Introduction
Fructus Ligustri Lucidi (FLL), the vernacular name
for the fruit of Ligustrum lucidum, is a herb used for
treating age-associated diseases (1). FLL ethanol extract modulates the
turnover of bone and the calcium balance in ovariectomized rats
(2). FLL may improve the uptake of
calcium from the diet, predominantly via its actions on increasing
the levels of 1,25-dihydroxyvitamin D3
(1,25(OH)2D3) serum and vitamin D-dependent
calcium binding proteins (CaBP) (3).
Lower back pain is one of the most prevalent and
costly health problems in developed countries. For example, lower
back pain was found to be one of the most expensive diseases in
Australia, with an estimated cost in 2001 of $9.17 billion
(4). The predominant cause of
lower back pain is lumbar disc herniation (LDH). LDH is a term
referring to a group of conditions, including back pain, femoral
nerve pain and sciatica. LDH may be caused by the compression of
dural or spinal nerve roots, associated with rupturing of the
annulus fibrosus or by a herniated nucleus forcing pressure on the
spinal canal (5). Current
treatments and surgeries for patients with LDH include conventional
open discectomy, microdiscectomy, percutaneous laser discectomy,
percutaneous discectomy and microendoscopic discectomy (6). Although there are numerous cases with
successful outcomes for patients with LDH following disc surgery,
there remain a significant number of patients who do not benefit
from this procedure (7,8).
To the best of our knowledge, little is known as to
whether FLL may improve the prognosis for patients with LDH
following disc surgery. In the present study, the efficacy of FLL
to reduce pain was evaluated using a lumbar disc herniation rat
model.
Materials and methods
Tissue samples
Intervertebral disc tissue samples were collected
from 51 patients who had undergone posterior open discectomy for
LDH in the Department of Orthopedics (The First Affiliated Hospital
of China Medical University, Shenyang, China) between May 2010 and
May 2013. Five cadaveric tissue samples were also obtained from
cancer patients within 36 h of mortality with no previously known
spinal pathology. Blood samples from the patients with LDH and 20
healthy volunteers were collected in ethylenediamine tetraacetic
acid on ice. Samples were immediately centrifuged at 3,000 × g for
20 min. Plasma samples were frozen at −70°C. Patients or relatives
signed an informed consent form approved by the China Medical
University ethics committee prior to participation in the study.
The study design was in accordance with the Declaration of
Helsinki. Patient and control group data are provided in Table I.
 | Table IPatient characteristics. |
Table I
Patient characteristics.
| Feature | Control group | LDH group |
|---|
| Male/Female (n) | 4/1 | 38/13 |
| Mean age (years) | 40 | 42 |
| Body mass index | | |
| Mean | 27.7 | 26.8 |
| Range | 21.5–33.2 | 20.22–31.25 |
| Position of herniated
disc | | |
| L3-L4 | NA | 15 |
| L4-L5 | NA | 18 |
| L5-S1 | NA | 18 |
| Type of
herniation | | |
| Protrusion | NA | 22 |
| Extrusion | NA | 18 |
| Sequestration | NA | 11 |
| Duration of
symptoms | | |
| <3 months | NA | 7 |
| 3–12 months | NA | 19 |
| >12 months | NA | 25 |
| Pain intensity
(visual analog scale) | | |
| 0–5 | NA | 15 |
| 5–7 | NA | 16 |
| 8–10 | NA | 20 |
Cytokine assay
Enzyme-linked immunosorbent assay (ELISA) kits for
inflammatory cytokines: Interleukin-2 (IL-2), IL-6, IL-8 and tumor
necrosis factor-α (TNF-α) were purchased from R&D Systems, Inc.
(Minneapolis, MN, USA). ELISA was conducted according to the
manufacturer’s instructions. Cytokine levels were expressed as
pg/ml of sample homogenate.
Preparation of FLL extract
Ligustrum lucidum plants were obtained from
Liaoning, China. Dried and powered crude plant material was
extracted twice using 70% ethanol. The crude plant was boiled with
distilled water twice, each lasting 2 h. The mixture was filtered
to collect the filtrate, which was evaporated using a rotary
evaporator (rotary evaporator; Shyarong Biochemical Instruments
Inc., Shanghai, China) under reduced pressure, yielding 23.5% of
the weight of the starting materials.
Operation procedure
The study protocol was approved by the Animal Ethics
Committee of China Medical University. Male Sprague-Dawley rats
(n=90; weight, 200–250 g; Harlan Sprague Dawley Inc.; Indianapolis,
IN, USA) were used in the present study. According to methods
described previously by Obata et al, rats were anesthetized
using intraperitoneal injection of sodium pentobarbital (40 mg/kg;
Beijing Propbs Biotechnology Co., Ltd., Beijing, China) (9). Laminectomies were performed in the
left L5 nerve roots and dorsal root ganglions (DRG) were exposed.
Nucleus pulposuses, harvested from the second and third coccygeal
intervertebral discs, were implanted next to the left L5 nerve
roots, near to the DRG.
Treatment groups
Rats were separated into three groups: Control
group, normal diet; mock group, normal diet mixed with ethanol (200
mg/kg) and FLL group, normal diet mixed with FLL extract (200
mg/kg). Rats were provided with sterile deionized water, ad
libitum.
Evaluation of mechanical allodynia and
thermal hyperalgesia
Evaluations were performed prior to surgery (day 0)
and on days 5, 10, 15, 20, 25 and 30 after surgery.
Mechanical allodynia was evaluated by measuring the
withdrawal response of the hind paw to a mechanical stimulation,
using a Von Frey assay (North Coast Medical, Inc., Gilroy, CA, USA)
that had been calibrated to the force required to elicit a
withdrawal response (g) (10).
Rats were placed in a clear plastic cage with a metal mesh floor
and allowed to acclimatize to the testing environment for 15 min.
The plantar surface of each hind paw was stimulated five times
using Von Frey filaments, beginning with a 0.1 g filament, the
thickness was increased or decreased, until a withdrawal response
was observed in three of the five stimuli. Filaments were increased
every 6–8 mins with logarithmically incremental rigidity of 0.41,
0.70, 1.20, 2.00, 3.63, 5.50, 8.50 and 15.1 g, to calculate the
mechanical threshold. Fifty percent probability thresholds of
mechanical paw withdrawal were calculated. If no withdrawal
response of the hind paw was observed prior to stimulation with a
26-g filament, 26 g was assigned as the mechanical threshold.
Thermal hyperalgesia was determined by measuring paw
withdrawal latency in a thermal stimulation system (XR1102;
Shanghai Xin Ruan Information Technology Co., Ltd., Shanghai,
China) consisting of a clear plastic chamber (10 × 20 × 24 cm) on a
clear smooth glass floor, at 30°C. Rats were placed individually in
the chamber for ~15 min, in order to acclimatize to the chamber
conditions. A heat stimulus (150 mcal/sec/cm2) was
delivered using a 0.5-cm diameter radiant heat source positioned
under the plantar surface of the paw. Once a rat withdrew its paw
from the heat stimulus, a photocell detected the interruption of a
light beam, which automatically switched off the infrared generator
and stopped the timer, providing the value for paw withdrawal
latency. This method exhibits a 0.1 sec precision level for the
measurement of paw withdrawal latency. If a rat failed to withdraw
its paw the heat stimulus was automatically discontinued after 25
sec.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total tissue RNA was isolated using
TRIzol® reagent (Invitrogen Life Technologies, Carlsbad,
CA, USA) and was reverse transcribed using SuperScript II reverse
transcriptase (Invitrogen Life Technologies) according to the
manufacturer’s instructions. RT-qPCR analysis was performed using
an ABI prism 7500 sequence detection system (Applied Biosystems
Life Technologies, Foster, CA, USA) and an SYBR Green PCR Master
mixture (Takara Biotechnology Co., Ltd., Dalian, China). Primer
sequences are shown in Table II.
PCR conditions were as follows: One cycle at 95°C for 10 min
followed by 40 cycles of 95°C for 15 sec and 60°C for 1 min.
Relative quantification was calculated by the ΔΔCt method using
High Resolution Melt v.2.0 software from Applied Biosystems Life
Technologies.
 | Table IIPrimers used in RT-qPCR analyses. |
Table II
Primers used in RT-qPCR analyses.
| Gene | Sequence (5′–3′,
forward and reverse) |
|---|
| MMP-1 |
GGCCCACAAACCCCAAAAG |
|
ATCTCTGTCGGCAAATTCGTAAGC |
| MMP-3 |
GATGCCCACTTTGATGATGATGAA |
|
AGTGTTGGCTGAGTGAAAGAGACC |
| MMP-8 |
TGGGGCTCGCTCACTCCTC |
|
ATCAAATGTCAAACTGGGGTCAC |
| MMP-9 |
TGCCCGGACCAAGGATACAGTTT |
|
AGGCCGTGGCTCAGGTTCAGG |
| MMP-13 |
CCCCAACCCTAAACATCCAAAAAC |
|
TTAAAAACAGCTCCGCATCAACCT |
| GAPDH |
TGGTATCGTGGAAGGACTCATGAC |
|
ATGCCAGTGAGCTTCCCGTTCAGC |
Western blotting
Specimens from the patients and the mouse model were
lysed using a lysis buffer (50 mM Tris-HCl, pH 7.4; 150 mM NaCl, 1%
Triton X-100, 0.1% sodium dodecyl sulfate, 1 mM
ethylenediaminetetraacetic acid, 1 mM Na3VO4
and 1 mM NaF, protease inhibitor cocktail). The extracts were
incubated on ice for 20 min and centrifuged at 12,000 × g for 20
min at 4°C, and the supernatants were collected. Protein
concentrations were determined using a Bradford assay (Bio-Rad
Laboratories, Hercules, CA, USA) and proteins were separated using
10% Bis-Tris gel (Bio-Rad Laboratories) electrophoresis,
transferred to a nitrocellulose membrane (Bio-Rad Laboratories),
and western blot analysis was performed. The primary antibodies are
summarized in Table III. The
secondary mouse monoclonal antibodies antibodies included
anti-mouse IgG (A0216), anti-rabbit IgG (A0239) or anti-goat IgG
(A0182; determined by primary antibodies) at a dilution of
1:1,000-2,000 (Amersham Biosciences, Needham, MA, USA).
Subsequently, the results were detected by enhanced
chemiluminescence (Amersham Pharmacia, Piscataway, NJ, USA).
 | Table IIIAntibodies used in western blot
analysis. |
Table III
Antibodies used in western blot
analysis.
| Protein | Catalogue number | Dilution |
|---|
| MMP-1 | ab52631 | 1:100 |
| MMP-3 | ab18898 | 1:100 |
| MMP-8 | ab81286 | 1:100 |
| MMP-9 | ab38898 | 1:100 |
| MMP-1 | ab39012 | 1:100 |
| β-actin | ab8227 | 1:500 |
GeneChip technology
Total RNA was extracted from cells as described
above. Total RNA samples were then analyzed using a GeneChip assay
(Affymetrix, Inc., Santa Clara, CA, USA). Three replicates were
performed for each experimental group. Gene expression analysis was
performed using GeneChip (Affymetrix), according to the
manufacturer’s instructions. Gene expression analysis was performed
using three arrays and three independent mRNA samples for each
treatment. Microarray data were analyzed using Bio MAS 3.0 software
(CapitalBio Corporation, Beijing, China). A fold change of ≥2 or
≤0.5 and a Q-value of ≤5% were used as cutoff criteria The value of
the control group was set as a standard of 1.0 and the value of the
other two groups was plotted with respect to the control group.
Differentially expressed genes were screened and clustered among
the control group, mock group and FLL group using an Affymetrix
Rat230.2 array.
Gene ontology (GO) analysis
The associations between gene expression and
biological processes, molecular functions, and cellular
compartments, were annotated using the GOTree Machine (11). GOTree Machine uses a hypergeometric
test to evaluate the significance of gene enrichment for each
category by determining whether the observed number of gene counts
exceed the expected counts. Results were visualized as a directed
acyclic graph in order to demonstrate the relationships among the
enriched GO categories.
Statistical analysis
Using GraphPad 5.0 software (GraphPad Software,
Inc., La Jolla, CA, USA), data were analyzed using Student’s
t-test. P<0.05 was considered to indicate a statistically
significant difference.
Results
MMP and inflammatory cytokine levels in
healthy and LDH tissues
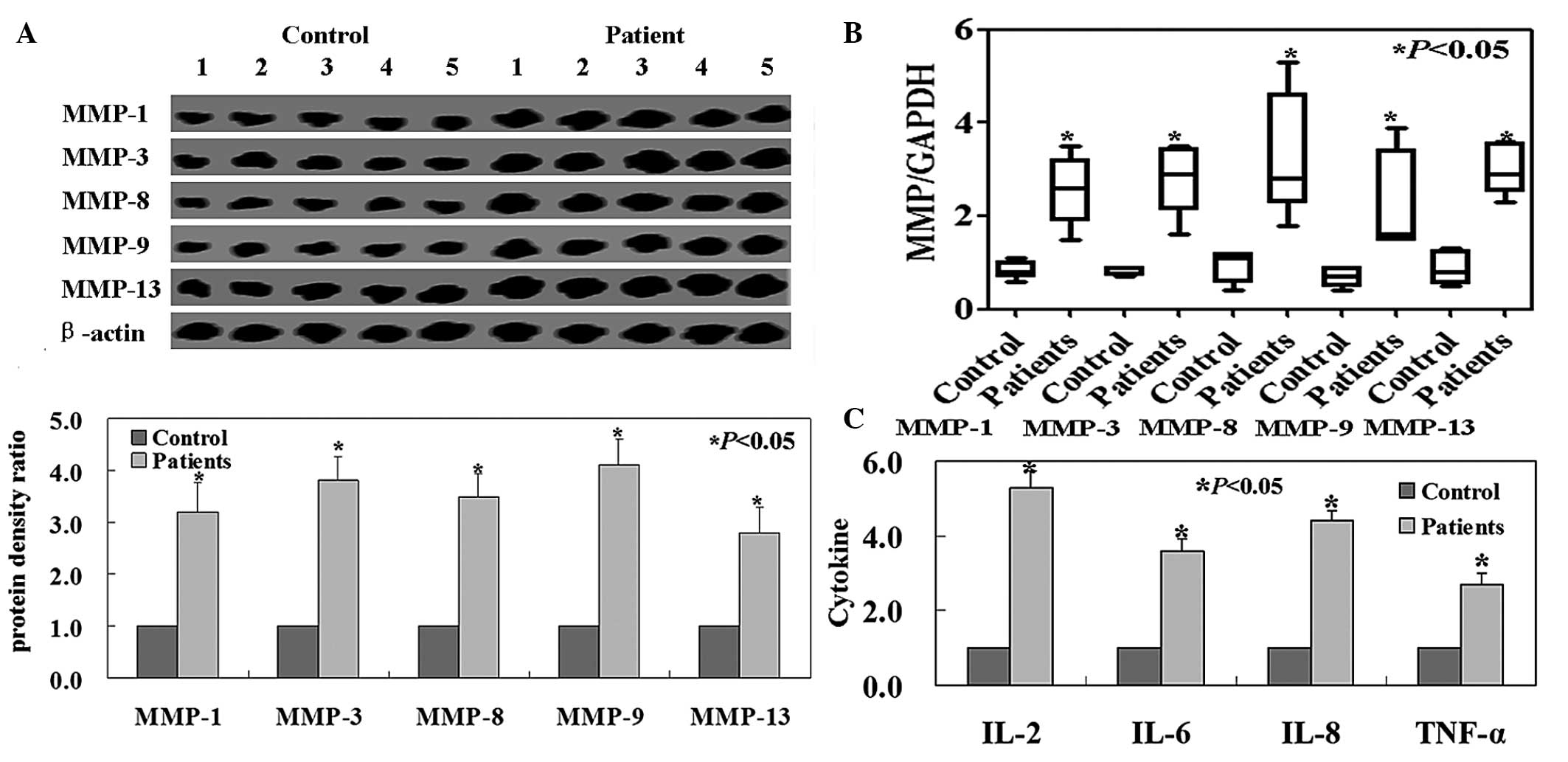
MMP-1, -3, -8, -9 and -13 protein expression levels
were downregulated in healthy tissues compared with those in LDH
tissues (P<0.05, Fig. 1A).
Transcript levels of MMPs were significantly higher in LDH tissues
than in healthy tissues (P<0.05, Fig. 1B). Inflammatory cytokine expression
levels were significantly lower in the healthy control group
compared with those in the LDH group (Fig. 1C; P<0.05).
Effects of FLL on mechanical allodynia
and thermal hyperalgesia
Paralysis was not observed in rats throughout the
experiments. Significant attenuation of mechanical allodynia was
observed in the FLL-treated group compared with the control and
mock groups (P<0.05, Fig. 2A).
Mean withdrawal latency in rats in the FLL-treated group was
significantly higher than in rats in the control and mock groups
(P<0.05, Fig. 2B). IL-2, IL-6,
IL-8 and TNF-α expression levels were significantly higher in the
control and mock group compared with those in the FLL-treated group
(P<0.05; Fig. 2C). Protein
expression levels of MMPs in the FLL-treated group were lower than
those in the mock and control groups (Fig. 2D).
Affymetrix GeneChip analysis
The expression of 645 rat transcripts was analyzed
using the GeneChip Rat Genome array (Affymetrix). The expression of
125 genes was upregulated and that of 345 genes was downregulated
in the FLL-treated group compared with the control and mock groups
(P<0.05, Fig. 3). A GO analysis
was conducted using the GOTree Machine. The GO analysis produced
clusters of enriched differentially expressed genes (P<0.01) in
three categories: Biological processes, cellular component and
molecular function. In the GO terms tree (Figs. 4 and 5) the association and overlap of MMPs and
inflammatory cytokines are demonstrated. In total, enriched GO
terms were found in 71 subcategories under biological process, 16
subcategories under cellular component and 51 subcategories under
molecular function. In biological process ontology, the results
indicated that cytokine production, cellar localization, protein
secretion are predominantly associated with FLL treatment.
Discussion
In the present study, downregulation of the protein
expression of MMPs and upregulation of IL-2, IL-6, IL-8 and TNF-α
expression was observed in the patients with LDH compared with that
in control group. Weiler et al (12) demonstrated a significant
correlation between MMP protein activity and histological signs of
degeneration in patients with LDH. Similarly, Tsarouhas et
al (13) found higher MMP mRNA
levels in invertebral disc herniation samples compared with samples
from other disc disorders and control specimens. A previous study
has demonstrated that inflammatory cytokines are produced by
infiltrating mononuclear cells after the onset of disc herniation
(14). Consistent with previous
studies, increased levels of inflammatory cytokines were observed
in the serum of patients with LDH compared with the control
group.
Furthermore, the present study demonstrated that FLL
ethanol extract may be used for the treatment of LDH. An et
al (15) found that FLL
treatment reduced inflammation via inhibition of nuclear factor-κB
in mouse peritoneal macrophages. The results of the present study
also demonstrated that daily FLL ethanol extract treatment
decreased the levels of inflammatory cytokines in serum in rats
with LDH. Previous studies have also demonstrated that FLL promotes
bone development with increased mineral density and improved bone
mechanical properties (16,17).
It is hypothesized that bone development in response to FLL
treatment may benefit LDH treatment. However, the mechanisms
underlying these processes require further investigation.
Gene expression profiling and GO analysis were
conducted in order to explore the changes of mRNA expression in the
FLL-treated group. No changes among associated genes were observed.
However, the results of the GO analyses suggested that FLL may be
associated with a number of biological processes, including
cytokine production and secretion, cellular components, including
the proteasome, and molecular functions, including proteolysis. GO
terms for cytokine secretion and cytokine production were highly
enriched in the FLL treated group. Therefore, we focused further on
cytokine secretion, which were induced by FLL. The limitation of
this method is conventional gene expression signatures are simpler
as it requires less transformation of the data. Therefore, the
mechanistic meaning of their co-regulation remains difficult to
interpret.
In conclusion, the present study was the first to
demonstrate the potential of FLL treatment for LDH. The
pharmacological effects and active ingredients of FLL should be
investigated in future studies.
Acknowledgments
The authors would like to thank Dr Yu-Min Sun for
technical assistance.
References
|
1
|
Li ML: Progress in the study on fructus
Ligustri lucidi. Zhongguo Zhong Yao Za Zhi. 19:504–506. 1994.In
Chinese. PubMed/NCBI
|
|
2
|
Zhang Y, Lai WP, Leung PC, Wu CF, Yao XS
and Wong MS: Effects of Fructus Ligustri Lucidi extract on bone
turnover and calcium balance in ovariectomized rats. Biol Pharm
Bull. 29:291–296. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhang Y, Lai WP, Leung PC, Che CT and Wong
MS: Improvement of Ca balance by Fructus Ligustri Lucidi extract in
aged female rats. Osteoporos Int. 19:235–242. 2008. View Article : Google Scholar
|
|
4
|
Hoy D, March L, Brooks P, Woolf A, Blyth
F, Vos T and Buchbinder R: Measuring the global burden of low back
pain. Best Pract Res Clin Rheumatol. 24:155–165. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Golob AL and Wipf JE: Low back pain. Med
Clin North Am. 98:405–428. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Iwamoto J, Sato Y, Takeda T and Matsumoto
H: The return to sports activity after conservative or surgical
treatment in athletes with lumbar disc herniation. Am J Phys Med
Rehabil. 89:1030–1035. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Daneyemez M, Sali A, Kahraman S, Beduk A
and Seber N: Outcome analyses in 1072 surgically treated lumbar
disc herniations. Minim Invasive Neurosurg. 42:63–68. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Findlay GF, Hall BI, Musa BS, Oliveira MD
and Fear SC: A 10-year follow-up of the outcome of lumbar
microdiscectomy. Spine (Phila Pa 1976). 23:1168–1171. 1998.
View Article : Google Scholar
|
|
9
|
Obata K, Tsujino H, Yamanaka H, Yi D, et
al: Expression of neurotrophic factors in the dorsal root ganglion
in a rat model of lumbar disc herniation. Pain. 99:121–132. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sasaki N, Sekiguchi M, Kikuchi S and Konno
S: Anti-nociceptive effect of bovine milk-derived lactoferrin in a
rat lumbar disc herniation model. Spine (Phila Pa 1976).
35:1663–1667. 2010. View Article : Google Scholar
|
|
11
|
Zhang B, Schmoyer D, Kirov S and Snoddy J:
GOTree Machine (GOTM): A web-based platform for interpreting sets
of interesting genes using Gene Ontology hierarchies. BMC
Bioinformatics. 5:162004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Weiler C, Nerlich AG, Zipperer J,
Bachmeier BE and Boos N: 2002 SSE award competition in basic
science: Expression of major matrix metalloproteinases is
associated with intervertebral disc degradation and resorption. Eur
Spine J. 11:308–320. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tsarouhas A, Soufla G, Katonis P, Pasku D,
Vakis A and Spandidos DA: Transcript levels of major MMPs and
ADAMTS-4 in relation to the clinicopathological profile of patients
with lumbar disc herniation. Eur Spine J. 20:781–790. 2011.
View Article : Google Scholar :
|
|
14
|
Yoshida M, Nakamura T, Sei A, Kikuchi T,
Takagi K and Matsukawa A: Intervertebral disc cells produce tumor
necrosis factor alpha, interleukin-1beta, and monocyte
chemoattractant protein-1 immediately after herniation: An
experimental study using a new hernia model. Spine (Phila Pa 1976).
30:55–61. 2005.
|
|
15
|
An HJ, Jeong HJ, Um JY, Park YJ, Park RK,
Kim EC, Na HJ, Shin TY, Kim HM and Hong SH: Fructus Ligustrum
lucidi inhibits inflammatory mediator release through inhibition of
nuclear factor-kappaB in mouse peritoneal macrophages. J Pharm
Pharmacol. 59:1279–1285. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Feng X, Lyu Y, Wu Z, Fang Y, Xu H, Zhao P,
Xu Y and Feng H: Fructus ligustri lucidi ethanol extract improves
bone mineral density and properties through modulating calcium
absorption-related gene expression in kidney and duodenum of
growing rats. Calcif Tissue Int. 94:433–441. 2014. View Article : Google Scholar
|
|
17
|
Lyu Y, Feng X, Zhao P, et al: Fructus
Ligustri Lucidi (FLL) ethanol extract increases bone mineral
density and improves bone properties in growing female rats. J Bone
Miner Metab. 32:616–626. 2014. View Article : Google Scholar
|