Introduction
An increased level of serum homocysteine (Hcy) is
closely associated with the development of juvenile cataracts and
age-related cataracts (ARCs). Recent studies have demonstrated
statistically significant increases in Hcy and decreases in vitamin
B2, B6, B12 and folic acid in the plasma of 40 adult patients with
ARCs (1). Additionally, a total of
24 of 29 children with homocystinuria type 1 were also diagnosed
with cataracts (2). Homocystinuria
in adults has been revealed to be acquired through chronic kidney
disease, cigarette smoking and alcoholism in addition to dietary
deficiencies of vitamin B2, B6, B12 and folic acid (3). Hcy is biosynthesized from methionine
in a multistep process (4,5). The co-enzymes involved in this
process are folic acid and the vitamins B2, B6 and B12. Under the
conditions of normal metabolism, the formation and elimination of
Hcy is balanced. Under higher levels of Hcy, misfolded proteins
have been observed to accumulate in the endoplasmic reticulum (ER),
which can then induce the unfolded protein response (UPR) (6,7).
Recent studies have found the cataractogenic
stressors, including hypoxia with low glucose (8) or high glucose (9), Hcy (10) and galactose (9), induce ER stress-mediated activation
of the UPR and overproduction of reactive oxygen species (ROS),
resulting in death of lens epithelial cells (LECs). The ROS that
have been generated then decrease the levels of cytosolic
glutathione and contribute to an additional source of ROS from the
mitochondria (11), which leads to
cell death. In addition, Hcy was also reported to suppress nuclear
factor erythroid-2-related factor 2 (Nrf2; also termed as NFE2L2)
dependent antioxidant protection leading to the overproduction of
ROS in LECs (8,10). Nrf2 leads to the transcription of
~200 protective genes, including 20 enzymatic antioxidant genes
(12,13). Recently, Gao et al (14) reported promoter DNA demethylation
of Kelch-like ECH-associated protein 1 (Keap1), which is a negative
regulator of Nrf2, in human lens epithelial cells (HLECs) and in
age-related cataractous lenses. These sequential events may be
responsible for aging and cataract formation in humans.
Acetyl-L-carnitine
(γ-trimethyl-β-acetyl-butyrrobetaine) is the acetyl ester of the
trimethylated amino acid L-cartinine. Carnitine and its short-chain
esters facilitate transport of long-chain fatty acids across the
inner mitochondrial membrane for β-oxidation, thereby promoting
energy availability and preventing toxic accumulation of long-chain
fatty acids (15). The enzyme
carnitine acetyltransferase catalyses the formation of
acetyl-L-carnitine from carnitine and acetyl-coenzyme A
(acetyl-CoA) and also the reversible reaction. The modulation of
the intracellular concentration of free CoA and acetyl-CoA has been
established to be a common mechanism for the various physiological
activities of acetyl-L-carnitine, such as the acetylation of H4
histones (15,16). It has been reported that ALCAR may
be a promising candidate for arresting Hcy-induced Alzheimer
disease-like pathological and behavioral impairments (17). In addition, ALCAR has been
previously revealed to exhibit an anti-cataractogenic effect in
against selenite-injection and buthionine sulfoximine
(BSO)-injection rat models (18,19).
The aim of the present study was to investigate the efficacy of
ALCAR in the protection against the adverse effect of Hcy in
HLECs.
Materials and methods
HLEC culture
HLECs (HLEC-SRA 01/04; Clonetics, Walkersville, MD,
USA) were cultured overnight in Dulbecco’s modified Eagle’s medium
(DMEM) high glucose (Gibco Life Technologies, Carlsbad, CA, USA)
supplemented with 10% fetal bovine serum under 20% atmospheric
oxygen at 37°C. The cells were harvested and used for the
measurement of ROS levels, cell death analyses, western blotting,
reverse transcription-quantitative polymerase chain reaction
(RT-qPCR) and bisulfite genomic DNA sequencing.
Measurement of ROS and cell death
Using fluorescence-activated cell sorting (FACSDiva
v4.0.1; BD Biosciences, Sydney, Australia), ROS production and cell
death rates were measured. Cells were plated 24 h prior to the
experiment, maintained in DMEM low glucose (Gibco Life
Technologies) and cultured with different concentrations of Hcy
(50, 75, 100 or 125 μM) or acetyl-l-carnitine (50, 100 or
150 μM) for 6, 12 and 24 h in order to determine the optimum
concentration and time period for experimentation. Further
experiments were then performed for 24 h with the determined
optimum dosage.
Western blot analysis
At the end of experimental treatments, HLECs were
harvested, washed with ice-cold phosphate-buffered saline (PBS) and
lysed with radioimmunoprecipitation assay buffer (Cell Signaling
Technology, Danvers, MA, USA). The lysates were centrifuged at
13,000 × g for 10 min and the protein content of the supernatant
was determined using the Bradford method (20). Soluble proteins (10–20 μg)
were loaded and separated by 10% SDS-PAGE and blotted onto a
polyvinylidene fluoride membrane (Sigma-Aldrich, St. Louis, MO,
USA). Subsequently, the membranes were blocked with 5% non-fat dry
milk powder solution for 1 h at room temperature prior to an
overnight incubation with the following primary antibodies (1:1,000
dilution): Rabbit polyclonal anti-catalase (CAT; cat. no.
NBP2-24916; Novus Biologicals, LLC, Littleton, CA, USA), rabbit
polyclonal anti-glutathione reductase (GR; cat. no. NBP2-24940;
Novus Biologicals, LLC), rabbit polyclonal
anti-glutathione-s-trans-ferase (GST; cat. no. NBP2-16686; Novus
Biologicals, LLC), mouse monoclonal anti-immunoglobulin heavy-chain
binding protein (BiP; cat. no. 610978; BD Biosciences), rabbit
polyclonal anti-inositol-requiring enzyme 1α (IRE1α; cat. no.
PA5-20189; Thermo Fisher Scientific, Waltham, MA, USA), rabbit
polyclonal anti-activating transcription factor 6 (ATF6; cat. no.
PA5-20215; Thermo Fisher Scientific), rabbit polyclonal anti-Nrf2
(cat. no. PA5-27882; Thermo Fisher Scientific) and goat polyclonal
Keap1 (cat. no. sc-15246; Santa Cruz Biotechnology, Inc., Santa
Cruz, CA, USA) at 4°C. Following rinsing of the membranes with
Tris-buffered saline with Tween 20, they were incubated with the
secondary antibody (1:5,000 dilution) for 1 h at room temperature
and the bands were visualized using enhanced chemiluminescence as
described previously (10). The
intensity of each band was normalized to that of β-actin and
quantified using ImageJ version 1.48 software (National Institutes
of Health, Bethesda, MD, USA).
RT-qPCR analysis
Total RNA was extracted from the HLECs with
Quick-RNA MicroPrep solution (Zymo Research Corporation, Orange,
CA, USA) according to the manufacturer’s instructions.
Subsequently, the purified total RNA was reverse-transcribed using
iScript reverse transcription supermix for real-time PCR (Bio-Rad,
Hercules, CA, USA) according to the manufacturer’s instructions.
The reverse transcribed RNA was analyzed using qPCR using the
SsoFast EvaGreen supermix (Bio-Rad). The optimal qPCR assay for
Nrf2 and Keap1 genes was performed according to Roche’s ProbeFinder
(http://qpcr.probefinder.com/organism.jsp). The primers
were purchased from Generay (Shanghai) Biotech Co., Ltd. (Shanghai,
China). The primer sequences were as follows: Forward:
5′-ACACGGTCCACAGCTCATC-3′ and reverse: 5′-TGCCTC
CAAAGTATGTCAATCA-3′ for Nrf2, with a product size of 96 bp;
forward: 5′-GGGTCCCCTACAGCCAAG-3′ and reverse: 5′-TGG
GGTTCCAGAAGATAAGC-3′ for Keap1, with a product size of 66 bp; and
forward: 5′-CCAACCGCGAGA AGATGA-3′ and reverse:
5′-CCAGAGGCGTACAGGGATAG-3′ for β-actin, with a product size of 97
bp. Each reaction was conducted in triplicate and a standard curve
was prepared using a serial dilution of a reference sample. The
relative copy numbers were obtained from the standard curve and
were normalized to the values obtained for β-actin as the internal
control. The results of the PCR were quantified using the
2−ΔΔCT (21).
Bisulfite conversion and DNA
sequencing
The genomic DNA of HLECs was subjected to bisulfite
conversion using the EZ DNA Methylation-Direct™ kit (Zymo Research
Corporation). The bisulfite converted DNA was then used for
bisulfite genomic DNA sequencing. The bisulfite-modified DNA was
amplified via bisulfite sequencing PCR using Platinum PCR SuperMix
High Fidelity (Invitrogen Life Technologies, Carlsbad, CA, USA)
with primers specific to the human Keap1 promoter region (−430 to
−110) with a product of 330 bp. The sequences were as follows:
forward: 5′-TTAGTTATTTAGGAGGTTGT-3′ and reverse:
5′-AACCCCCCTTCTCACTA-3′. The primers were designed using the Methyl
Primer Express v1.0 software from Applied Biosystems (Foster City,
CA, USA). The PCR products were purified by gel extraction using
the Zymoclean™ Gel DNA recovery kit (Zymo Research Corporation) and
then cloned into pCR4-TOPO vectors using the TOPO TA Cloning kit
(Invitrogen Life Technologies). The recombinant plasmids were
transformed into One Shot TOP10 chemically competent Escherichia
coli (Invitrogen Life Technologies) using the regular chemical
transformation method as described in the manufacturer’s
instructions. Plasmid DNA was prepared from ~12 independent clones
of each amplicon with the PureLink Quick Plasmid Miniprep kit
(Invitrogen Life Technologies) and sequenced to determine the
status of CpG methylation. Subsequently, the sequenced data of each
clone was analyzed for DNA methylation in the Keap1 promoter using
BISMA software (http://biochem.jacobs-university.de/BDPC/BISMA/) using
the default filtering threshold settings (22).
Statistical analysis
The results were expressed as the mean ± standard
deviation, and statistical significance was evaluated by Student’s
t-test using SPSS version 15.0 (SPSS Inc., Chicago, IL, USA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
Dosage determination
The optimum concentration was obtained by
administering various concentrations of Hcy (50, 75, 100 and 125
μM) and ALCAR (50, 100 and 150 μM) in HLECs at
different time intervals, including 6, 12 and 24 h. The dosage of
Hcy was determined by the level of ROS production and cell death.
Similarly, the dosage of ALCAR was determined by its protective
effects against ROS and cell death resulting from Hcy exposure. The
Hcy concentration of 100 μM exhibited a low level of ROS
production from 6 h and increased considerably at 24 h (Fig. 1A). Whereas concentrations of 50 and
75 exhibited a low level of ROS production and 125 μM
demonstrated a high level of ROS production and cell death at 24 h.
ALCAR exhibited a greater protective effect at a concentration of
150 μM, as compared with the other concentrations assessed
(Fig. 1B). Considering these
results, the experiment was performed for 24 h with the optimum
concentration of Hcy (100 μM) and ALCAR (100 μM),
from which ALCAR exhibited a significant protective effect against
Hcy in HLECs (Fig. 1C).
ALCAR increases the levels of antioxidant
proteins and decreases the levels of ER stress-associated
proteins
Following the dosage determination, the antioxidant
and ER stress-associated protein levels in experimental groups,
including control, Hcy alone and Hcy with ALCAR were investigated.
Western blotting analysis of the Nrf2/Keap1-dependent antioxidant
protection system in HLECs treated with 100 μM Hcy for 24 h
was performed as Nrf2 is a nuclear transcriptional factor, which
controls >200 stress-associated genes and Keap1 is a negative
regulator of the Nrf2 protein. Notably, the protein levels of Nrf2
and its downstream enzymes, including GR, GST and CAT were
significantly decreased in HLECs treated with 100 μM Hcy for
24 h, with a marked increase in the level of Keap1 (Fig. 2). However cells treated with Hcy
and ALCAR (100 μM) exhibited a significantly increased level
of Nrf2 and its downstream antioxidant proteins. Notably, ALCAR had
a significant effect on the level of Keap1 protein. Similarly, Hcy
induced an increase in the ER stress-associated proteins, BiP, ATF6
and IRE1α, which were also inhibited in the presence of ALCAR
(Fig. 2). These results suggested
that Hcy induced suppression of Nrf2/Keap1-dependent antioxidant
protection through ER stress mediated ROS production and this was
inhibited by treatment with ALCAR.
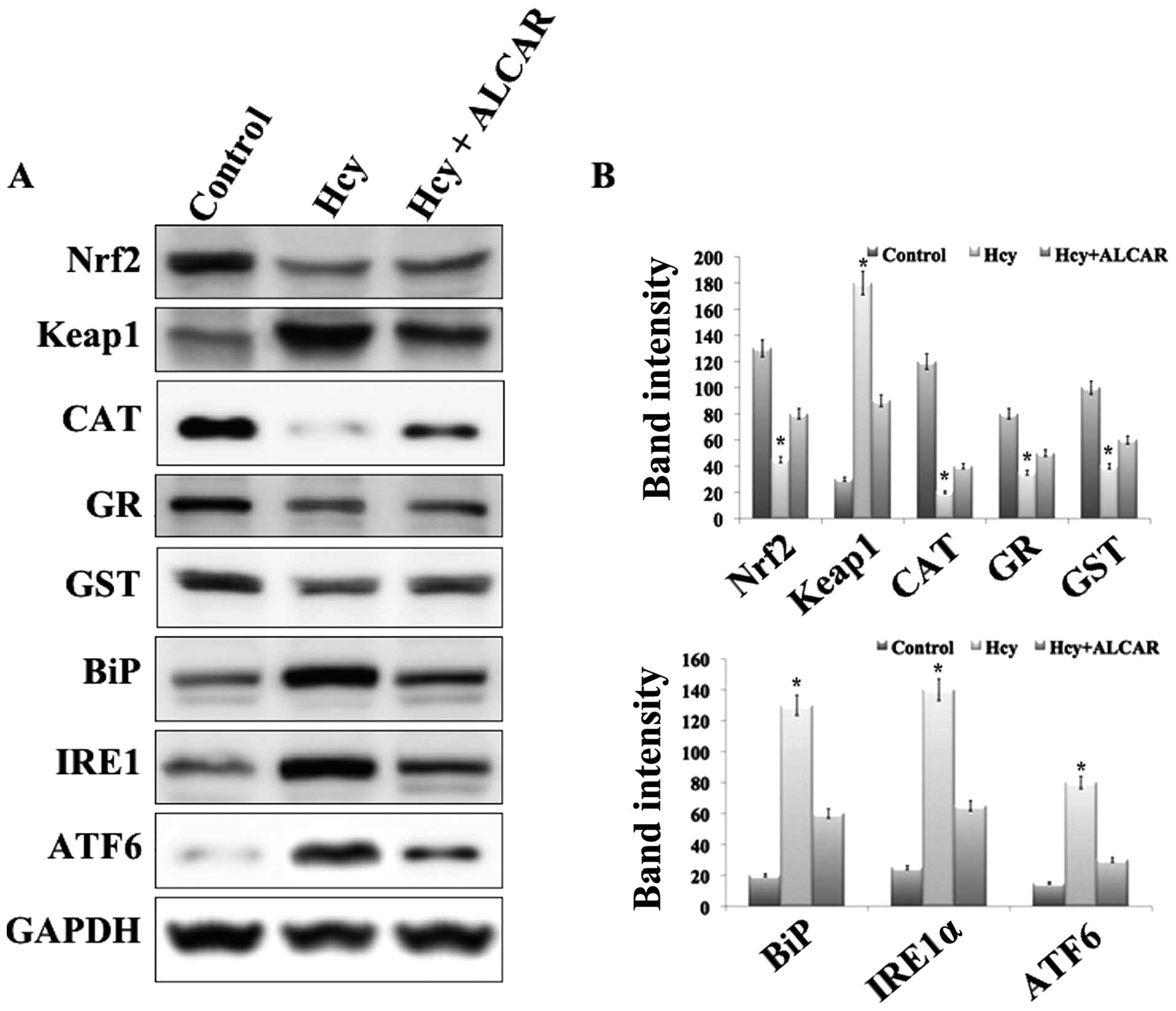 | Figure 2Results of western blot analysis. (A)
Representative western blot analysis of the Nrf2/Keap1 mediated
antioxidant system and endoplasmic reticulum stress-associated
proteins in human lens epithelial cells of the experimental groups:
Control, Hcy alone, and Hcy and ALCAR in combination at a
concentration of 100 μM each. (B) Representive
quantification of band intensity of the corresponding western
blots. *P<0.05, compared with control and Hcy+ALCAR treated
group. Keap1, Kelch-like ECH-associated protein 1; Hcy,
homocysteine; ALCAR, acetyl-l-carnitine; Nrf2, nuclear factor
erythroid-2-related factor 2; CAT, catalase; GR, glutathione
reductase; GST, glutathione-s-transferase; BiP, immunoglobulin
heavy-chain binding protein; IRE1, inositol-requiring enzyme 1;
ATF6, activating transcription factor 6. |
ALCAR treatment increases Nrf2/Keap1 mRNA
levels
Following the protein blot results, subsequent
quantification of the mRNA levels of expression of Nrf2 and Keap1
genes in the experimental groups was conducted. RT-qPCR was
performed to analyze or quantify the mRNA transcript level.
Notably, these results reflected the protein immunoblot results. A
decrease in the level of Nrf2 and significant increase in Keap1
mRNA levels in the Hcy exposed cells was observed. However, these
changes in mRNA levels were inhibited by ALCAR treatment (Fig. 3A). These results confirmed that Hcy
mediated Keap1 promoter DNA demethylation, which significantly
increased Keap1 transcription and this is controlled by ALCAR
treatment.
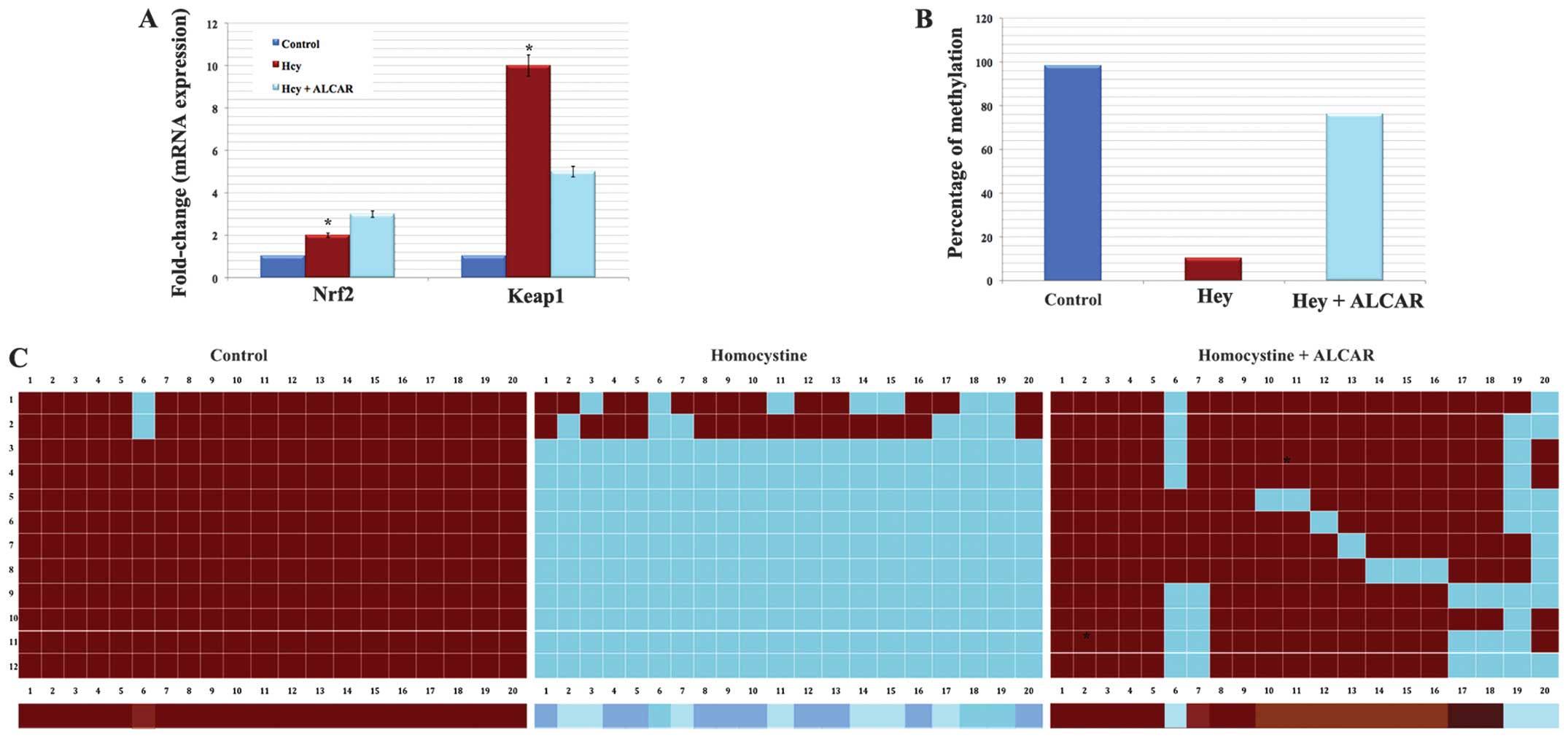 | Figure 3Protective effect of ALCAR in gene
expression and promoter DNA demethylation. (A) Representative of
the reverse transcription-quantitative polymerase chain reaction
results demonstrating the mRNA levels of the Nrf2 and Keap1 gene in
the experimental groups. *P<0.05, compared with control and
Hcy+ALCAR treated group. (B) Representative percentage of
demethylation based on the sequencing results. (C) Methylation
pattern in the Keap1 promoter region between −430 and −110 of
control HLECs exhibit highly methylated CpG dinucleotides,
Hcy-treated cells exhibit highly demethylated CpG dinucleotides and
HLECs treated with Hcy and ALCAR exhibit moderately demethylated
CpG dinucleotides. Red colored boxes denote methylcytosine and blue
colored boxes denote unmethylated cytosine, respectively. The color
gradient bar, which represents the red region, has more methylated
CpGs and the blue region has more unmethylated CpGs. Keap1,
Kelch-like ECH-associated protein 1; Hcy, homocysteine; ALCAR,
acetyl-l-carnitine; Nrf2, nuclear factor erythroid-2-related factor
2; HLECs, human lens epithelial cells. |
ALCAR prevents Hcy-induced DNA
demethylation
Bisulphite genomic DNA sequencing of the Keap1
promoter region (20 CpGs; between −430 to −110) was performed in
HLECs of the experimental groups to investigate whether the marked
Keap1 gene expression may be due to demethylation. The normal HLECs
(control) exhibited ~100% methylation of Keap1 promoter DNA.
Notably, HLECs treated with Hcy alone exhibited ~90% loss of
5-methylcytosine in the Keap1 promoter region than that of the
control HLECs (Fig. 3B and C).
However, HLECs exposed to Hcy and ALCAR exhibited ~80% methylation.
The present results suggested that loss of Keap1 promoter DNA
methylation eventually leads to over-expression of Keap1 resulting
in Nrf2 suppression, as Keap1 is a negative regulator of Nrf2.
Thus, it was hypothesized that ALCAR prevented the Hcy induced
promoter DNA demethylation of Keap1 (Fig. 3B and C).
Discussion
The present study demonstrated that Hcy induces ER
stress-mediated UPR activation, ROS overproduction and suppression
of Nrf2/Keap1 dependent antioxidant protection by Keap1 promoter
demethylation, leading to cell death in HLECs. These sequential
events may be a critical mechanism for cataract formation. However,
it is hypothesized that ALCAR may protect against Hcy-induced
adverse effects in HLECs, thereby preventing the formation of
cataracts. Previously, Elanchezhian et al (18) demonstrated the protective effect of
ALCAR in selenite-induced cataractogenesis in a rat model.
Considering the potential of ALCAR treatment, the present study was
designed in HLECs against the adverse effect of Hcy. A high level
of serum Hcy is closely associated with juvenile and age-associated
cataracts. Recent studies have revealed statistically significant
increases of Hcy and decreases in vitamins B2, B6, B12 and folic
acid in the plasma of 40 adult patients with ARCs (1). In addition, 24 of 29 children with
homocystinuria type 1 were also diagnosed with cataracts (2). Consistent with these studies, the
present study revealed that HLECs exposed to 100 μM Hcy for
24 h was capable of inducing the production of ROS and cell
death.
In addition, the induction of ER stress mediated UPR
activation by various ER stressors, such as Hcy (10), tunicamycin (9), galactose (9) and hypoxia with low or high glucose
(8,9), eventually results in UPR activation,
ROS overproduction and cell death of LECs. In the present study,
the immunoblot analysis of Nrf2 mediated antioxidant proteins were
observed to be decreased and ER stress-associated proteins were
found to be increased in Hcy treated cells. Following treatment
with ALCAR, these changes were prevented. A similar effect was
observed in Nrf2 and Keap1 gene expression. The action of ALCAR in
protecting the expression of genes involved in apoptosis suggested
an additional pathway for its effect in retarding selenite
cataractogenesis by protecting against the abnormal expression of
genes involved in apoptosis and of antioxidant genes (23). Administration of ALCAR was observed
to prevent the decreased expression of calpain Lp82 proteins and
decreased levels of m-calpain mRNA transcripts in selenite-induced
cataractogenesis (19), again
suggesting the anti-apoptotic effect of ALCAR.
It has been well-established that the development of
ARCs is tightly coupled with lens oxidation and aging (24,25).
The present results are concomitant with the previous findings, as
ER stress induced by Hcy establishes production of ROS and
suppresses the Nrf2/Keap1 mediated antioxidant system, promoting
DNA demethylation in the Keap1 promoter region. The promoter DNA
demethylation of the Keap1 gene resulted in the overexpression of
the Keap1 protein and, as a result, the Nrf2 levels were ultimately
decreased through proteasomal degradation. Thereby, Nrf2/Keap1
mediated antioxidant protection deteriorated and the cellular redox
balance in the HLECs were shifted towards lens oxidation leading to
cataract formation. Furthermore, aging is associated with a gradual
progression of epigenetic promoter DNA demethylation of the Keap1
gene in human lenses, whereas, ER stressors are associated with
rapid, radical and severe loss of Keap1 promoter demethylation
leading to ARC formation (14).
The treatment of ALCAR was observed to significantly protect the
cells from these effects of Hcy. It has been reported that ALCAR
may be a promising candidate for arresting Hcy-induced Alzheimer
disease-like pathological and behavioral impairments (17). In addition, ALCAR has been
previously revealed to exhibit an anti-cataractogenic effect in rat
models against selenite-injection and BSO-injection (18,19).
The present investigation further demonstrated its protective
effect in HLECs.
In conclusion, the present study identified that
simultaneous supplementation of exogenous ALCAR significantly
rescued HLECs from Hcy-induced ER stress and DNA demethylation of
the Keap1 gene. ALCAR controlled the production of ROS, protein
levels of antioxidants, ER stress signaling, and mRNA levels of
Nrf2 and Keap1 gene in HLECs from Hcy mediated adverse effects. The
present findings suggested that ALCAR is a potential component in
protecting the human lens from oxidative stress-induced damage,
thereby preventing the formation of ARCs.
References
|
1
|
Sen SK, Pukazhvanthen P and Abraham R:
Plasma homo-cysteine, folate and vitamin B(12) levels in senile
cataract. Indian J Clin Biochem. 23:255–257. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sulochana KN, Amirthalakshmi S, Vasanthi
SB, et al: Homocystinuria with congenital/developmental cataract.
Indian J Pediatr. 67:725–728. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cumurcu T, Sahin S and Aydin E: Serum
homocysteine, vitamin B 12 and folic acid levels in different types
of glaucoma. BMC Ophthalmol. 6:62006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Selhub J: Homocysteine metabolism. Annu
Rev Nutr. 19:217–246. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Williams KT and Schalinske KL:
Homocysteine metabolism and its relation to health and disease.
Biofactors. 36:19–24. 2010.PubMed/NCBI
|
|
6
|
Ron D and Walter P: Signal integration in
the endoplasmic reticulum unfolded protein response. Nat Rev Mol
Cell Biol. 8:519–529. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Todd DJ, Lee AH and Glimcher LH: The
endoplasmic reticulum stress response in immunity and autoimmunity.
Nat Rev Immunol. 8:663–674. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Elanchezhian R, Palsamy P, Madson CJ, et
al: Low glucose under hypoxic conditions induces unfolded protein
response and produces reactive oxygen species in lens epithelial
cells. Cell Death Dis. 3:e3012012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ikesugi K, Yamamoto R, Mulhern ML and
Shinohara T: Role of the unfolded protein response (UPR) in
cataract formation. Exp Eye Res. 83:508–516. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Elanchezhian R, Palsamy P, Madson CJ,
Lynch DW and Shinohara T: Age-related cataracts: homocysteine
coupled endoplasmic reticulum stress and suppression of
Nrf2-dependent antioxidant protection. Chem Biol Interact.
200:1–10. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Huang HC, Nguyen T and Pickett CB:
Phosphorylation of Nrf2 at Ser-40 by protein kinase C regulates
antioxidant response element-mediated transcription. J Biol Chem.
277:42769–42774. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Enomoto A, Itoh K, Nagayoshi E, et al:
High sensitivity of Nrf2 knockout mice to acetaminophen
hepatotoxicity associated with decreased expression of
ARE-regulated drug metabolizing enzymes and antioxidant genes.
Toxicol Sci. 59:169–177. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kaspar JW, Niture SK and Jaiswal AK:
Nrf2:INrf2 (Keap1) signaling in oxidative stress. Free Radic Biol
Med. 47:1304–1309. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gao Y, Yan Y and Huang T: Human
age-related cataracts: Epigenetic suppression of the nuclear factor
erythroid 2-related factor 2-mediated antioxidant system. Mol Med
Rep. 11:1442–1447. 2015.
|
|
15
|
Peluso G, Petillo O, Barbarisi A, et al:
Carnitine protects the molecular chaperone activity of lens
alpha-crystallin and decreases the protein post-translational
modifications induced by oxidative stress. FASEB J. 15:1604–1606.
2001.PubMed/NCBI
|
|
16
|
Riccioloni R, Scalibastri M, Kelleher JK,
et al: Role of acetyl-L-carnitine in rat brain lipogenesis:
implications for polyunsaturated fatty acid biosynthesis. J
Neurochem. 71:2510–2517. 1998. View Article : Google Scholar
|
|
17
|
Zhou P, Chen Z, Zhao N, et al:
Acetyl-L-carnitine attenuates homocysteine-induced Alzheimer-like
histopathological and behavioral abnormalities. Rejuvenation Res.
14:669–679. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Elanchezhian R, Ramesh E, Sakthivel M, et
al: Acetyl-L-carnitine prevents selenite-induced cataractogenesis
in an experimental animal model. Curr Eye Res. 32:961–971. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Elanchezhian R, Sakthivel M, Geraldine P
and Thomas PA: The effect of acetyl-L-carnitine on lenticular
calpain activity in prevention of selenite-induced
cataractogenesis. Exp Eye Res. 88:938–944. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bradford MM: A rapid and sensitive method
for the quantitation of microgram quantities of protein utilizing
the principle of protein-dye binding. Anal Biochem. 72:248–254.
1976. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
22
|
Rohde C, Zhang Y, Reinhardt R and Jeltsch
A: BISMA - fast and accurate bisulfite sequencing data analysis of
individual clones from unique and repetitive sequences. BMC
Bioinformatics. 11:2302010. View Article : Google Scholar :
|
|
23
|
Elanchezhian R, Sakthivel M, Geraldine P
and Thomas PA: Regulatory effect of acetyl-l-carnitine on
expression of lenticular antioxidant and apoptotic genes in
selenite-induced cataract. Chem Biol Interact. 184:346–351. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Brennan LA and Kantorow M: Mitochondrial
function and redox control in the aging eye: role of MsrA and other
repair systems in cataract and macular degenerations. Exp Eye Res.
88:195–203. 2009. View Article : Google Scholar :
|
|
25
|
Lou MF: Redox regulation in the lens. Prog
Retin Eye Res. 22:657–682. 2003. View Article : Google Scholar : PubMed/NCBI
|

















