Introduction
Interferons (IFNs) are glycoproteins produced by the
immune system of host cells in response to infection of pathogens
(1–4). Various forms of interferons have been
evaluated as potential therapeutics in a number of solid tumors and
hematological malignancies, including renal anemia, renal cell
carcinoma, melanoma, acquired immune deficiency syndrome-associated
Kaposi’s sarcoma, follicular lymphoma, hairy cell leukemia and
chronic myelogenous leukemia (1).
INF-α and INF-β cytokines exert their antiviral function by
eliciting antiviral activity from target cells and inducing
apoptosis in infected cells. They also contribute to the immune
response by activation of natural killer cells and macrophages
(2–4). IFN-α was approved by the Food and
Drug Administration as a treatment for hairy cell leukemia in 1986
(1) and, using a gene cloning
method, pure IFN has been used in clinical investigations (1).
Erythropoietin (EPO) is an acidic glycoprotein
hormone with a molecular weight of 30–34 kDa. This protein
comprises 165 amino acids and contains multiple isoforms, which
differ in their carbohydrate structure (5). EPO is expressed in the liver during
the fetal stages. Following birth, it is predominantly synthesized
in the peritubular fibroblasts of the renal cortex (6). Under hypoxic conditions, EPO is
elevated in the plasma, and the induced EPO then stimulates the
synthesis of more red blood cells in the bone marrow and increases
the oxygen-carrying capacity of the blood (6,7). The
expression levels of EPO are inversely correlated with levels of
hematocrit and hemoglobin, and reflect the reciprocal association
between oxygen supply and EPO synthesis (8). EPO deficiency is the main cause of
anemia in patients with chronic kidney disease (CKD) and
contributes to anemia in patients with chronic inflammation and
cancer (8). According to a
previous study, anemia in patients with CKD or cancer may be
effectively corrected by treatment with recombinant EPO (9).
As cytokines, IFN-α and EPO have been used in the
treatment of certain hematological malignancies, including renal
anemia (1,8). Therefore, the production of
pharmaceutical recombinant human IFN-α and EPO is of value.
Importantly, these recombinant proteins require activation through
posttranslational modification (PTM) for purification and
therapeutic use (10). Due to the
absence of appropriate mechanisms for the PTM of exogenous protein
in prokaryotic cells, the production of recombinant proteins using
a prokaryotic cell culture system is less effective compared with
that of recombinant proteins using transgenic animals (11), which are termed bioreactors
expressing a transgene. Milk-producing animals, including cattle,
goats and sheep, are the optimal bioreactors as they produce large
quantities of a recombinant protein in their mammary glands
(10,12,13).
Using a promoter of milk-specific proteins,
including caseins and whey acidic proteins, numerous research
groups have generated various recombinant proteins (14–17).
In bovine milk, the quantity of αS1-, αS2-, β- and κ-casein,
α-lactalbumin and β-lactoglobulin comprises ~90% of the proteins
(18). The expression and
secretion of milk proteins in the mammary gland is regulated
through interactions between particular hormone-activated
transcription factors by steroid and peptide hormones, including
insulin, prolactin and hydrocortisone (19,20).
Promoters of the casein gene have binding sites for transcription
factors, including the glucocorticoid receptor and the
transcriptional activator (TA) CCAAT/enhancer binding protein
(21–24).
The unregulated expression of a target gene often
produces unexpected results through a constitutively active
promoter; for instance, overproduction of EPO results in
erythrocytosis (25). As excess
production of transgene can cause stillbirths and spontaneous
abortions in animal bioreactors, a conditional transgenic
technique, the tetracycline (tet)-on system, was used in the
present study for establishment of fibroblasts with transgenes
expressing mammary gland-specific human IFN-α or EPO. This system
enables temporal and spatial regulation of transgene expression and
requires a responder construct and activator construct in a single
cell (26). The activator
construct consists of a cytomegalovirus (CMV) promoter and TA,
which induces conformational change by tet or its analog,
doxycycline (dox) (27). The
responder construct contains the target gene and ZsGreen1 cDNA,
controlled by the TA-response element promoter. In the presence of
dox, conformationally changed TA binds to the tetracycline response
element (TRE) promoter of the responder construct. Finally, a
series of these processes induces the transcription of the target
gene and a green fluorescent protein reporter gene, as an indicator
of expression (28).
In the present study, a unitary vector system was
established, combining an activator cassette with a responder
cassette. The CMV promoter of the activator cassette was exchanged
for the bovine αS1-casein promoter, as the mammary tissue-specific
promoter. In addition, bovine transgenic fibroblasts were generated
containing a mammary gland-specific, dox-inducible human IFN-α or
EPO gene. These cells can be utilized as a source of somatic cell
nuclear transfer (SCNT) for the generation of animal bioreactors,
which produce large quantities of human IFN-α or EPO protein in
their milk.
Materials and methods
Animal care and ethics statement
Cattle were fed a standard commercial cow diet
(Suwon Purina, Suwon, Korea) and provided with water ad
libitum in accordance with the animal study guidelines of the
Sooam Biotech Research Foundation for Accreditation for Laboratory
Animal Care and Use. All surgery was performed under isoflurane
anesthesia (3–5%; Hana Pharm Co., Ltd., Seoul, South Korea), and
all efforts were made to minimize suffering. The current study was
approved by the ethics committee of Sooam Biotech Research
Foundation (Seoul, South Korea).
Cell culture
The MAC-T bovine mammary epithelial cell line, was
cultured in Dulbecco’s modified Eagle’s medium (DMEM; Gibco-BRL,
Gaithersburg, MD, USA) containing 25 mM glucose, supplemented with
10% fetal bovine serum (FBS; Welgene, Daejeon, South Korea), 100
U/ml penicillin and 100 μg/ml streptomycin (Welgene). Bovine
fibroblasts were obtained from a bovine fetus (Deutsche
Schwarzbunte) on day 30 of pregnancy through disaggregation by
0.05% trypsin (Welgene) treatment, and were maintained in DMEM
containing 25 mM glucose, supplemented with 10% FBS, 100 U/ml
penicillin and 100 μg/ml streptomycin. All the cells were
grown in a humidified 5% CO2 atmosphere at 37°C.
Vector construction
All restriction enzymes were obtained from Takara
Bio, Inc. The αS1-casein promoter region between nucleotides −175
and +796 demonstrated the highest transcriptional activity in a
previous study (29) and were
prepared by long-range PCR using the genomic DNA of bovine
fibroblasts as the template and specific primers containing
restriction enzyme sites (SpeI at the 5′ end or SacII
at the 3′ end), using an identical procedure to that described
above. The amplified fragments were digested using SpeI and
SacII and replaced with the CMV promoter of the TA plasmid,
pCMV-Tet3 G, purchased from Clontech (Mountain View, CA, USA). The
human IFN-α or EPO cDNA was prepared via PCR using cDNA amplified
from total human RNA (Clontech) as a template and was inserted into
pTRE3G-ZsGreen1, which contained the ZsGreen1 green fluorescence
protein (Clontech), through either MluI at the 5′ end or
BamHI at the 3′ end. The human IFN-α or EPO expressing
tTA-response region, obtained from the recombinant
pTRE3G-ZsGreen1-human IFN-α/EPO construct by PCR, was then digested
with either Bst1107I or SpeI and combined with the
recombinant pαS1P-Tet3 G vector, controlled by the bovine
αS1-casein promoter.
Establishment of transgenic cell
lines
The fibroblasts were transfected with the linearized
targeting vector using Lipofectamine™ 2000 (Invitrogen Life
Technologies, Carlsbad, CA, USA) according to the manufacturer’s
instruction and incubated for 4 h in a humidified CO2
atmosphere at 37°C, then the media was replaced with fresh complete
media (DMEM with 10% FBS and antibiotics). After one day, the
medium was replaced with DMEM supplemented with 10% FBS,
antibiotics and 500 μg/ml G-418 (Roche) for 4 weeks. The
antibiotic-resistant cells were further selected using PCR-based
genotyping (cycling conditions: Denaturation at 95°C for 30 sec,
annealing at 62°C for 30 sec and 30 cycles of extension at 72°C for
2 min)with confirming primers (Table
I) to identify the integration of target genes into the genomic
DNA of bovine fibroblasts.
 | Table IPrimer sequences for reverse
transcription polymerase chain reaction. |
Table I
Primer sequences for reverse
transcription polymerase chain reaction.
| Primer | Restriction
enzyme | Direction | Sequence
(5′-3′) |
|---|
| αS1-casein promoter
(−175 nt) | SpeI | Forward |
GGGACTAGTTAGAACAATGCCATTCCATTTCC |
| αS1-casein promoter
(+796 nt) | SacII | Reverse |
CCGCGGTGTGCTGGAAAAATGCGTTT |
| hIFN-α | MluI | Forward |
ACGCGTATGGCCTTGACCTTTGCTTTA |
| hIFN-α | BamHI | Reverse |
CCCGGATCCTCATTCCTTACTTCTTAAACTTTCT |
| hEPO | MluI | Forward | ACGCGTATG
GGGGTGCACGAATGTCC |
| hEPO | BamHI | Reverse |
GGATCCTCATCTGTCCCCTGTCCTGCA GG |
| Tet-response
element | BstZ17I | Forward |
GTATACCGAGGCCCTTTCGTCTTCAAGAATTC |
| Tet-response
element | SpeI | Reverse |
ACTAGTGCCGCAGACATGATAAGATACATTGA |
| Confirming primer
a | – | Forward |
TAGAACAATGCCATTCCATTTCC |
| Confirming primer
a′ | – | Reverse |
TTTCAGAAGTGGGGGCATAG |
| Confirming primer
b | – | Forward |
GAGGATGGAGCAGTTTGCAT |
| Confirming primer
b′ | – | Reverse |
GCATTCCACCACTGCTCCCA |
| Bovine GAPDH | – | Forward |
GGGTCATCATCTCTGCACCT |
| Bovine GAPDH | – | Reverse |
GGTCATAAGTCCCTCCACGA |
| hIFN-α | – | Forward |
TCCAAAAGGCTGAAACCATC |
| hIFN-α | – | Reverse |
CAGGCACAAGGGCTGTATTT |
| hEPO | – | Forward |
TCACTGTCCCAGACACCAAA |
| hEPO | – | Reverse |
CACTGACGGCTTTATCCACA |
Genomic DNA extraction and polymerase
chain reaction (PCR)
Genomic DNA from the cells was isolated using a
G-DEX™ IIc genomic DNA extraction kit (iNtRON Biotechnology, Seoul,
South Korea). Genomic DNA (0.1 μg) was amplified in a 20
μl PCR reaction containing 1 U i-Start Taq polymerase
(iNtRON Biotechnology), 2 mM dNTPs (Takara Bio Inc., Otsu, Japan)
and 10 pmol of each specific primer (Macrogene, Inc., Seoul, South
Korea). The details of all the primers are presented in Table I. The PCR reactions were as
follows: Denaturation at 95°C for 30 sec, annealing at 62°C for 30
sec, extension at 72°C for 2 min and 30 cycles of amplification
were conducted. The PCR products were then subjected to cloning
processes and/or separated on a 1% agarose gel (Roche,
Indianapolis, IN, USA), stained with ethidium bromide (Amresco
Inc., Solon, OH, USA) and images were captured and scanned under UV
illumination using a Bio-Rad GelDoc EQ System (Bio-Rad
Laboratories, Inc., Hercules, CA, USA).
Transient transfection and dox
treatment
Transient transfection was performed using
Lipofectamine™ 2000 (Invitrogen Life Technologies), according to
the manufacturer’s instructions. Briefly, 1.2×105 cells
were seeded in 6-well tissue culture plates 1 day prior to
transfection. In total, 4 μg of the recombinant constructs
was transfected into the cells in serum-free DMEM. Following
incubation for 4 h at 37°C, the medium was replaced with DMEM
containing 10% FBS, 100 U/ml penicillin and 100 μg/ml
streptomycin. Subsequently, various concentrations (0, 0.1 and 1.0
μg/ml) of dox were administered in transiently transfected
cells for an additional 24 h.
RNA preparation and reverse transcription
(RT)-PCR
The total RNA from the MAC-T cells was extracted
using TRIzol reagent (Invitrogen Life Technologies), according to
the manufacturer’s instructions. The concentration of the total RNA
was determined by measuring the absorbance at 260 nm using an
Epoch™ Multi-Volume Spectrophotometer System (BioTek, Inc.,
Winooski, VT, USA). First-strand cDNA was prepared by subjecting
total RNA (1 μg) to RT using Moloney murine leukemia virus
reverse transcriptase (Invitrogen Life Technologies) and random
primers (9-mers; Takara Bio, Inc.). To determine the optimal
conditions for logarithmic phase PCR amplification for target cDNA,
aliquots of total cDNA (1 μg) were amplified using different
numbers of cycles. The bovine GAPDH gene was amplified as the
internal control to rule out the possibility of RNA degradation and
to control for variations in mRNA concentrations. A linear
association between the PCR product band visibility and the number
of amplification cycles was observed for the target mRNA. The
bovine GAPDH gene and target genes were quantified using 25 and 30
cycles, respectively. The PCR reactions were denatured at 94°C for
30 sec, annealed at 58°C for 30 sec, and extended at 72°C for 30
sec. The PCR products were then separated on a 2.3% agarose gel and
stained with ethidium bromide. Images were then captured under UV
illumination and the images were scanned using GelDoc EQ (Bio-Rad
Laboratories, Inc.).
Results
Establishment of a unitary tet-on
IFN-α/EPO expression vector with a milk protein gene promoter
The constructed unitary tet-on system was composed
of the activator cassette and the responder cassette (Fig. 1A and B). The activator cassette
contained a TA under the control of the bovine αS1-casein promoter
(between −175 and +796 nt), which demonstrated the highest promoter
activity in a bovine MAC-T cell line in our previous study. The
responder cassette was composed of either the human IFN-α or EPO
gene, and linked with a gene for ZsGreen1 green fluorescence
protein. Following transcription of the TA in mammary gland cells
by tissue-specific activity of the bovine αS1-casein promoter, it
binds to the TRE promoter of the responder cassette following
conformational change by dox (26–28).
In this system, the human IFN-α/EPO was specifically expressed in
mammary tissue and expression of the ZsGreen1 green fluorescence
marker was observed in the presence of dox.
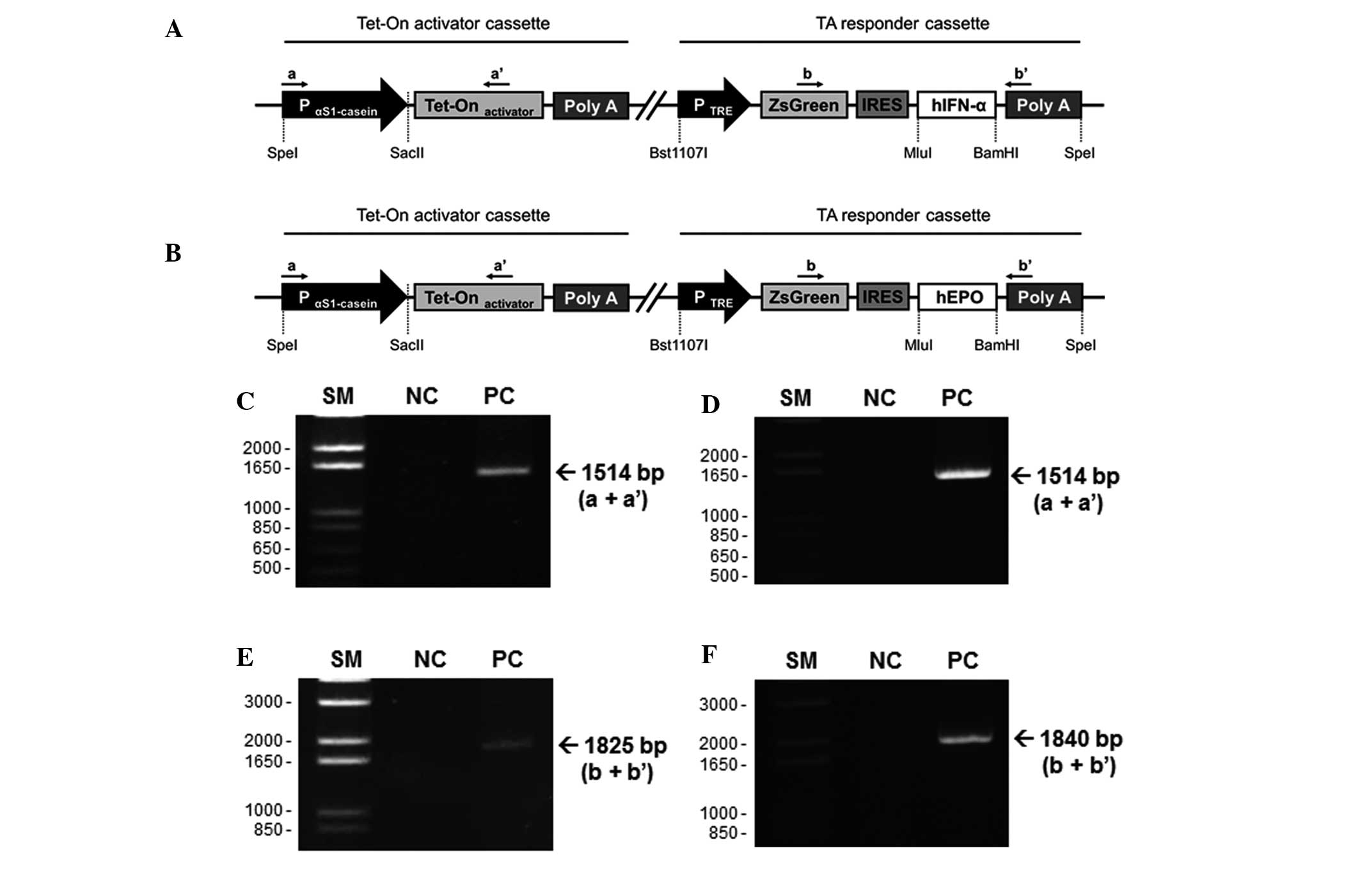 | Figure 1Schematic structure of the targeting
vector and PCR-based confirmation of transgenic fibroblasts. The
unitary tet-on system was composed of two regions in a vector; an
activator cassette and a responder cassette. The activator cassette
has tet-controlled TA under the control of the bovine αS1-casein
promoter (between −175 and +796 nt). The responder cassette
contained the target gene, (A) human IFN-α or (B) EPO, and ZsGreen1
cDNA, expressing green fluorescence protein, controlled by the
TA-response element promoter. These unitary vectors were linearized
and integrated into the genomic DNA of the bovine fibroblasts.
Genomic sDNA was isolated from the G418-resistant fibroblast
colonies and was identified using specific primer sets (a, a′, b
and b′; Table I). (C and D) PCR
products using primer a and a′ indicated the chromosomal insertion
of the activator cassette. (E and F) primers b and b′ were used to
confirm whether the same fibroblast colonies had the responder
cassette (IFNα, 1825 bp; EPO, 1840 bp). IRES, internal ribosomal
entry site; SM, size marker; NC, negative control; PC, positive
clone; PCR, polymerase chain reaction; EPO, erythropoietin; TA,
tet-on transcriptional activator; IFN, interferon. |
Generation of fibroblast cell lines
expressing the dox-inducible human IFN-α/EPO protein
The dox-inducible human IFN-α/EPO construct was
digested and linearized using Bst1107I. These constructs
were introduced into bovine fibroblasts using a liposomal-mediated
gene delivery system. The transfected fibroblasts were maintained
in medium containing G418 (500 μg/ml) for four weeks, and
the G418-resistant and stably cloned fibroblasts were selected. To
confirm chromosomal integration of the targeting vector, PCR was
performed using primer sets specific for the vector (Table I). The selected clones were
amplified using a and a′ primers (product size, 1,514 bp; Fig. 1C and D). The genomic DNA extracted
from the clones expressing human IFN-α was analyzed using b and b′
primers (product size, 1,825 bp; Fig.
1E). In addition, genomic DNA obtained from clones expressing
human EPO was evaluated using b and b′ primers (product size, 1,840
bp; Fig. 1F). Based on the results
of the gene cloning, five positive clones from the 29
G418-resistant and IFN-α-transfected clones, and 12 positive clones
from the 28 G418-resistant and EPO-transfected clones were obtained
in the two rounds of transfection, respectively (Table II). The fibroblasts from the
positive clones may serve as a cell source for SCNT for the
generation of a bovine bioreactor to produce recombinant human
IFN-α or EPO in its milk.
 | Table IITransfection efficiencies of the
porcine fibroblasts. |
Table II
Transfection efficiencies of the
porcine fibroblasts.
| Cell line | Trials (n) | G418-resistant
colony (n) | PCR-positive colony
(n) |
|---|
| Recombinant
hIFN-α | 2 | 29 | 5 |
| Recombinant
hEPO | 2 | 28 | 12 |
Observation of dox-inducible fluorescence
in MAC-T cells and bovine fetal fibroblasts
For the observation of tissue-specific expression,
transient transfection was performed in the MAC-T bovine mammary
epithelial cell line and bovine fibroblasts. These cells were
cotransfected with pCMV-TA and pTRE-ZsGreen1-IFN-α/EPO constructs
as a positive control to confirm the activity of the dox-inducible
expression system. No fluorescence was observed in either the
untreated or dox-treated bovine fetal fibroblasts transiently
transfected with pαS1CP-TA-TRE-ZsGreen-IFNα/EPO as a result of the
mammary gland specific casein promoter (Fig. 2A–D). However, in the MAC-T cells
expressing pαS1CP-TA-TRE-ZsGreen-IFNα/EPO, green fluorescence was
observed, indicating the expression of dox-inducible and mammary
gland-specific IFN-α/EPO (Fig.
2E–H). Green fluorescence was also confirmed following dox
treatment in a dose-dependent manner (0, 0.1, and 1
μg/ml).
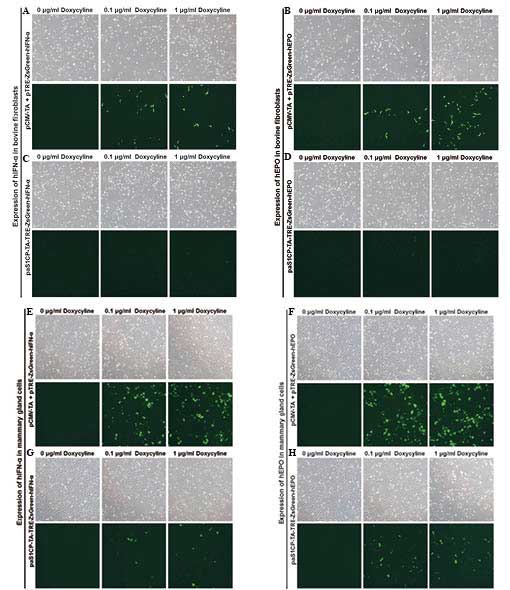 | Figure 2Observation of dox-inducible green
fluorescence in the (A–D) bovine fibroblasts and (E–H) MAC-T cells
using transient transfection of (A, B, E and F) pCMV-TA with
pTRE-ZsGreen-IFN-α/EPO or (C, D, G and H) unitary
pαS1CP-TA-TRE-ZsGreen-IFN-α/EPO (magnification, ×40). The Dox
concentration was increased between 0, 0.1 and 1 μg/ml. In
the MAC-T cells, dox-inducible and mammary gland specific
expression of green fluorescence by bovine α1S casein promoter was
observed. Additionally, the green fluorescence revealed a
dose-dependent increase in expression as the dox concentration
increased between 0, 0.1 and 1 μg/ml. Image were captured
using a bright field microscope and are representative of the cell
density and degree of fluorescent to non-fluorescent cells. CMV,
cytomegalovirus promoter; TA, tet-on transcription activator;
αS1CP, bovine α1S casein promoter; EPO, erythropoietin; IFN-α,
interferon-α; Dox, doxycycline; MAC-T, mammary epithelial cell
line. |
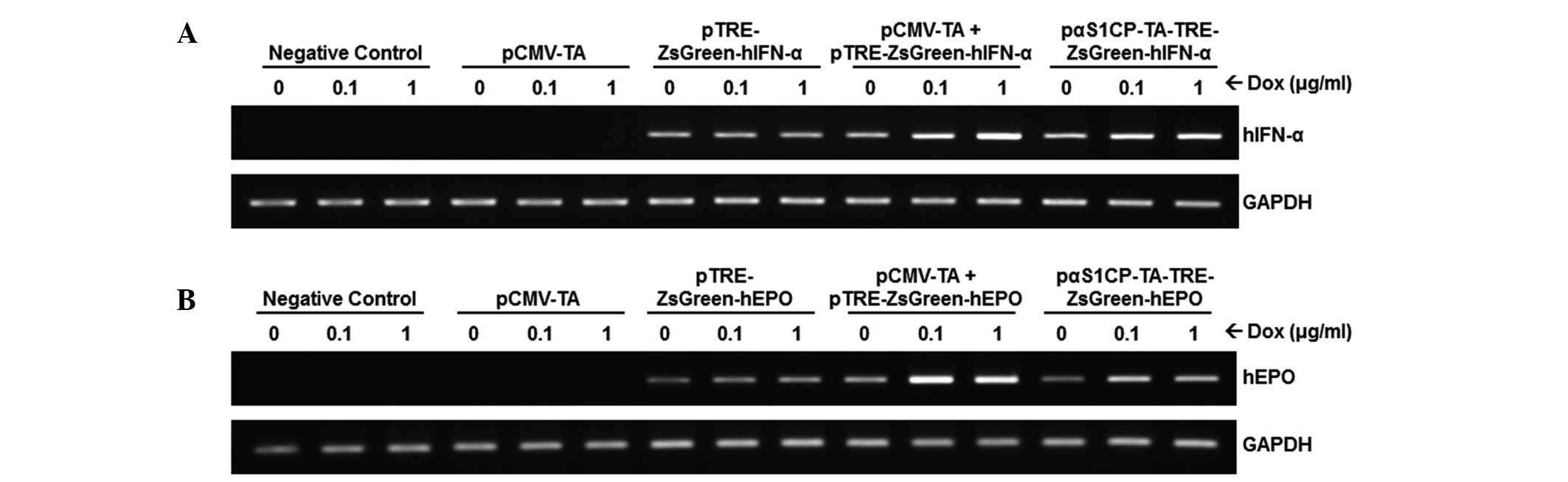
Dox-inducible mRNA expression of human
IFNα/EPO in MAC-T cells
To evaluate the dox-inducible expression of IFNα/EPO
under the control of the αS1 casein promoter, RT-PCR was performed
using mRNA obtained from transiently transfected MAC-T cells and
bovine fetal fibroblasts (data not shown). In parallel with the
results observed in the fluorescence microscopy, dox-dose dependent
and mammary gland-specific transcripts of IFNα/EPO were observed in
the MAC-T cells (Fig. 3) following
dox treatment, however, this was not observed in the bovine
fibroblasts (data not shown). In addition, the expression of
IFNα/EPO in cells transfected with pCMV-TA or
pTRE-ZsGreen1-IFNα/EPO exhibited no notable changes with or without
dox. Although the expression of IFNα/EPO in the MAC-T cells
expressing the pTRE-ZsGreen1-IFNα/EPO transgene was observed, its
level was consistently low and unaffected by dox treatment. Factors
in the transcriptional environment, which remain to be elucidated,
may control the gene expression in an in vitro system
introducing the target gene.
Discussion
IFN is an important cytokine for immune responses
against viral and bacterial infections and the mediation of
anticancer activity either indirectly, through regulation of
anti-inflammatory and anti-angiogenic responses, or directly, by
affecting the proliferation and differentiation of cancer cells
(30). IFN-α/β is known to be
essential in the activation of natural killer cells and macrophages
(2–4). As key cytokines, IFN-α/β links the
innate and adaptive immune systems (31,32).
Another cytokine involved in the maintenance of red blood cell mass
is EPO, which is involved in the proliferation and differentiation
of erythrocytic progenitors (8).
If plasma oxygen levels are low, EPO, which is released by the
interstitial cells of the kidney, stimulates bone marrow to produce
more red blood cells and increases the aerobic capacity of blood
(6,7).
IFN-α and EPO are glycoproteins, which are major
therapeutic agents in the treaent of certain hematological
malignancies, including chronic renal anemia and other diseases
(1,8). The mass production of these
therapeutic agents is important in the pharmaceutical industry.
Recombinant DNA technology has been widely used to obtain
therapeutic agents in eukaryotic cell-based culture systems
(33,34). However, the productivity of these
systems is limited in large-scale production. Although the
productivity of a prokaryotic system is sufficient for the
generation of recombinant proteins, the functional activity of
recombinant proteins is restricted due to an absence of protein
post-translational modification (11). Therefore, it was hypothesized that
the generation of transgenic cattle, termed bioreactors, is a more
effective method for the production of relatively large quantities
of recombinant therapeutic agents. This technology using livestock
has significant potential and economic merit in biomedicine,
agriculture, human health industries and environmental
sustainability (35,36).
In the present study, bovine fibroblast cell lines
expressing human IFN-α/EPO transgenes were developed in order to
generate transgenic cattle. Milk is the easiest body fluid to
obtain from ruminants (35). The
capacity for mass-production of the mammary gland, coupled with the
relative ease of harvesting milk, means it is the organ of choice
for the production of pharmaceutical products from animals. In our
previous study, a αS1-casein promoter region spanning between −175
and +796 nt was assessed, which demonstrated the highest expression
activity among all the assessed promoters (29). Therefore, this promoter was applied
in the present study for the generation of bovine transgenic
fibroblasts containing dox-inducible human IFN-α and EPO genes, as
a material for SCNT procedures in the production of an animal
bioreactor.
IFN-α and EPO are useful therapeutic agents, As
over-expression by constitutively active promoters may cause
unexpected side effects, including erythrocytosis involved with the
induction of EPO (25), a
dox-inducible expression system was adopted in parallel with a
tissue-specific promoter in the present study. The expression of
mammary gland-specific and dox-inducible human IFN-α or EPO was
observed in an MAC-T bovine mammary epithelial cell line, which is
an immortalized epithelial cell line isolated from bovine mammary
tissue (37). Mammary epithelial
cells are the functional unit of the mammary gland. MAC-T cells
provide a useful tool in the evaluation of foreign gene expression
and the assessment of mammary tissue-specific expression in
vitro, as they retain a number of biochemical and morphological
characteristics of the mammary gland in vivo (38). The unitary tet-on IFN-α/EPO
induction system has mammary gland-specific and dox-inducible
traits. When generating animal bioreactors, these cellular traits
may reduce rates of stillbirth during pregnancy and unwanted
outcomes caused by uncontrollable gene expression.
For the generation of transgenic cattle, these
transgenic fibroblasts may be used in SCNT, a method used to
perform sequential genetic modifications, targeted DNA insertions
and artificial chromosome transfer using long-term cultured somatic
cells. The human IFN-α/EPO-transgenic fibroblasts established in
the present study may be used for the generation of transgenic
cattle using SCNT technology. In this process, the nucleus
containing chromosomal DNA in an egg cell may be removed and
replaced with a nucleus containing genetically modified chromosomal
DNA obtained from the transgenic fibroblast. Fusion between the
enucleated egg cell and the donor somatic nucleus creates a new
cell, which gains a complete set of chromosomes derived from the
donor nucleus. The SCNT method has several advantages in the
genetic cloning of valuable transgenic animals. The production of
live offspring by SCNT using adult skin fibroblast cells was
reported in goats in 1999 (39)
and in cattle in 2000 (40),
demonstrating the potential for use in the agricultural and
pharmaceutical industries. If the animal bioreactors in the present
study are born, they may produce recombinant human IFN-α and EPO
proteins in milk on a daily basis and serve as a promising model
for the production of recombinant therapeutic agents.
Acknowledgments
The present study was supported by a grant from the
Next-Generation BioGreen 21 Program (grant no. PJ00956301), Rural
Development Administration, Republic of Korea.
References
|
1
|
Jonasch E and Haluska FG: Interferon in
oncological practice: review of interferon biology, clinical
applications and toxicities. Oncologist. 6:34–55. 2001. View Article : Google Scholar
|
|
2
|
Belardelli F: Role of interferons and
other cytokines in the regulation of the immune response. APMIS.
103:161–179. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Biron CA, Nguyen KB, Pien GC, Cousens LP
and Salazar-Mather TP: Natural killer cells in antiviral defense:
function and regulation by innate cytokines. Annu Rev Immunol.
17:189–220. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bogdan C: The function of type I
interferons in antimicrobial immunity. Curr Opin Immunol.
12:419–424. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jelkmann W: Erythropoietin after a century
of research: younger than ever. Eur J Haematol. 78:183–205. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Neumann E: Regulation of erythropoiesis.
Acta Med Austriaca Supp. 6:360–363. 1979.In German.
|
|
7
|
Argenti M: Hematosis and erythropoiesis in
guinea pigs exposed to low oxygen pressure. Riv Med Aeronaut.
14:283–313. 1951.In Undetermined Language. PubMed/NCBI
|
|
8
|
Jelkmann W: Regulation of erythropoietin
production. J Physiol. 589:1251–1258. 2011. View Article : Google Scholar :
|
|
9
|
Macdougall IC and Ashenden M: Current and
upcoming erythropoiesis-stimulating agents, iron products and other
novel anemia medications. Adv Chronic Kidney Dis. 16:117–130. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yang X and Carter MG: Transgenic animal
bioreactors: a new line of defense against chemical weapons? Proc
Natl Acad Sci USA. 104:13859–13860. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Baneyx F: Recombinant protein expression
in Escherichia coli. Curr Opin Biotechnol. 10:411–421. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ebert KM, Selgrath JP, DiTullio P, et al:
Transgenic production of a variant of human tissue-type plasminogen
activator in goat milk: generation of transgenic goats and analysis
of expression. Biotechnology (NY). 9:835–838. 1991. View Article : Google Scholar
|
|
13
|
Krimpenfort P, Rademakers A, Eyestone W,
et al: Generation of transgenic dairy cattle using ‘in vitro’
embryo production. Biotechnology (NY). 9:844–847. 1991. View Article : Google Scholar
|
|
14
|
Buhler TA, Bruyere T, Went DF, Stranzinger
G and Burki K: Rabbit beta-casein promoter directs secretion of
human interleukin-2 into the milk of transgenic rabbits.
Biotechnology (NY). 8:140–143. 1990. View Article : Google Scholar
|
|
15
|
Cerdan MG, Young JI, Zino E, et al:
Accurate spatial and temporal transgene expression driven by a
3.8-kilobase promoter of the bovine beta-casein gene in the
lactating mouse mammary gland. Mol Reprod Dev. 49:236–245. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ebert KM, DiTullio P, Barry CA, et al:
Induction of human tissue plasminogen activator in the mammary
gland of transgenic goats. Biotechnology (NY). 12:699–702. 1994.
View Article : Google Scholar
|
|
17
|
Gordon K, Lee E, Vitale JA, Smith AE,
Westphal H and Hennighausen L: Production of human tissue
plasminogen activator in transgenic mouse milk. 1987.
Biotechnology. 24:425–428. 1992.PubMed/NCBI
|
|
18
|
Ikonen T, Ojala M and Ruottinen O:
Associations between milk protein polymorphism and first lactation
milk production traits in Finnish Ayrshire Cows. J Dairy Sci.
82:1026–1033. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Buser AC, Gass-Handel EK, Wyszomierski SL,
et al: Progesterone receptor repression of prolactin/signal
transducer and activator of transcription 5-mediated transcription
of the beta-casein gene in mammary epithelial cells. Mol
Endocrinol. 21:106–125. 2007. View Article : Google Scholar
|
|
20
|
Doppler W, Windegger M, Soratroi C, et al:
Expression level-dependent contribution of glucocorticoid receptor
domains for functional interaction with STAT5. Mol Cell Biol.
21:3266–3279. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Raught B, Liao WS and Rosen JM:
Developmentally and hormonally regulated CCAAT/enhancer-binding
protein isoforms influence beta-casein gene expression. Mol
Endocrinol. 9:1223–1232. 1995.PubMed/NCBI
|
|
22
|
Malewski T and Zwierzchowski L:
Computer-aided analysis of potential transcription-factor binding
sites in the rabbit beta-casein gene promoter. Biosystems.
36:109–119. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rosen JM, Rodgers JR, Couch CH, et al:
Multihormonal regulation of milk protein gene expression. Ann NY
Acad Sci. 478:63–76. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Rosen JM, Wyszomierski SL and Hadsell D:
Regulation of milk protein gene expression. Annu Rev Nutr.
19:407–436. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hodges VM, Rainey S, Lappin TR and Maxwell
AP: Pathophysiology of anemia and erythrocytosis. Crit Rev Oncol
Hematol. 64:139–158. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gossen M and Bujard H: Tight control of
gene expression in mammalian cells by tetracycline-responsive
promoters. Proc Natl Acad Sci USA. 89:5547–5551. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhou X, Vink M, Klaver B, Berkhout B and
Das AT: Optimization of the Tet-On system for regulated gene
expression through viral evolution. Gene Ther. 13:1382–1390. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gossen M, Freundlieb S, Bender G, Müller
G, Hillen W and Bujard H: Transcriptional activation by
tetracyclines in mammalian cells. Science. 268:1766–1769. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Jung EM, An BS, Kim YK, et al:
Establishment of transgenic fibroblasts for producing recombinant
human interferon-α and erythropoietin in bovine milk. Mol Med Rep.
7:406–412. 2013.
|
|
30
|
Wang CJ, Xiao CW, You TG, et al:
Interferon-alpha enhances antitumor activities of oncolytic
adenovirus-mediated IL-24 expression in hepatocellular carcinoma.
Mol Cancer. 11:312012. View Article : Google Scholar
|
|
31
|
Biron CA: Interferons alpha and beta as
immune regulators - a new look. Immunity. 14:661–664. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gallucci S, Lolkema M and Matzinger P:
Natural adjuvants: endogenous activators of dendritic cells. Nat
Med. 5:1249–1255. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
33
|
Son YD, Jeong YT, Park SY and Kim JH:
Enhanced sialylation of recombinant human erythropoietin in Chinese
hamster ovary cells by combinatorial engineering of selected genes.
Glycobiology. 21:1019–1028. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tuite MF, Dobson MJ, Roberts NA, et al:
Regulated high efficiency expression of human interferon-alpha in
Saccharomyces cerevisiae. EMBO J. 1:603–608. 1982.PubMed/NCBI
|
|
35
|
Wall RJ, Kerr DE and Bondioli KR:
Transgenic dairy cattle: genetic engineering on a large scale. J
Dairy Sci. 80:2213–2224. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zuelke KA: Transgenic modification of cows
milk for value-added processing. Reprod Fertil Dev. 10:671–676.
1998. View
Article : Google Scholar
|
|
37
|
Huynh HT, Robitaille G and Turner JD:
Establishment of bovine mammary epithelial cells (MAC-T): an in
vitro model for bovine lactation. Exp Cell Res. 197:191–199. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Berry SD, Weber Nielsen MS, Sejrsen K,
Pearson RE, Boyle PL and Akers RM: Use of an immortalized bovine
mammary epithelial cell line (MAC-T) to measure the mitogenic
activity of extracts from heifer mammary tissue: effects of
nutrition and ovariectomy. Domest Anim Endocrinol. 25:245–253.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Baguisi A, Behboodi E, Melican DT, et al:
Production of goats by somatic cell nuclear transfer. Nat
Biotechnol. 17:456–461. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kubota C, Yamakuchi H, Todoroki J, et al:
Six cloned calves produced from adult fibroblast cells after
long-term culture. Proc Natl Acad Sci USA. 97:990–995. 2000.
View Article : Google Scholar : PubMed/NCBI
|