Introduction
Sporadic colorectal cancer accounts for 75% of total
cases of colorectal cancer, and is the third most common cancer in
the world as well as the second leading cause of cancer mortality
(1). Screening tests are able to
reduce the mortality rate of colorectal cancer; however, a large
proportion of individuals do not undergo colorectal cancer
screening due to its inconvenience and high cost (2–5). The
increasing frequency of colorectal cancer worldwide makes the
prevention of this disease an urgent problem to be solved.
Previous studies suggested that
fructooligosaccharides (FOS) can inhibit the process of colon
cancer development, mainly by its prebiotic effect and the
short-chain fatty acids (SCFA) produced during the fermentation of
FOS in the colon (6–9). FOS are important prebiotics, since
FOS cannot be digested in the small intestine of humans, but can be
fermented in the colon to form short-chain fatty acids (SCFA) and
lactic acid (10). FOS have
recently attracted attention due to their beneficial physiological
effects. These include relief of constipation, improved mineral
absorption, as well as reduced blood levels of total cholesterol,
triglycerides and phospholipids (8,11,12).
FOS are classified into three categories:
1F-, 6F- and 6G-FOS (13–15).
Among these FOS, only 1F-FOS is commercially available.
However, 6G-FOS, also called neo-FOS, has fructosyl
units bound at the β (2→6) position in the glucose moiety of
sucrose. Hence, neo-FOS has superior bifidogenecity as well as
better heat and chemical stability than 1F-FOS (16,17).
Neo-FOS comprises neokestose and neonystose (18); the structure of neokestose,
together with the structures of 1-kestose (a 1F-FOS) and
6-kestose (a 6F-FOS), is shown in Fig. 1.
When FOS are ingested by patients with colorectal
cancer, they may come into contact with cancer cells prior to being
fermented by bifidobacteria in the colon. Among various FOS,
neokestose was selected as the candidate in the present study, as
it is the prebiotic with the highest potency, as mentioned above.
The effects of neokestose on the colon cancer cell line Caco-2 were
investigated, and the potential applications of neokestose for the
dietary chemoprevention of colorectal cancer were also evaluated.
The present study aimed to determine the biological anti-cancer
activity of neokestose towards the colon cancer cell line Caco-2,
since neokestose is the most potential prebiotic among the
fructooligosaccharides. For this, neokestose was produced using
yeast fermentation of sucrose, isolated using high-performance
liquid chromatography (HPLC), and its anti-cancerous effect against
Caco-2 cells was assessed using MTT and flow cytometric assays as
well as western blot analysis. The results indicated that
neokestose may be an effective dietary chemopreventive against
colorectal cancer.
Materials and methods
Preparation of neokestose
As previously reported, neo-FOS was produced by the
culture of Xanthophyllomyces dendrorhous (BCRC 22367;
Bioresource Collection and Research Center, Hsinchu, Taiwan), using
250 g/l sucrose as the substrate (15). Neokestose was purified by HPLC on a
semipreparative YMC-pack ODS-AQ column (20×250 mm; YMC Co., Ltd.,
Kyoto, Japan) with a Waters 410 differential refractive index
detector (Waters Corporation, Milford, MA, USA). Water at a flow
rate of 8 ml/min was used as the mobile phase. A 200-μl
sample of neo-FOS (containing 109 g/l neokestose) was injected for
the purification of neokestose. The respective peak fractions were
collected and concentrated by rotary evaporation.
Cell culture
The Caco-2 cell line (BCRC 60182) was obtained from
the Bioresource Collection and Research Center. Cells were
routinely maintained and subcultured in 10 cm2-dishes at
37°C in a humidified CO2 incubator (95% air and 5%
CO2). The medium for cell maintenance consisted of 10%
heat-inactivated fetal bovine serum and 100 U/ml penicillin and 100
g/ml streptomycin in Dulbecco’s modified Eagle’s medium (DMEM)
solution, which are all from HyClone Laboratories, Inc. (Logan, UT,
USA). When cells were 80% confluent, they were subcultured using
0.25% trypsin and 0.02% EDTA in D-Hanks’ balanced salt solution
(HyClone Laboratories, Inc.). The medium was replaced every 48
h.
MTT assay
MTT (Sigma, St Louis, MO) is a tetrazolium salt that
can be cleaved by mitochondrial dehydrogenase in living cells. The
effects of neokestose (0, 0.5, 1.0 or 2.5 mg/ml) on cell growth
were tested using the MTT assay. Caco-2 cells were cultured in
96-well plates at a density of 1.0×104 cells/well for
two days, then the medium was discarded, 20 μl MTT solution
[0.5 mg/ml in phosphate-buffered saline (PBS)] was added to all
wells, and the cells were incubated for 2 h. At the end of the
incubation, 100 μl dimethylsulfoxide was added to each well
to lyse the cells, and the plates were transferred to a microplate
reader (Synergy™ HT; BioTek, Winooski, VT, USA). Absorbance was
recorded at 570 nm. All experiments were performed at least four
times with four wells of each concentration.
Cell cycle analysis
To analyze the cell cycle distribution, cells were
washed twice with PBS, collected by centrifugation at 725 x g for 5
min at 4°C, and fixed in 70% (v/v) ethanol at 4°C for 30 min. After
fixation, cells were resuspended in PBS and stained with propidium
iodide (PI; Sigma-Aldrich, St. Louis, MO, USA) solution (containing
48 μg/ml propidium iodide and 48 μg/ml RNase A;
Invitrogen Life Technologies, Carlsbad, CA, USA) for 20 min. The
DNA content of the cells was examined by flow cytometry
(FACSCalibur; BD Biosciences, Franklin Lakes, NJ).
Apoptosis analysis
Apoptosis was measured by using a fluorsecein
isothiocyanate (FITC) Annexin V Apoptosis Detection Kit I (BD
Biosciences) according to the manufacturer’s instructions. Briefly,
cells were washed twice with PBS and collected by centrifugation at
725 x g for 5 min at 25°C. Cells were resuspended in 100 μl
of binding buffer and labeled with 5 μl FITC Annexin V and 5
μl PI solution for 15 min in the dark. After labeling, cells
were resuspended in 400 μl binding buffer and detected by
flow cytometry (FACSCalibur).
Western blot analysis
Caco-2 cells were lysed with Cell Extraction Buffer
(M-PER® Mammalian Protein Extraction Reagent, Thermo
Fisher Scientific, Waltham, MA, USA). The bicinchoninic acid
protein assay kit (cat. no. 23225; Thermo Fisher Scientific) was
used to measure the protein concentration. The cell extracts were
subjected to 10% SDS-PAGE. Following electrophoresis, the proteins
were transferred onto a nitrocellulose membrane (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). After blocking with 3%
bovine serum albumin (Sigma-Aldrich) in Tris-buffered saline (20 mM
Tris, pH 7.5, 150 mM NaCl) containing 0.1% Tween 20 (T-TBS) for 1
h, nitrocellulose membranes were incubated with the following
primary antibodies (all diluted 1:1,000): Rabbit monoclonal
anti-COX-2 (cat. no. 12282; Cell Signaling Technology, Danvers, MA,
USA) mouse monoclonal anti-NF-κB (cat. no. MA5-16160; Thermo Fisher
Scientific) and mouse monoclonal anti-actin (cat. no. MA1-744;
Thermo Fisher Scientific), for 8 h at 4°C and then washed in T-TBS.
The secondary antibody, horseradish peroxidase-conjugated rabbit
anti-mouse IgG(H+L) (cat. no. 315-035-003; Jackson ImmunoResearch
Laboratories, Inc., West Grove, MA, USA) or mouse anti-rabbit
IgG(H) (cat. no. GTX628140-01; GeneTex, Inc., Irvine, CA, USA), was
incubated with the membranes at a dilution of 1:2,000 in T-TBS.
After washing, the antibody complexes were detected by
chemiluminescence using ECL reagents (Clarity™ Western ECL
Substrate; Bio-Rad Laboratories, Inc.) and ImageQuant LAS 4000 (GE
Healthcare Bio-Sciences, Pittsburgh, PA, USA).
Statistical analysis
The results of multiple experiments are expressed as
the mean ± standard error. Differences between the treatment and
control groups were analyzed using Student’s t-test. A
P<0.05 was considered to indicate a statistically significant
difference, which was denoted by asterisks in the figures.
Results
Preparation of neokestose
Neokestose was isolated from neo-FOS, which is
produced from sucrose by the culture of Xanthophyllomyces
dendrorhous BCRC 22367, which has
6G-fructofuranosidase (6G-FFase) activity
(15). Fig. 2 shows the HPLC chromatogram of the
neo-FOS mixture. Peak separation for the neo-FOS components was
achieved. In Fig. 2, peak 6
indicates the neokestose fraction, which was the major component of
the neo-FOS mixture. Approximately 20 mg pure neokestose was
obtained from every HPLC run. The neo-FOS product consisted of
77.0% neokestose, 10.4% neonystose, 2.8% 1-kestose, 4.5%
monosaccharides (glucose and fructose) and 5.3% sucrose on a dry
weight basis. This product is adequate for large-scale preparation
of neokestose.
Effect of neokestose on cell
viability
The antiproliferative effect of neokestose on Caco-2
cells was measured by an MTT assay. Caco-2 cells were treated with
different concentrations (0–2.5 mg/ml) of neokestose for 48 h, and
the obtained results are shown in Fig.
3. In Fig. 3, the cell
viability of the Caco-2 cells was decreased from 100% to ~59% as
the concentration of neokestose was increased from 0 to 2.5 mg/ml.
The results showed that Caco-2 cells treated with neokestose
exhibited a significant and dose-dependent loss of viability.
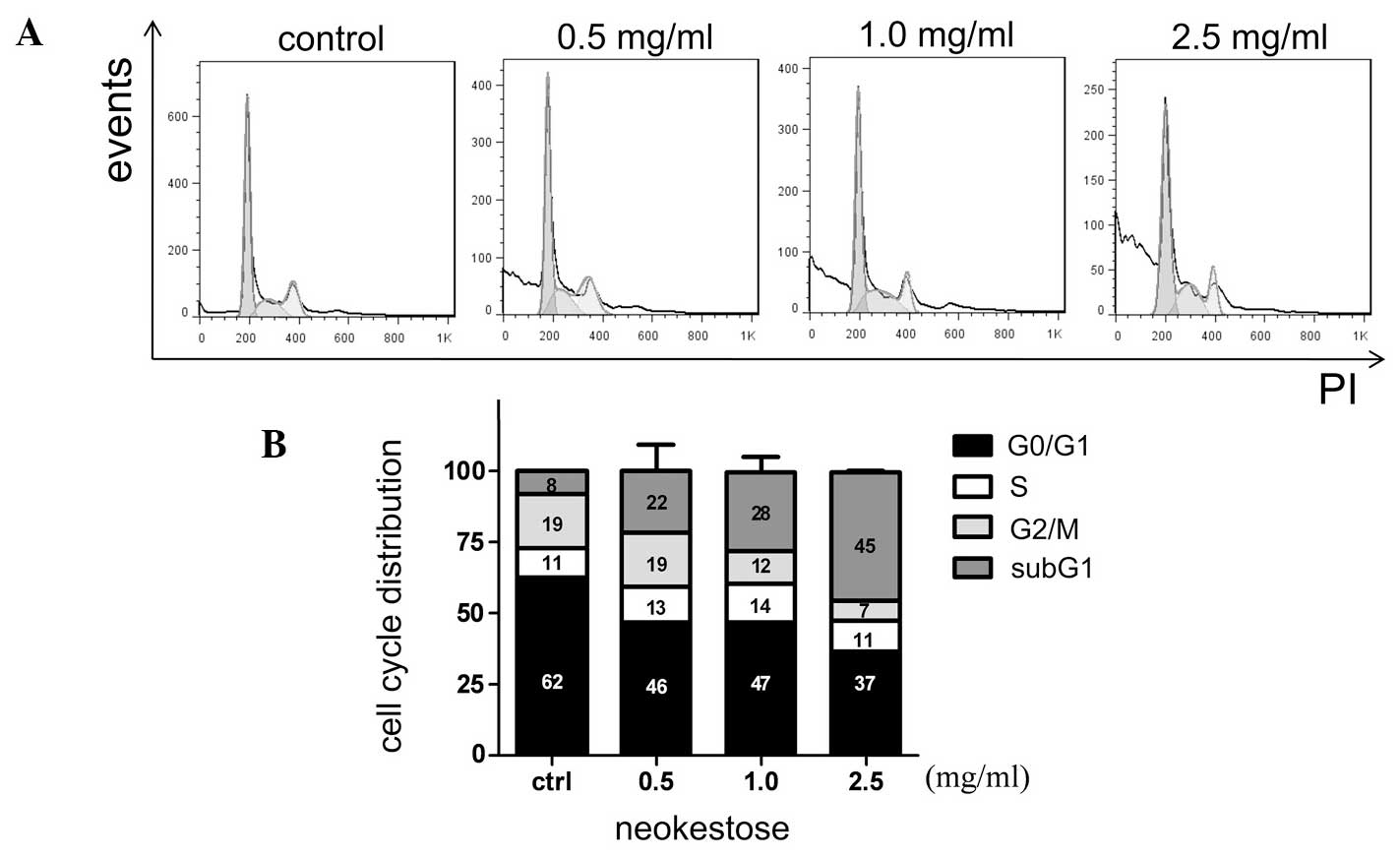
Effect of neokestose on the sub-G1
population and tumor cell apoptosis
Caco-2 cells were treated with neokestose for 48 h,
and the cell cycle distribution and apoptosis were measured by flow
cytometric analysis. Neokestose treatment significantly increased
the sub-G1 phase population (Fig.
4). Similarly, neokestose treatment also dramatically increased
the percentage of the late apoptotic cells, according to the
PI-Annexin V staining assay (Fig.
5).
Fig. 4 clearly
indicates that the sub-G1 phase population increased from 8, 22, 28
and 45% when the Caco-2 cells were treated by an increasing
concentrations of neokestose from 0 (control), 0.5, 1.0 and 2.5
mg/ml. In the flow cytometric cell cycle distribution curve, the
sub-G1 area represents a population of cells with a reduced DNA
content, which is a marker for apoptosis. Therefore, the
significantly increased sub-G1 phase population indicates
increasing apoptosis in the Caco-2 cells.
In addition, as mentioned above, apoptosis was
further verified by annexin V-FITC/PI double staining using a flow
cytometry. Therefore, neokestose-treated Caco-2 cells were stained
with PI and annexin V-FITC to determine the apoptotic rate of the
cells. Cells were distiguished as viable (annexin V-FITC, PI double
negative), early apoptotic (annexin V-FITC positive, PI negative)
and late apoptotic (annexin V-FITC, PI double positive) cells. In
Fig. 6, it can be clearly seen
that when the Caco-2 cells were treated with neokestose at
concentrations of 0 (control), 0.5, 1.0 and 2.5 mg/ml, the
percentage of early apoptotic cells was 8.3, 10.9, 9.0 and 8.7%,
respectively, along with percentages of late apoptotic cells of
15.3, 25.3, 27.2 and 31.2%, respectively. This indicated that
neokestose efficiently induced apoptosis of Caco-2 cells.
From the results, it was evident that neokestose
affected cell cycle progression and apoptosis in colorectal cancer
cells.
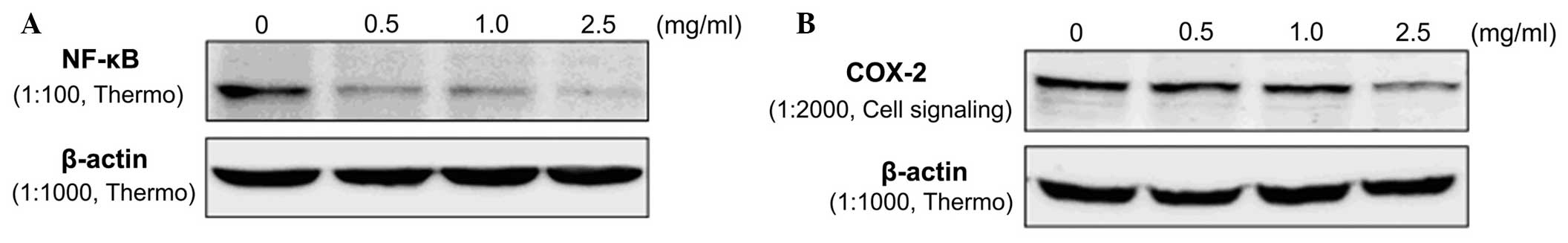
Effect of neokestose on expression of
NF-κB and COX-2
To evaluate potential molecular mechanisms causing
apoptosis, the expression of NF-κB and COX-2 was assessed by
western blot analysis (19,20).
Caco-2 cells were treated with neokestose at concentrations of 0
(control), 0.5, 1.0 or 2.5 mg/ml for 48 h. Protein was then
extracted and analyzed by western blotting. Fig. 6 shows that NF-κB and COX-2 were
overexpressed in the Caco-2 cell line; however, following treatment
with neokestose, the expression of NF-κB and COX-2 protein was
significantly reduced in a dose-dependent manner.
Discussion
In the present study, neokestose produced by the
conversion of sucrose by Xanthophyllomyces dendrorhous BCRC
22367 induced apoptosis in the colorectal cancer cell line Caco-2.
Flow cytometric analysis showed that neokestose increased the
population of Caco-2 cells in sub-G1 phase, and increase the
percentage of annexin V-FITC-positive cells. These results
indicated that neokestose induced apoptosis of Caco-2 cells in a
dose-dependent manner, which is consistent with the results of the
cell viability test.
NF-κB and COX-2 are commonly used markers of cell
apoptosis. Therefore, the protein expression of NF-κB and COX-2 in
Caco-2 cells treated with various concentrations of neokestose was
evaluated. The results showed that the expression of NF-κB and
COX-2 was decreased in Caco-2 cells treated with neokestose. NF-κB
is a central molecule responsible for the transition from
inflammation to cancer (19), and
COX-2 has an important role in colorectal tumorigenesis (4,19).
Accordingly, the results of the present study demonstrated that
neokestose has a potent bifidogenic effect directed against
colorectal cancer.
In conclusion, the results of the present study
demonstrated that neokestose significantly induces apoptotic cell
death in a dose-dependent manner via inhibition of NF-κB and COX-2
expression in colorectal carcinoma cells. Accordingly, neokestose
may be used as a chemopreventive agent for colorectal cancer.
Ideally, an anti-cancer agent or chemopreventive should inhibit the
growth of cancer cells without affecting normal cell growth. To the
best of our knowledge, the present study was the first to provide
in vitro evidence that neokestose may serve as a potential
dietary chemopreventive agent for colorectal cancer.
Acknowledgments
The authors are grateful for the financial supports
provided by Tatung University and Taipei Medical University.
References
|
1
|
Pisani P, Bray F and Parkin DM: Estimates
of the world-wide prevalence of cancer for 25 sites in the adult
population. Int J Cancer. 97:72–81. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Arber N and Levin B: Chemoprevention of
colorectal cancer: ready for routine use? Curr Top Med Chem.
5:517–525. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dvory-Sobol H and Arber N:
Cyclooxygenase-2 as target for chemopreventive interventions: new
approaches. Cancer Biomark. 3:153–161. 2007.PubMed/NCBI
|
|
4
|
Manzano A and Pérez-Segura P: Colorectal
cancer chemo-prevention: is this the future of colorectal cancer
prevention? Scientific World Journal. 2012:327–341. 2012.
View Article : Google Scholar
|
|
5
|
Temraz S, Mukherji D and Shamseddine A:
Potential targets for colorectal cancer prevention. Int J Mol Sci.
14:17279–17303. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pool-Zobel B, van Loo J, Rowland I and
Roberfroid MB: Experimental evidences on the potential of prebiotic
fructans to reduce the risk of colon cancer. Brit J Nutr. 87(Suppl
2): 273–281. 2002. View Article : Google Scholar
|
|
7
|
Swennen K, Courtin CM and Delcour JA:
Non-digestible oligosaccharides with prebiotic properties. Crit Rev
Food Sci Nutr. 46:459–471. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sabater-Molina M, Larque E, Torrella F and
Zamora S: Dietary fructooligosaccharides and potential benefits on
health. J Physiol Biochem. 65:315–328. 2009. View Article : Google Scholar
|
|
9
|
Vargas AJ and Thompson PA: Diet and
nutrient factors in colorectal cancer risk. Nutr Clin Pract.
27:613–623. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Oku T, Tokunaga T and Hosoya N:
Nondigestibility of a new sweetener, ‘Neosugar’ in the rat. J Nutr.
114:1574–1581. 1984.PubMed/NCBI
|
|
11
|
Hidaka H, Eida T, Takizawa T, Tokunaga T
and Tashiro Y: Effects of fructooligosaccharides on intestinal
flora and human health. Bifidobacteria Microflora. 5:37–50. 1986.
View Article : Google Scholar
|
|
12
|
Ooi LG and Liong MT: Cholesterol-lowering
effects of probiotics and prebiotics: a review of in vivo and in
vitro findings. Int J Mol Sci. 11:2499–2522. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Maiorano AE, Piccoli RM, da Silva ES and
de Andrade Rodrigues MF: Microbial production of
fructosyltransferases for synthesis of prebiotics. Biotechnol Lett.
30:1867–1877. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
de Abreu M, Alvaro-Benito M, Sanz-Aparicio
J, Plou FJ, Fernandez-Lobato M and Alcalde M: Synthesis of
6-kestose using an efficient β-fructofuranosidase engineered by
directed evolution. Adv Synth Catal. 355:1698–1702. 2013.
View Article : Google Scholar
|
|
15
|
Sheu DC, Chang JY, Chen YJ and Lee CW:
Production of high-purity neofructooligosaccharides by culture of
Xanthophyllomyces dendrorhous. Bioresour Technol. 132:432–435.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kilian S, Kritzinger S, Rycroft C, Gibson
G and du Preez J: The effects of the novel bifidogenic
trisaccharide, neokestose, on the human colonic microbiota. World J
Microbiol Biotechnol. 18:637–644. 2002. View Article : Google Scholar
|
|
17
|
Lim JS, Lee JH, Kang SW, Park SW and Kim
SW: Studies on production and physical properties of neo-FOS
produced by co-immobilized Penicillium citrinum and
neo-fructosyltransferase. Eur Food Res Technol. 225:457–462. 2007.
View Article : Google Scholar
|
|
18
|
Linde D, Rodríguez-Colinas B, Estévez M,
Poveda A, Plou FJ and Fernández-Lobato M: Analysis of
neofructooligosaccharides production mediated by the extracellular
β-fructofuranosidase from Xanthophyllomyces dendrorhous. Biores
Technol. 109:123–130. 2012. View Article : Google Scholar
|
|
19
|
Karin M, Cao Y, Greten FR and Li ZW:
NF-kappaB in cancer: from innocent bystander to major culprit. Nat
Rev Cancer. 2:301–310. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Oshima M, Dinchuk JE, Kargman SL, Oshima
H, Hancock B, Kwong E, Trzaskos JM, Evans JF and Taketo MM:
Suppression of intestinal polyposis in Apc delta716 knockout mice
by inhibition of cyclooxygenase 2 (COX-2). Cell. 87:803–809. 1996.
View Article : Google Scholar : PubMed/NCBI
|