Introduction
Arsenic trioxide (As2O3) has
been used medicinally for >2,400 years for conditions ranging
from infectious diseases to cancer (1). It was not until 1970, that
researchers at Harbin Medical University observed its ability to
treat acute promyelocytic leukemia (2,3).
As2O3 has also been demonstrated to exert
inhibitory effects in various types of cancer. In MGC-803 human
gastric cancer cells, As2O3 was shown to
inhibit cell growth and to induce cell apoptosis (4). Similar findings were observed in
esophageal carcinoma (5),
neuroblastoma (6), prostate and
ovarian carcinoma (7), and breast
cancer (8) cells. Several
mechanism of action involved in the
As2O3-induced apoptosis of cancer cells have
been identified (8,9).
The human ether-à-go-go-related gene (hERG) is
expressed in a number of tumor cell lines of various histogenetic
origins, although is not present in the corresponding healthy
cells, which indicates an association between hERG expression and
the development of cancer (10–12).
In a previous study by this group, it was identified that
As2O3 induces the apoptosis of MCF-7 breast
cancer cells via inhibition of hERG channels (13). To date, the molecular mechanisms
underlying the regulation of hERG expression in breast cancer cells
remain to be elucidated.
MicroRNAs (miRNAs/miRs) are a class of
evolutionarily conserved, endogenous non-coding RNAs of ~22
nucleotides in length. They regulate gene expression at the
post-transcriptional level by binding to the 3′-untranslated region
(UTR) of the target mRNA. Altered expression of miRNAs has been
demonstrated to be involved in cancer development and progression.
To date, a number of downregulated miRNAs identified in breast
cancer have also been shown to be associated with apoptosis
(14,15) and metastasis (16,17).
Through computational prediction, it was shown that
miR-328 may target the 3′-UTR of hERG mRNA. These findings prompted
the hypothesis that As2O3 may inhibit breast
cancer cell growth via the miR-328/hERG pathway. The present study
was conducted to assess this hypothesis.
Materials and methods
Materials
As2O3 was obtained from
Jiangsu Yida Chemical Co., Ltd. (Jiangyin, China) and was diluted
with phosphate-buffered saline (PBS) to prepare a 10 mM stock
solution, which was stored at 4°C in darkness.
Cell line and cell culture
The MCF-7 human breast cancer cell line was obtained
from the American Type Culture Collection (Manassas, VA, USA). The
cells were maintained in RPMI-1640 (Hyclone, Logan, UT, USA)
supplemented with 10% fetal bovine serum (Hyclone) in a humidified
atmosphere with 5% CO2 at 37°C.
In vivo tumor xenograft model
A total of 30 female BALB/c nude mice (nu/nu, 8
weeks old) were obtained from the Third Affiliated Hospital of
Harbin Medical University (Harbin, China). The MCF-7 cells were
inoculated subcutaneously into the flank of each mouse
(5×106 cells in 200 μl PBS) (18). At 2 weeks following tumor
implantation, the mice were randomly divided into three groups each
containing 10 mice. The As2O3-treated groups
were injected daily with 4 or 8 mg/kg of
As2O3 for 7 days The control group was
administered with an equal volume of saline. Tumor size was
measured using calipers and the volume was calculated according to
the formula: V = L × W2/2, where L
and W represent the length and width, respectively. The mice
were weighed twice a week. Any investigations using the mice were
performed in compliance with the guidelines of the Institute for
Laboratory Animal Research, Mudangjiang Medical University.
Luciferase assay
The 3′-UTR of the hERG mRNA was obtained by
polymerase chain reaction (PCR) amplification, and the PCR product
was inserted into the firefly luciferase gene reporter construct
(pMIR-REPORTTM; Ambion Life Technologies, Austin, TX, USA) as
described previously (19).
Mutation of the hERG sequence was created using a Quick Change
Site-Directed Mutagenesis kit (Stratagene, Santa Clara, CA, USA).
The miR-328 precursor (pre-miR-328) and a control precursor
(scramble) were purchased from Ambion Life Technologies.
For the luciferase assay, HEK-293 cells were
co-transfected with wild-type or mutant hERG 3′-UTR luciferase
reporter plasmid, and pS-Neg or miR-328 expression plasmid, using
Lipofectamine® 2000 (Invitrogen Life Technologies,
Carlsbad, CA, USA). The luciferase activity was determined using a
dual luciferase reporter assay system (Promega Corporation,
Madison, WI, USA) at 48 h following transfection, according to the
manufacturer’s instructions.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from MCF-7 cells using
TRIzol® reagent (Invitrogen Life Technologies) according
to the manufacturer’s instructions. miR-328 levels were quantified
using the mirVana qRT-PCR miRNA detection kit (Ambion Life
Technologies), according to the manufacturer’s instructions.
Variations in the expression of miR-328 between different RNA
samples were calculated following normalization to levels of
U6.
Western blot analysis
Western blot analysis was conducted as described
previously (20). The cells were
washed with cold PBS and lysed in lysis buffer [western blot
lysate: protease inhibitor cocktail (100:1); Beyotime Institute of
Biotechnology, Haimen, China]. The concentration of proteins was
determined using a bicinchoninic protein assay kit (Beyotime
Institute of Biotechnology). The samples were electrophoresed using
10% SDS-PAGE. Following electrophoresis, the gels were transferred
onto nitrocellulose membranes via electroblotting and blocked in 5%
non-fat milk in PBS for 2 h. Subsequently, the membrane was
incubated with polyclonal rabbit anti-hERG antibody (cat.no.
sc-20130; 1:200 dilution; Santa Cruz Biotechnology, Inc., Santa
Cruz, CA, USA) and polyclonal rabbit anti-GAPDH antibody (cat. no.
sc-25778; 1:1,000 dilution; Santa Cruz Biotechnology, Inc.) in PBS
and incubated at 4°C overnight. The following day, the membranes
were washed three times with PBS for 5 min at room temperature and
subsequently incubated with HRP-conjugated anti-rabbit IgG
polyclonal secondary antibodies (1:10,000 dilution; cat. no.
sc-2357, Santa Cruz Biotechnology, Inc.) for 2 h at room
temperature. The Odyssey infrared fluorescence scanning system
(LI-COR Biosciences, Lincoln, NE, USA) was used to detect protein
bands. The intensity of the bands was determined by densitometry
using Odyssey version 1.2 software (LI-COR Biosciences, Lincoln,
NE, USA).
Statistical analysis
All data are presented as the mean ± standard
deviation for three repeated experiments. Student’s t-test and SPSS
16.0 (SPSS, Inc., Chicago, IL, USA) were used for statistical
analysis. P<0.05 was considered to indicate a statistically
significant difference.
Results
As2O3 inhibits
tumor growth in MCF-7-inoculated nude mice
In order to examine the effect of
As2O3 on breast cancer cells in vivo,
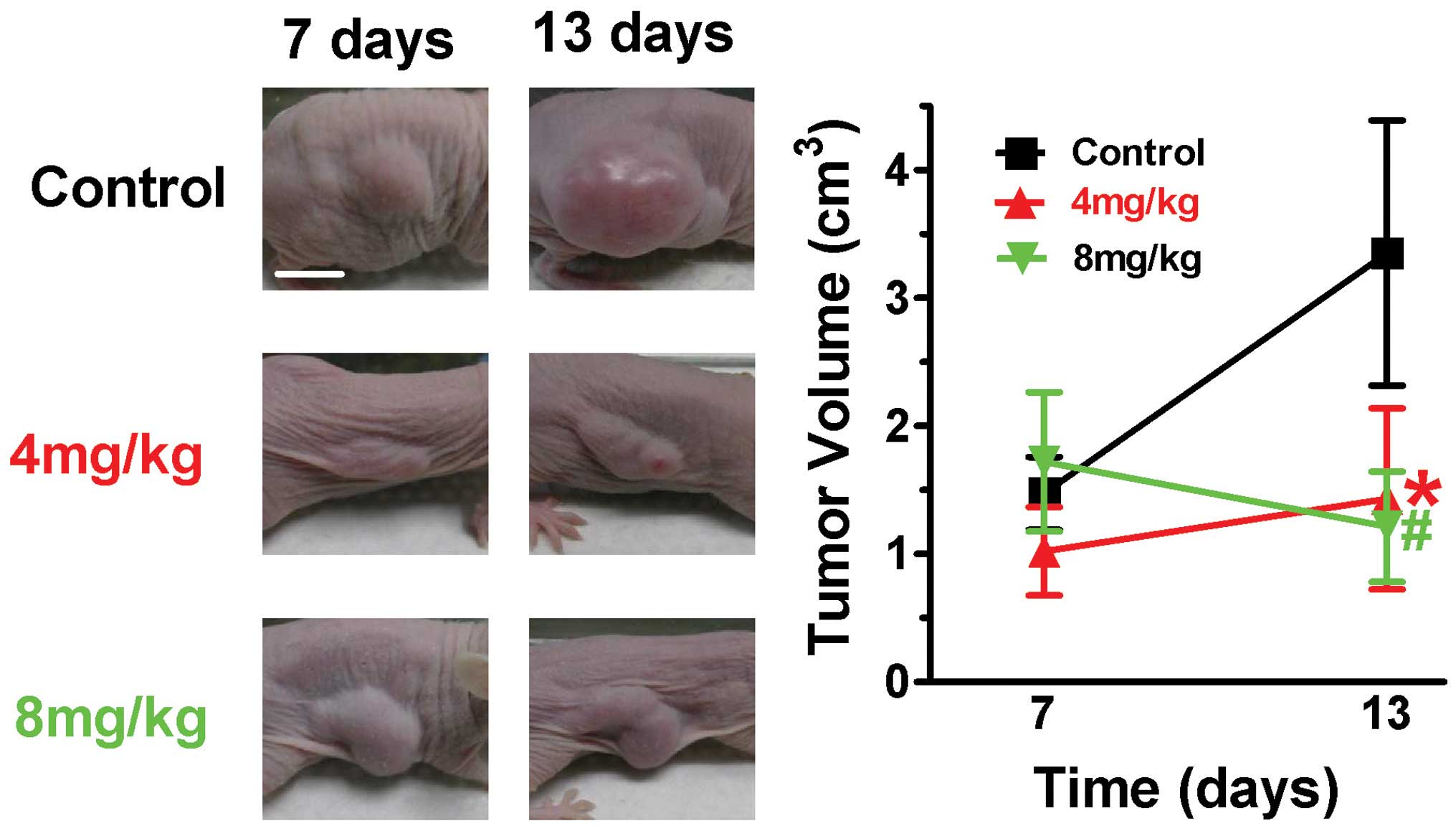
human tumor xenografts were investigated in nude mice. The size of
the tumor was measured, as shown in Fig. 1, in mice treated with 4 and 8
mg/kg, and As2O3-treated mice exhibited
significant tumor growth retardation. The tumor volume of the
non-treated mice increased between 1.19±0.31 and 3.18±0.67
cm3, in comparison with mice treated with 4 mg/kg
As2O3, which exhibited significant growth
retardation, with tumor growth of between 0.74±0.30 and 0.97±0.57
cm3. Notably, for the groups treated with 8 mg/kg
As2O3, the tumor volume decreased between
1.44±0.55 and 1.02±0.42 cm3. Regarding side effects of
As2O3, no signs of toxicity were observed
between the different groups, as judged at autopsy (data not
shown). The present results indicated that
As2O3 is effective in inhibiting tumor growth
in MCF-7-inoculated nude mice.
As2O3 upregulates
expression of miR-328 and downregulates expression of hERG
In order to further examine the mechanism underlying
the regulation of hERG expression, the 3′-UTR of hERG was analyzed
using the online databases, TargetScan Human version 6.2
(http://www.targetscan.org) and miRBase
version 20.0 (http://www.mirbase.org), to identify
potential interacting miRNAs. miR-133 and miR-328 were selected as
likely interactors. Of the two selected miRNAs, miR-133 has been
previously described as a muscle-specific miRNA, which has been
investigated primarily with regard to the heart (21,22).
Therefore, miR-328, a known tumor suppressor, was selected for
further analysis.
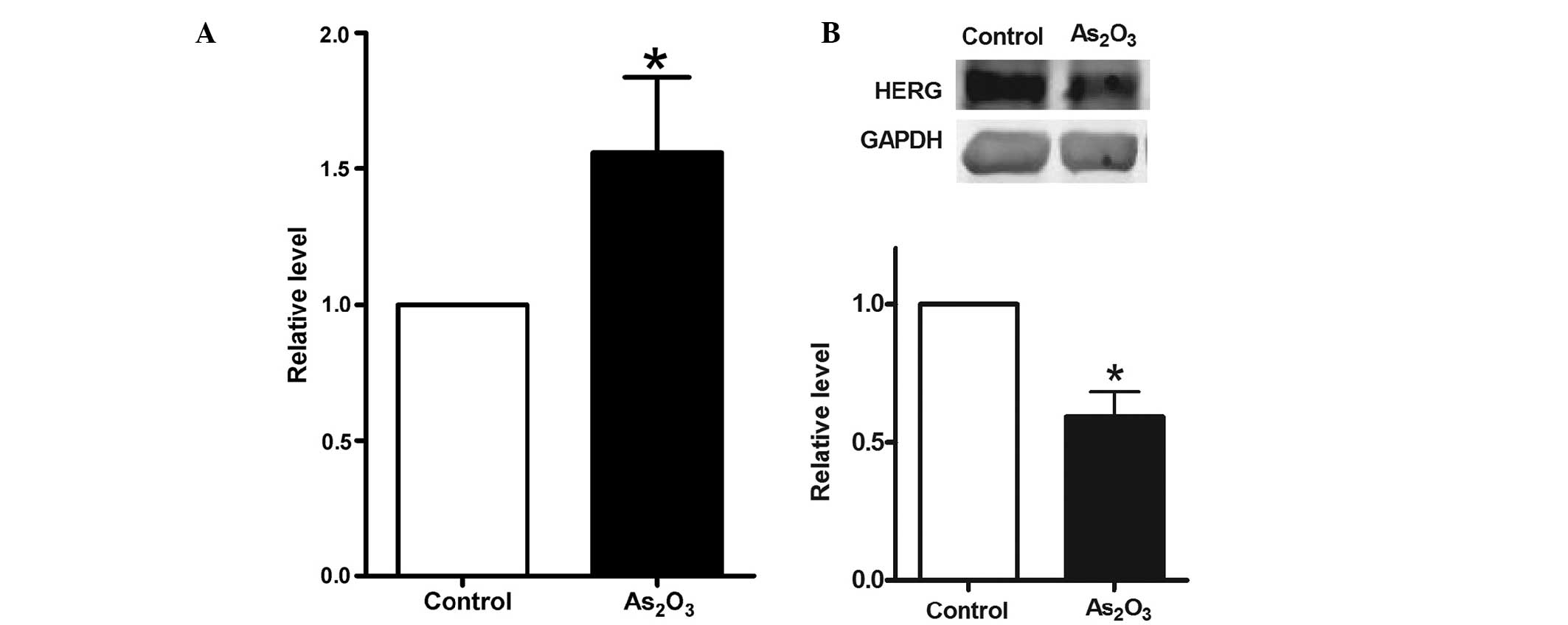
Initially, the expression of miR-328 and hERG was
detected in MCF-7 cells. The level of miR-328 was determined using
RT-qPCR after MCF-7 cells had been treated with 8 μM
As2O3 for 48 h. As shown in Fig. 2A, the expression of miR-328 in the
treated cells increased by 56±0.34% compared with the level in
non-treated cells. Subsequently, the level of hERG was determined
using western blot analysis, following treatment of MCF-7 cells
with 8 μM As2O3 for the same time
period. As shown in Fig. 2B, the
expression of hERG in the treated group decreased by 41±0.11%
compared with that in the control group. It was therefore
hypothesized that there is an inverse correlation between miR-328
and hERG protein expression levels.
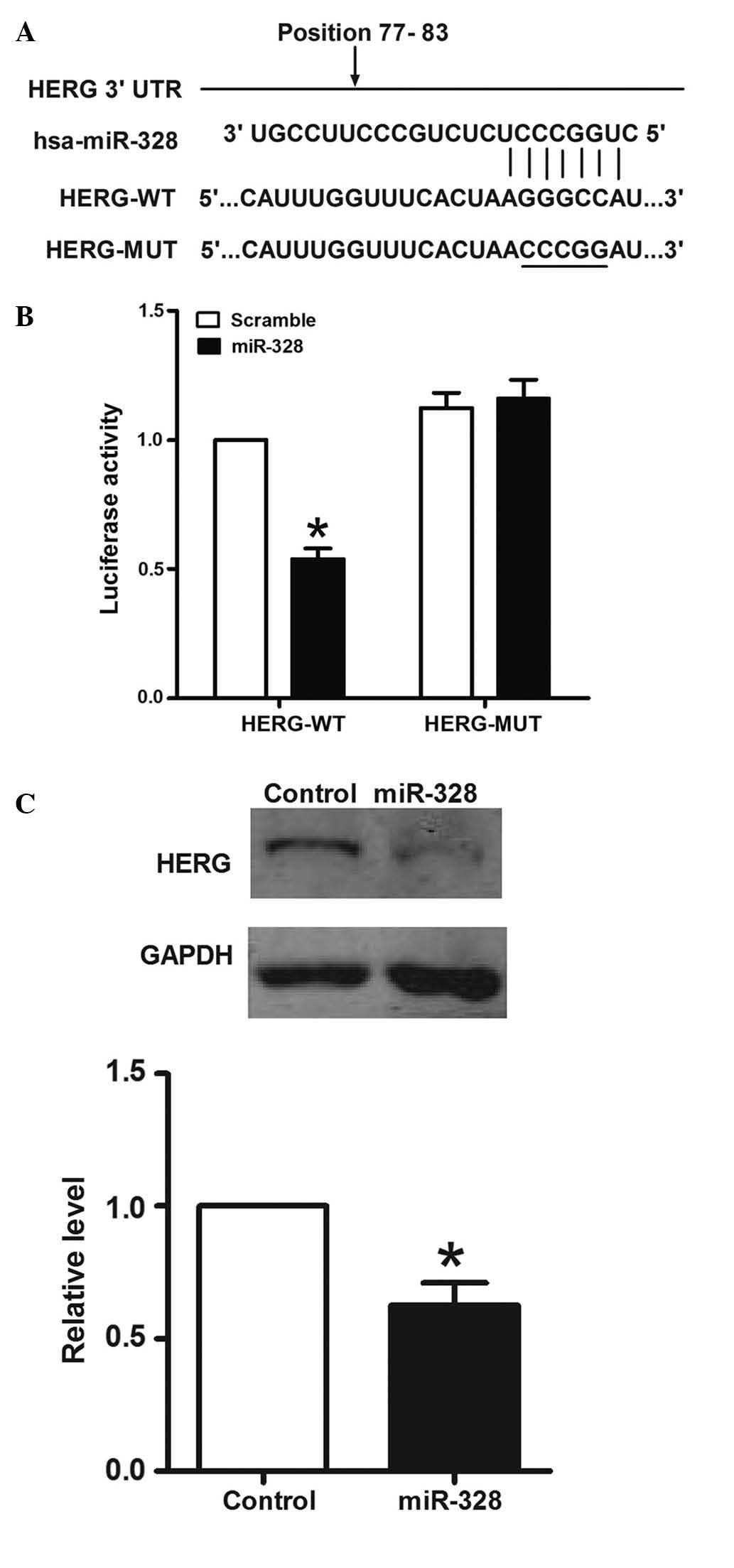
hERG is a direct target of miR-328
It was hypothesized that miR-328 interacts directly
with the 3′-UTR of hERG mRNA to suppress hERG expression. In order
to assess this hypothesis, the ability of miR-328 to regulate the
3′-UTR of hERG was evaluated using luciferase reporter assays. The
region from nucleotide +77 to nucleotide +83 of the hERG sequence
(NM-001204798) was cloned downstream of a reporter luciferase gene
(Fig. 3A).
HEK-293 cells were co-transfected with reporter
plasmid and pre-miR-328/scramble miR. As a result, co-transfection
of synthetic miR-328 and hERG-wildtype reduced the luciferase
activity by ~46%, which was a significant difference compared with
the scramble group. However, co-transfection of miR-328 in cells
transfected with hERG-mutant did not significantly affect
luciferase activity (Fig. 3B).
The most straightforward prediction from the
luciferase reporter assay would be that ectopic expression of
miR-328 should reduce hERG protein levels in MCF-7 cells. In order
to further investigate the interaction between miR-328 and hERG,
MCF-7 cells were transfected with pre-miR-328. Following
pre-miR-328 transfection in MCF-7 cells (Fig. 3C), western blotting was conducted
to measure the level of hERG protein. It was identified that the
expression of hERG protein was downregulated by ~37% in
pre-miR-328-treated MCF-7 cells. These data suggested that miR-328
directly recognizes the 3′-UTR of hERG mRNA and inhibits hERG
translation.
Discussion
Breast cancer is the most common type of cancer and
is the leading cause of cancer-associated mortality in females,
accounting for 25% of new cancer cases and 15% of the total global
cancer-associated mortality in 2012 (23). Although important advances have
been made in the early detection, prevention and treatment of
breast cancer, chemotherapy remains the primary method of
treatment, which leads to cumulative toxicity and has additional
tolerability problems (24). An
improved knowledge of tumor biology is providing the opportunity to
treat breast cancer with a new class of anticancer drugs.
hERG is overexpressed in numerous types of cancer in
humans, including endometrial cancer, leukemia, melanoma and
neuroblastoma, and inhibition of the hERG channel may reduce cancer
cell growth and proliferation (25–27).
Furthermore, this group has recently demonstrated that
As2O3 induces the apoptosis of MCF-7 cells
through inhibition of the hERG channel (13). However, the mechanisms that
regulate the expression of the hERG channel remain to be
elucidated. Therefore, an objective of the present study was to
identify potential miRNAs that are able to regulate hERG expression
in human breast cancer cells.
The studies discussed thus far on miRNAs, reflect
their ability to act as onco-miRNAs or oncosuppressor-miRNAs, by
favoring or inhibiting tumor progression. In the present study, it
was identified that in MCF-7 breast cancer cells, miR-328 is able
to target the 3′UTR of hERG and to decrease its level of
expression, thus suggesting that by maintaining hERG expression at
a low level, the activity of miR-328 may contribute to tumor
suppression.
In previous years, a number of miRNAs have been
implicated in the development of human breast cancer. Iorio et
al (28) first demonstrated
miRNA dysregulation in human breast cancer. The authors found that
miR-10b, miR-125b and miR-145 were downregulated, while miR-21 and
miR-155 were upregulated, suggesting that they may act as potential
tumor suppressor genes or oncogenes. Subsequently, additional
functional studies were conducted in order to identify specific
miRNAs involved in breast cancer. Wang et al (29) observed that miR-145 was
downregulated in MCF-7 cells, and overexpression of miR-145
suppressed MCF-7 cell growth and induced apoptosis. Kong et
al (30) demonstrated that
miR-155 induces cell survival by targeting forkhead box O3a in
breast cancer cells. Song et al (31) revealed that miR-21 negatively
regulates TIMP metallopeptidase inhibitor 3 expression in breast
cancer cells and promotes breast cancer invasion in multiple cell
lines in vitro.
miR-328 expression in human cancer has not been
extensively investigated thus far. Previously, miR-328 has been
shown to be expressed in the small intestine and liver (32). Furthermore, it appears to be
downregulated in high-grade gliomas (33) and a previous study demonstrated
that it was also downregulated in the human colorectal cancer cell
lines, HT29, HCT116 and HCT8 (32). In 5-Fluorouracil-treated MCF-7
cells, the expression of miR-328 increased in comparison with
control cells (34). Similar
results were observed in the present study, where miR-328
expression was upregulated in As2O3-treated
MCF-7 cells.
Notably, miR-328 has been demonstrated to have dual
actions in the regulation of cell functions, through base pairing
with mRNA targets and via a decoy activity that interferes with the
function of regulatory proteins (35). A recent study demonstrated that
miR-328 was able to target the 3′UTR of the breast cancer
resistance protein, ATP-binding cassette sub-family G member 2
(ABCG2) and, consequently, repress ABCG2 protein expression and
increase cancer cell sensitivity to drug treatment (36). miR-328 is also closely associated
with cell cycle progression. It may increase proliferation of HeLa
and SKBr3 cells, via downregulation of protein tyrosine
phosphatase, receptor type, J expression (37). Furthermore, ectopic miR-328
expression in glioblastoma cells may significantly suppress cell
proliferation (33). Thus, the
role of miR-328 in a appears to be dependent on the particular cell
type involved.
The present data revealed that miR-328 expression is
inversely correlated with hERG expression in MCF-7 breast cancer
cells. However, the mechanisms underlying the regulation of miR-328
in human cancer remain to be elucidated and therefore require
further investigation.
In conclusion, the results of the present study
demonstrated a novel mechanism for the regulation of hERG
expression by miR-328 in breast cancer cells, indicating that
miR-328-hERG signaling mediated by As2O3 may
represent a novel therapeutic target for breast cancer.
Acknowledgments
The present study was supported by the Scientific
Research Fund of Heilongjiang Provincial Health Department (grant
no. 2011-323), the Scientific Research Fund of Mudanjiang Medical
University (grant no. 2011-07), the Fund for Emergency Management
of the National Natural Science Foundation of China (grant no.
81441113) and the Youth Science and Technology Project of
Heilongjiang Traditional Chinese Medicine (grant no. ZQG-056).
References
|
1
|
Klaassen CD: Heavy metals and heavy-metal
antagonists. The Pharmacological Basis of Therapeutics. Gilman:
McGraw-Hill; New York, NY: pp. 649–1672. 1996
|
|
2
|
Cyranoski D: Arsenic patent keeps drug for
rare cancer out of reach of many. Nat Med. 13:10052007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sun HD, Ma L, Hu XC and Zhang TD: Ai-Lin I
treated 32 cases of acute promyelocytic leukemia. Chin J Integr
Chin W Med. 12:170–171. 1992.
|
|
4
|
Zhang TC, Cao EH, Li JF, Ma W and Qin JF:
Induction of apoptosis and inhibition of human gastric cancer
MGC-803 cell growth by arsenic trioxide. Eur J Cancer.
35:1258–1263. 1999. View Article : Google Scholar
|
|
5
|
Shen ZY, Shen J, Cai WJ, Hong C and Zheng
MH: The alteration of mitochondria is an early event of arsenic
trioxide induced apoptosis in esophageal carcinoma cells. Int J Mol
Med. 5:155–158. 2000.PubMed/NCBI
|
|
6
|
Akao Y, Nakagawa Y and Akiyama K: Arsenic
trioxide induces apoptosis in neuroblastoma cell lines through the
activation of caspase 3 in vitro. FEBS Lett. 455:59–62. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Uslu R, Sanli UA, Sezgin C, Karabulut B,
Terzioglu E, Omay SB and Goker E: Arsenic trioxide-mediated
cytotoxicity and apoptosis in prostate and ovarian carcinoma cell
lines. Clin Cancer Res. 6:4957–4964. 2000.
|
|
8
|
Chow SK, Chan JY and Fung KP: Inhibition
of cell proliferation and the action mechanisms of arsenic trioxide
(As2O3) on human breast cancer cells. J Cell Biochem. 93:173–187.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ye J, Li A, Liu Q, Wang X and Zhou J:
Inhibition of mitogen-activated protein kinase kinase enhances
apoptosis induced by arsenic trioxide in human breast cancer MCF-7
cells. Clin Exp Pharmacol Physiol. 32:1042–1048. 2005. View Article : Google Scholar
|
|
10
|
Crociani O, Guasti L, Balzi M, et al: Cell
cycle-dependent expression of HERG1 and HERG1B isoforms in tumor
cells. J Biol Chem. 278:2947–2955. 2003. View Article : Google Scholar
|
|
11
|
Guasti L, Crociani O, Redaelli E, Pillozzi
S, Polvani S, Masselli M, Mello T, Galli A, Amedei A, Wymore RS, et
al: Identification of a posttranslational mechanism for the
regulation of hERG1 K+ channel expression and hERG1 current density
in tumor cells. Mol Cell Biol. 28:5043–5060. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Arcangeli A: Expression and role of hERG
channels in cancer cells. Novartis Found Symp. 266:225–232.
2005.PubMed/NCBI
|
|
13
|
Wang Y, Zhang Y, Yang L, Cai B, Li J, Zhou
Y, Yin L, Yang L, Yang BF and Lu YJ: Arsenic trioxide induces the
apoptosis of human breast cancer MCF-7 cells through activation of
caspase-3 and inhibition of HERG channels. Exp Ther Med. 2:481–486.
2011.PubMed/NCBI
|
|
14
|
Wang S, Bian C, Yang Z, Bo Y, Li J, Zeng
L, Zhou H and Zhao RC: miR-145 inhibits breast cancer cell growth
through RTKN. Int J Oncol. 34:1461–1466. 2009.PubMed/NCBI
|
|
15
|
Kato M, Paranjape T, Müller RU, Nallur S,
Gillespie E, Keane K, Esquela-Kerscher A, Weidhaas JB and Slack FJ:
The mir-34 microRNA is required for the DNA damage response in vivo
in C. elegans and in vitro in human breast cancer cells. Oncogene.
28:2419–2424. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gregory PA, Bert AG, Paterson EL, Barry
SC, Tsykin A, Farshid G, Vadas MA, Khew-Goodall Y and Goodall GJ:
The miR-200 family and miR-205 regulate epithelial to mesenchymal
transition by targeting ZEB1 and SIP1. Nat Cell Biol. 10:593–601.
2008. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Valastyan S, Reinhardt F, Benaich N,
Calogrias D, Szász AM, Wang ZC, Brock JE, Richardson AL and
Weinberg RA: A pleiotropically acting microRNA, miR-31, inhibits
breast cancer metastasis. Cell. 137:1032–1046. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wu G, Qiu XL, Zhou L, Zhu J, Chamberlin R,
Lau J, Chen PL and Lee WH: Small molecule targeting the Hec1/Nek2
mitotic pathway suppresses tumor cell growth in culture and in
animal. Cancer Res. 68:8393–8399. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yang B, Lin H, Xiao J, Lu Y, Luo X, Li B,
Zhang Y, Xu C, Bai Y, Wang H, et al: The muscle-specific microRNA
miR-1 regulates cardiac arrhythmogenic potential by targeting GJA1
and KCNJ2. Nat Med. 13:486–491. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lu Y, Zhang Y, Wang N, Pan Z, Gao X, Zhang
F, Zhang Y, Shan H, Luo X, Bai Y, et al: MicroRNA-328 contributes
to adverse electrical remodeling in atrial fibrillation.
Circulation. 122:2378–2387. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shan H, Zhang Y, Lu Y, Zhang Y, Pan Z, Cai
B, Wang N, Li X, Feng T, Hong Y, et al: Downregulation of miR-133
and miR-590 contributes to nicotine-induced atrial remodelling in
canines. Cardiovasc Res. 83:465–472. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cai B, Pan Z and Lu Y: The roles of
microRNAs in heart diseases: A novel important regulator. Curr Med
Chem. 17:407–411. 2010. View Article : Google Scholar
|
|
23
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. Feb 4–2015.Epub ahead of print. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Vahdat LT, Pruitt B, Fabian CJ, Rivera RR,
Smith DA, Tan-Chiu E, Wright J, Tan AR, Dacosta NA, Chuang E, et
al: Phase II study of eribulin mesylate, a halichondrin B analog,
in patients with metastatic breast cancer previously treated with
an anthracycline and a taxane. J Clin Oncol. 27:2954–2961. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li H, Liu L, Guo L, et al: HERG K+ channel
expression in CD34+/CD38−/CD123(high) cells and primary leukemia
cells and analysis of its regulation in leukemia cells. Int J
Hematol. 87:387–392. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Afrasiabi E, Hietamäki M, Viitanen T,
Sukumaran P, Bergelin N and Törnquist K: Expression and
significance of HERG (KCNH2) potassium channels in the regulation
of MDA-MB-435S melanoma cell proliferation and migration. Cell
Signal. 22:57–64. 2010. View Article : Google Scholar
|
|
27
|
Zhao J, Wei XL, Jia YS and Zheng JQ:
Silencing of herg gene by shRNA inhibits SH-SY5Y cell growth in
vitro and in vivo. Eur J Pharmacol. 579:50–57. 2008. View Article : Google Scholar
|
|
28
|
Iorio MV, Ferracin M, Liu CG, Veronese A,
Spizzo R, Sabbioni S, Magri E, Pedriali M, Fabbri M, Campiglio M,
et al: MicroRNA gene expression deregulation in human breast
cancer. Cancer Res. 65:7065–7070. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang S, Bian C, Yang Z, Bo Y, Li J, Zeng
L, Zhou H and Zhao RC: miR-145 inhibits breast cancer cell growth
through RTKN. Int J Oncol. 34:1461–1466. 2009.PubMed/NCBI
|
|
30
|
Kong W, He L, Coppola M, Guo J, Esposito
NN, Coppola D and Cheng JQ: MicroRNA-155 regulates cell survival,
growth, and chemosensitivity by targeting FOXO3a in breast cancer.
J Biol Chem. 285:17869–17879. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Song B, Wang C, Liu J, Wang X, Lv L, Wei
L, Xie L, Zheng Y and Song X: MicroRNA-21 regulates breast cancer
invasion partly by targeting tissue inhibitor of metalloproteinase
3 expression. J Exp Clin Cancer Res. 29:292010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lee EJ, Baek M, Gusev Y, Brackett DJ,
Nuovo GJ and Schmittgen TD: Systematic evaluation of microRNA
processing patterns in tissues, cell lines, and tumors. RNA.
14:35–42. 2008. View Article : Google Scholar :
|
|
33
|
Wu Z, Sun L, Wang H, Yao J, Jiang C, Xu W
and Yang Z: MiR-328 expression is decreased in high-grade gliomas
and is associated with worse survival in primary glioblastoma. PLoS
One. 7:e472702012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Shah MY, Pan X, Fix LN, Farwell MA and
Zhang B: 5-Fluorouracil drug alters the microRNA expression
profiles in MCF-7 breast cancer cells. J Cell Physiol.
226:1868–1878. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Eiring AM, Harb JG, Neviani P, Garton C,
Oaks JJ, Spizzo R, Liu S, Schwind S, Santhanam R, Hickey CJ, et al:
miR-328 functions as an RNA decoy to modulate hnRNP E2 regulation
of mRNA translation in leukemic blasts. Cell. 140:652–665. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Pan YZ, Morris ME and Yu AM: MicroRNA-328
negatively regulates the expression of breast cancer resistance
protein (BCRP/ABCG2) in human cancer cells. Mol Pharmacol.
75:1374–1379. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Paduano F, Dattilo V, Narciso D, Bilotta
A, Gaudio E, Menniti M, Agosti V, Palmieri C, Perrotti N, Fusco A,
et al: Protein tyrosine phosphatase PTPRJ is negatively regulated
by microRNA-328. FEBS J. 280:401–412. 2013. View Article : Google Scholar
|