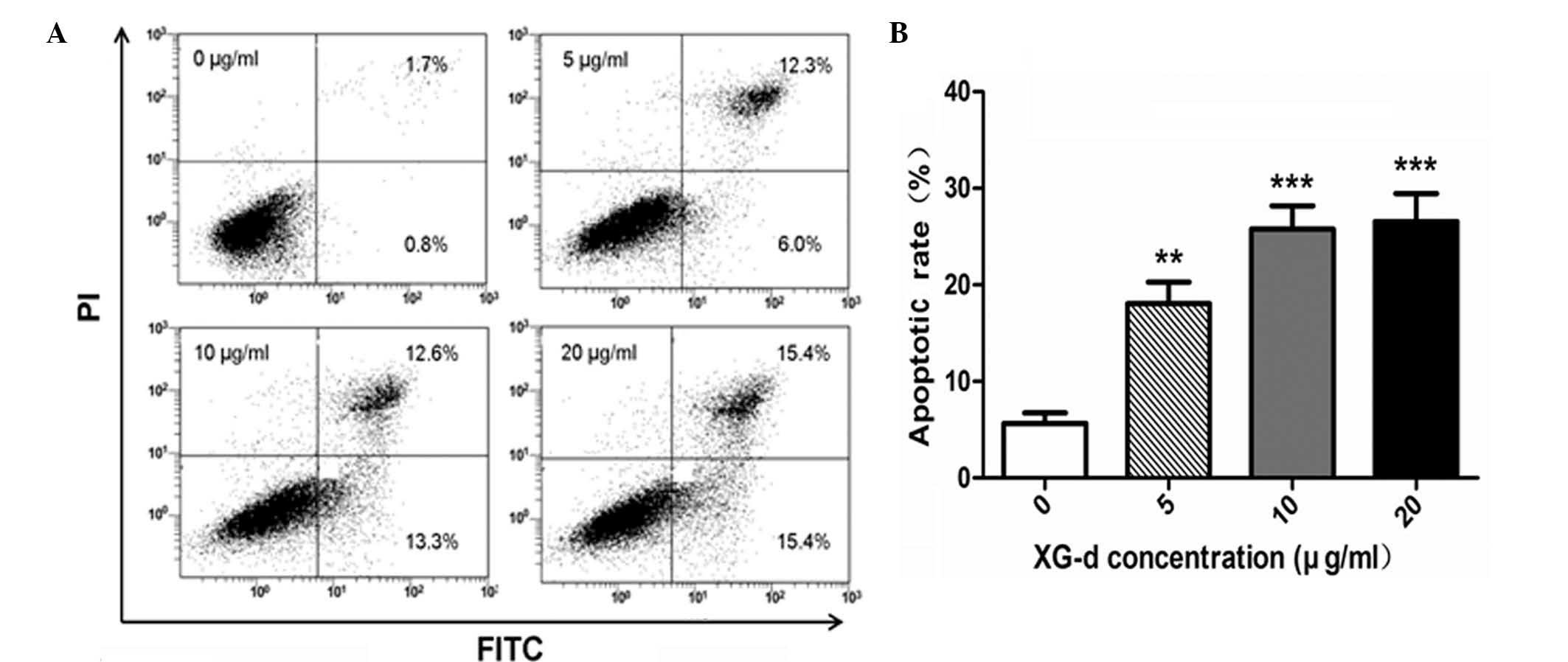
XG-d induces apoptosis
Flow cytometric analysis using an Annexin V-FITC
apoptosis kit was used to identify the levels of apoptosis induced
by XG-d in the C26 cells. As indicated in Fig. 4A, the C26 cells were exposed to
various concentrations of XG-d (5, 10 or 20 μg/ml) for 24 h.
The percentage of early apoptotic cells increased to 15.4%, while
the percentage of late apoptotic cells and necrotic cells increased
to 15.4% following treatment with 20 μg/ml XG-d, compared
with the control group. A significant increase in the apoptotic
rate was induced in the XG-d treated groups compared with the
untreated group (P<0.05; Fig.
4B). The results indicated that XG-d induced apoptosis in the
C26 cells.
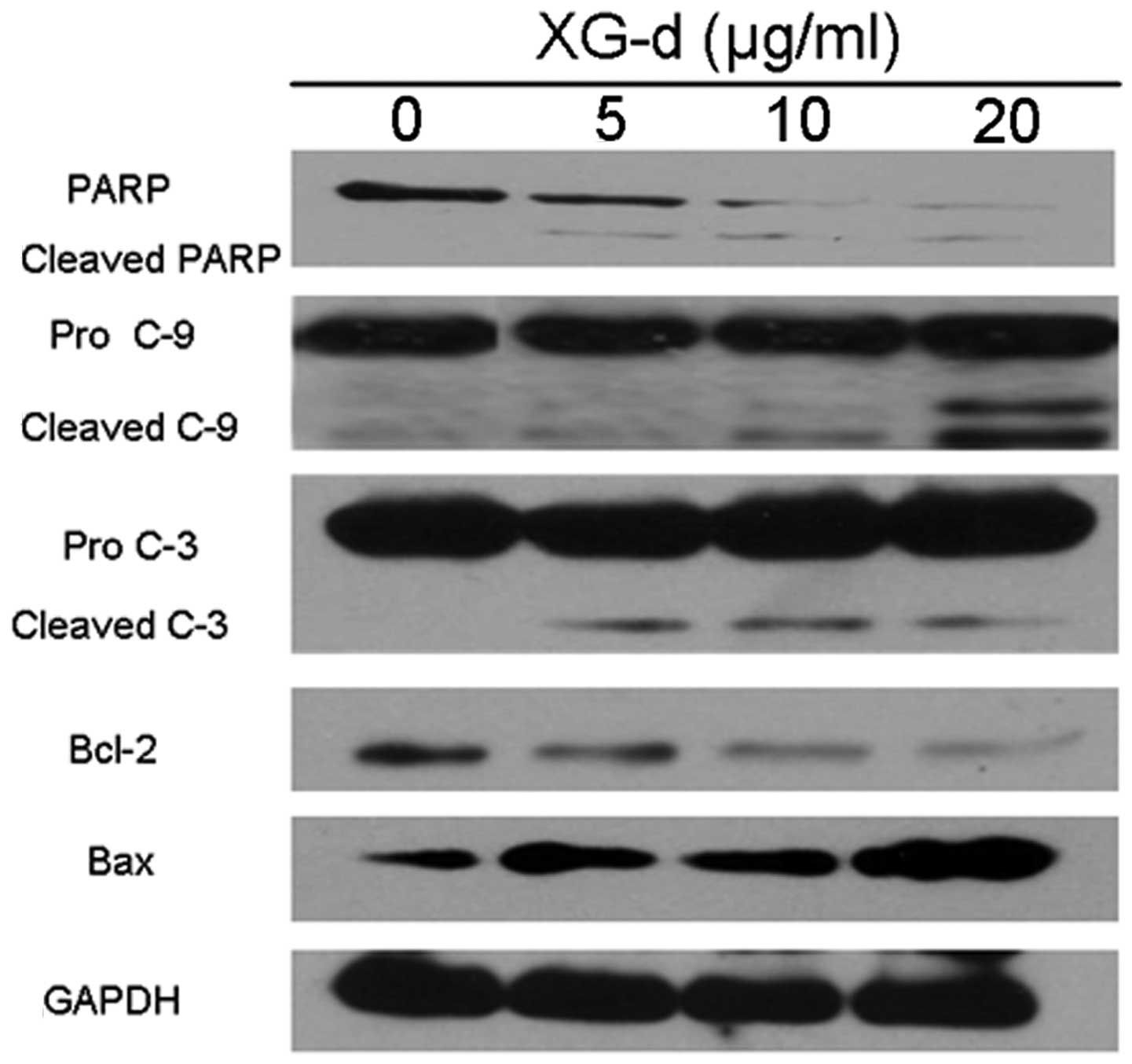
XG-d effects the expression of
apoptosis-associated proteins
To evaluate the potential pathways responsible for
the effects of XG-d, the expression of key apoptosis-associated
proteins in C26 cells were examined (Fig. 5). PARP, a nuclear enzyme involved
in DNA repair, was cleaved into 89 kDa fragments. Caspase-9 and
caspase-3 were also cleaved and accumulated following XG-d
treatment. Furthermore, the expression of Bcl-2 was decreased,
while the expression levels of Bax were upregulated. These results
indicated that XG-d induced the caspase-dependent and
mitochondria-mediated apoptotic pathways.
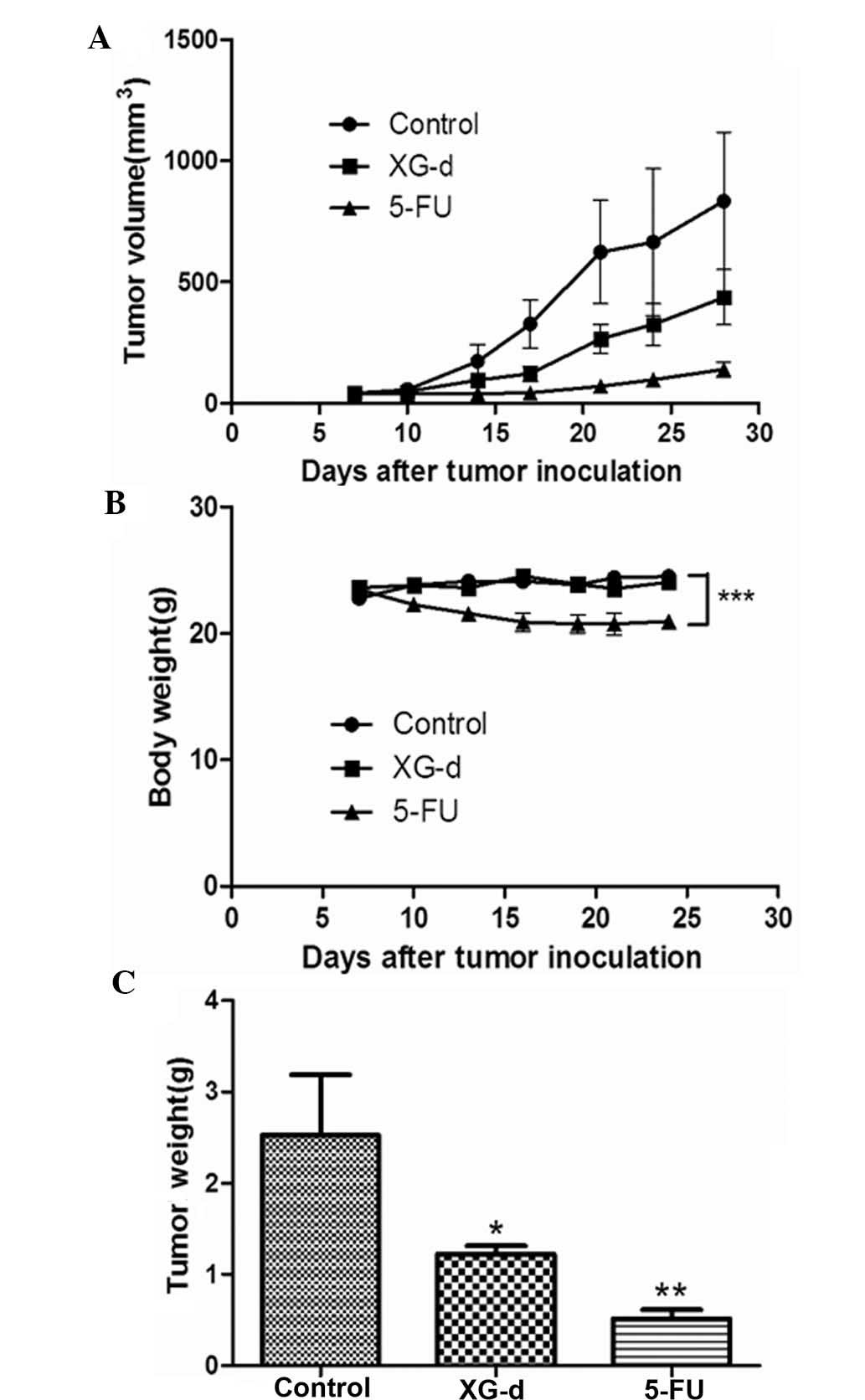
XG-d inhibits colon tumor growth in
vivo
In the present study, a mouse tumor model of colon
cancer was used to examine the effect of XG-d on tumor growth in
vivo. Each mice was subcutaneously injected with
1.0×106 C26 cells. Following establishment of the tumor
(~50 mm3), the anti-tumor effects of oral administration
of 100 mg/kg XG-d to the mice were evaluated. Oral administration
of 5-FU (30 mg/kg) was used as a positive control and oral
administration of 0.5% carboxymethyl cellulose sodium was used as
the normal control. As indicated in Fig. 6A, after 3 weeks treatment, the
average tumor size in the vehicle control group was 834.8±284.1
mm3, whereas the average tumor size in the XG-d- and
5-FU-treated groups were 436.8±114.1 and 138.9±31.3 mm3,
respectively. The weight of the mice was also measured over the
course of the treatment period. No significant difference was
observed between the body weights of the vehicle control or
XG-d-treated groups, however, the body weights of the mice in the
5-FU-treated group were decreased (Fig. 6B). The tumor weight of the XG-d-
and 5-FU-treated mice after 3 weeks of treatment were significantly
reduced compared with that of the vehicle control (Fig. 6C). These results revealed that the
oral administration of XG-d inhibited C26 cell tumor growth.
Discussion
In the last 20 years, herbal plant compounds have
been studied in order to elucidate their potential antitumor
activity (22). XG-d is an
effective tyrosinase inhibitor, which is frequently used to whiten
skin (23,24). In the present study, it was
revealed that XG-d potentially suppresses C26 cell proliferation
in vitro; in addition, these results indicated that XG-d
induces C26 cell apoptosis in a dose- and time-dependent manner.
Furthermore, XG-d upregulated the expression of Bax, downregulated
the expression of Bcl-2 and activated caspase-9, caspase-3 and PARP
proteins.
Inducing cellular apoptosis is an important strategy
in treating cancer. The anticancer action of numerous
chemotherapeutic agents have been found to cause cell death via the
induction of apoptosis (25,26).
Caspases have a critical role in apoptosis. In response to
apoptotic stimuli, the mitochondrial membrane becomes permeable,
leading to the release of cytochrome-C into the cytosol.
Subsequently, cytochrome-C activates the caspase-9 and caspase-3
pro-enzymes, inducing a caspase signaling cascade and resulting in
cell apoptosis (27,28). Among the known members of the
interleukin-1-converting enzyme family of proteases, the key
component of the apoptotic mechanism is caspase-3 (29). Caspase-3 cleaves multiple cellular
proteins, including PARP, the cleaved form of which is associated
with apoptosis (30). In the
present study caspase-9 and caspase-3 were found to be activated,
while PARP was cleaved. These findings suggested that XG-d
increased the percentage of apoptotic cells in a dose-dependent
manner. Therefore, XG-d may induce the apoptosis of C26 cells via
the activation of caspases.
The Bcl-2 family is one of the most important
regulators of apoptosis (31,32)
and has a critical role in the mitochondrion-mediated pathway. In
humans, >20 members of the Bcl-2 family have been identified,
including Bcl-2, Bcl-XL, Bcl-1, Bax and Bcl-2-antagonist/killer 1
(33–35). Changes in the expression levels of
Bcl-2, an anti-apoptotic protein, and Bax, a pro-apoptotic protein,
is often used as an index of apoptosis (36,37).
The results of the present study demonstrated that XG-d
downregulated the expression of Bcl-2 and enhanced the activity of
Bax. The ratio of Bax/Bcl-2, suggested that XG-d induced apoptosis
in the C26 cells via the mitochondrial pathway.
In conclusion, the results of the present study
indicated that XG-d induced cell death in the C26 cell line by
activating the regulation of caspases and the Bcl-2 family
member-dependent mitochondrial pathway. The cytotoxicity of XG-d
against several cancer cell lines, in particular the C26 murine
colon carcinoma cell line, was observed, however, it had low toxic
effects on two normal human cell lines. Furthermore, the effects of
XG-d on tumor growth in vivo revealed that XG-d may be a
promising novel anticancer agent.
Acknowledgments
The present study was supported by the China
National ‘12.5’ Foundation (no. 2011BAJ07B04) and the National
Natural Science Foundation of China (no. 20972105).
References
|
1
|
Jemal A, Murray T, Ward E, et al: Cancer
statistics, 2005. CA Cancer J Clin. 55:10–30. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Center MM, Jemal A, Smith RA and Ward E:
Worldwide variations in colorectal cancer. CA Cancer J Clin.
59:366–378. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jemal A, Bary F, Center MM, et al: Global
cancer statistic. CA Cancer J Clin. 61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Saif MW: Targeted agents for adjuvant
therapy of colon cancer. Clin Colorectal Canc. 6:46–51. 2006.
View Article : Google Scholar
|
|
5
|
Macdonald JS: Adjuvant therapy of colon
cancer. CA Cancer J Clin. 49:202–219. 1999. View Article : Google Scholar
|
|
6
|
Longley DB, Harkin DP and Johnston PG:
5-fluorouracil: mechanisms of action and clinical strategies. Nat
Rev Cancer. 3:330–338. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Delval L and Klastersky J: Optic
neuropathy in cancer patients. Report of a case possibly related to
5 fluorouracil toxicity and review of the literature. J Neurooncol.
60:165–169. 2002. View Article : Google Scholar
|
|
8
|
Meregalli M, Martignoni G, Frontini L, et
al: Increasing doses of 5-fluorouracil and high-dose folinic acid
in the treatment of metastatic colorectal cancer. Tumori.
84:662–665. 1998.
|
|
9
|
Ghobrial IM, Witzig TE and Adjei AA:
Targeting apoptosis pathways in cancer therapy. CA Cancer J Clin.
55:178–194. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ziegler DS and Kung AL: Therapeutic
targeting of apoptosis pathways in cancer. Curr Opin Oncol.
20:97–103. 2008. View Article : Google Scholar
|
|
11
|
Wong RSY: Apoptosis in cancer: from
pathogenesis to treatment. J Exp Clin Cancer Res. 30:872011.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Green DR and Kroemer G: The
pathophysiology of mitochondrial cell death. Science. 305:626–629.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Borner C: The Bcl-2 protein family:
sensors and checkpoints for life-or-death decisions. Mol Immunol.
39:615–647. 2003. View Article : Google Scholar
|
|
14
|
Adams JM and Cory S: The Bcl-2 apoptotic
switch in cancer development and therapy. Oncogene. 26:1324–1337.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Willis SN, Fletcher J, Kaufmann T, et al:
Apoptosis initiated when BH3 ligands engage multiple Bcl-2
homologs, not Bax or Bak. Science. 315:856–859. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tin MM, Cho CH, Chan K, et al: Astragalus
saponins induce growth inhibition and apoptosis in human colon
cancer cells and tumor xenograft. Carcinogenesis. 28:1347–1355.
2007. View Article : Google Scholar
|
|
17
|
Miller K, Wang ML, Gralow J, et al:
Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic
breast cancer. New Engl J Med. 357:2666–2676. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Phillips PA, Sangwan V, Borja-Cacho D, et
al: Myricetin induces pancreatic cancer cell death via the
induction of apoptosis and inhibition of the phosphatidylinositol
3-kinase (PI3K) signaling pathway. Cancer Lett. 308:181–188. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Morimoto S, Nonaka GI and Nishioka I:
Tannins and related compounds. LX. Isolation and characterization
of proanthocy-anidins with a doubly-linked unit from Vaccinium
vitis-idaea L. Chem Pharm Bull. 36:33–38. 1988. View Article : Google Scholar
|
|
20
|
Fokina GI, Roĭkhel’ VM, Frolova MP, et al:
The antiviral action of medicinal plant extracts in experimental
tick-borne encephalitis. Vopr Virusol. 38:170–173. 1993.In Russian.
PubMed/NCBI
|
|
21
|
Tong QY, Qing Y, Shu D, et al: Deltonin, a
steroidal saponin, inhibits colon cancer cell growth in vitro and
tumor growth in vivo via induction of apoptosis and
antiangiogenesis. Cell Physiol Biochem. 27:233–242. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jeone SJ, Koh W, Kim B and Kim SH: Are
there new therapeutic options for treating lung cancer based on
herbal medicines and their metabolites. J Ethnopharmacology.
138:652–661. 2011. View Article : Google Scholar
|
|
23
|
Wang J, Zhao XZ, Qi Q, et al:
Macranthoside B, a hederagenin saponin extracted from Lonicera
macranthoides and its anti-tumor activities in vitro and in vivo.
Food Chem Toxicol. 47:1716–1721. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hu M, Xu L, Yin LH, et al: Cytotoxicity of
dioscin in human gastric carcinoma cells through death receptor and
mitochondrial pathways. J Appl Toxicol. 33:712–722. 2013.
View Article : Google Scholar
|
|
25
|
Cai SX, Drewe J and Kasibhatla S: A
chemical genetics approach for the discovery of apoptosis inducers:
from phenotypic cell based HTS assay and structure-activity
relationship studies, to identification of potential anticancer
agents and molecular targets. Curr Med Chem. 13:2627–2644. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hickman JA: Apoptosis induced by
anticancer drugs. Cancer Metastasis Rev. 11:121–139. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Desagher S and Martinous JC: Mitochondria
as the central control point of apoptosis. Trends Cell Biol.
10:369–377. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gabriel B, Sureau F, Casselyn M, et al:
Retroactive pathway involving mitochondria in electroloaded
cytochrome c-induced apoptosis. Protective properties of Bcl-2 and
Bcl-XL. Exp Cell Res. 289:195–210. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Maianski NA, Roos D and Kuijpers TW: Bid
truncation, bid/bax targeting to the mitochondria, and caspase
activation associated with neutrophil apoptosis are inhibited by
granulocyte colony-stimulating factor. J Immunol. 172:7024–7030.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Nuñez G, Benedict MA, Hu Y and Inohara N:
Caspases: the proteases of the apoptotic pathway. Oncogene.
17:3237–3245. 1998. View Article : Google Scholar
|
|
31
|
Duriez PJ and Shah G: Cleavage of
poly(ADP-ribose) polymerase: a sensitive parameter to study cell
death. Biochem Cell Biol. 75:337–349. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Cory S and Adams JM: The Bcl2 family:
regulators of the cellular life-or-death switch. Nat Rev Cancer.
2:647–656. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
33
|
Oltersdorf T, Elmore SW, Shoemaker AR, et
al: An inhibitor of Bcl-2 family proteins induces regression of
solid tumours. Nature. 435:677–681. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Murray A: Cyclin ubiquitination: the
destructive end of mitosis. Cell. 81:149–152. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Antonsson B and Martinou JC: The Bcl-2
protein family. Exp Cell Res. 256:50–57. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Reed JC: Double identity for protein of
the Bcl-2 family. Nature. 387:773–776. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Mirjolet JF, Barberi-Heyob M, Didelot C,
et al: Bcl-2/Bax protein ratio predicts 5-fluorouracil sensitivity
independently of p53 status. Br J Cancer. 83:1380–1386. 2000.
View Article : Google Scholar : PubMed/NCBI
|