Introduction
Hepatitis C virus (HCV) infection affects 170
million people worldwide and as a major cause of liver disease
worldwide, HCV is a potential cause of substantial morbidity and
mortality in the future and therefore, a health serious health
concern (1). Approximately 80% of
patients with HCV fail to clear the virus and <10% may develop
severe liver diseases, such as chronic hepatitis, liver cirrhosis
and hepatocarcinoma, which reduce the chance of patient survival
(2). Understanding HCV pathology
and the development of novel drugs with which to treat patients
with HCV is an ongoing challenge.
Microarrays and different types of high-throughput
approaches, for example, high-throughput RNA-Seq and
high-throughput DNA-Seq, for gene expression analysis have led to
an improved understanding of the processes underlying HCV
infection. These approaches are advantageous compared with
traditional approaches, which are restricted to analyzing small
numbers of genes (3). Microarray
technology does not require gene selection in advance, meaning that
the method is less biased and is capable of identifying genes that
are modified when cells are exposed to environmental changes
(4). Microarray technology aims to
establish gene regulatory networks and to identify interactions
among genes and their products. Carefully analyzed networks are
used to identify correlated genes that are associated with the same
biological processes or pathways, and to infer interactions among
molecules, such as physical association, metabolite flow,
regulatory associations and co-expression (5). In the present study, GRNinfer
software was used in order to establish a gene regulatory network
and to identify molecular interactions, including activation and
inhibition, in the activating transcription factor 3 (AFT3)
signaling pathway in healthy Huh7 (Huh7) and HCV-infected
(HCV-Huh7) cell lines.
Materials and methods
Microarray
The gene expression profile data GSE 20948
(http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE20948)
were extracted from the public Gene Expression Omnibus database
(http://www.ncbi.nlm.nih.gov/gds/).
Microarray analysis was performed for 14 Huh7 samples (2 samples, 6
h; 3 samples, 12 h; 3 samples, 18 h; 3 samples, 24 h; 3 samples, 48
h.) and 14 HCV-Huh7 samples (2 samples, 6 h; 3 samples, 12 h; 3
samples, 18 h; 3 samples, 24 h; 3 samples, 48 h); the latter had
been infected with HCV for 6, 12, 18, 24 or 48 h. The platform of
the data was GPL 570 (HG-u133-plus-2) affymetrix human Genome u 133
2.0 array.
Gene selection algorithms
Fifty HCV-Huh7 molecular markers were identified
using Multiexperiment Viewer (http://www.tm4.org/mev.html; Version 4.8.1-windows),
which belongs to TM4, in order to conduct a significant analysis of
microarrays (SAM). The TM4 suite consists of four major
applications, including Microarray Data Manager, TIGR_Spotfinder,
Microarray Data Analysis System. This software is a free,
open-source software released under the Artistic license, and is
OSI certified. SAM is a statistical technique, used to identify
significant genes in a set of microarray experiments. The
explanatory variable is gene expression measurement from a set of
microarray experiments, and the response variable is a grouping,
such as mock-infected or infected. Microarray raw data CEL files
were processed using expression console software of Affymetrix,
Inc. (Santa Clara, CA, USA). In the present study, 50 significant
genes using ATF3 as a target gene, which was on interest, were
further analyzed.
Network establishment of candidate
genes
ATF3 gene networks were constructed using GRNinfer
(6) and GVedit (http://portableapps.com/node/38245; version:
2.38) tools. GRNinfer is a novel mathematical method base on linear
programming and a decomposition procedure that infers gene
networks. The method ensures derivation of the most consistent
network structure reducing the issues associated with data
scarcity, yet improving reliability. The following equation
represents all of the possible networks for the same dataset:
J=(X′−A)U∧1VT+YV=J+YVT.
Where J=(Jij)m×m=∂f(x)/∂x is an
n×m Jacobian matrix or connectivity matrix,
X=(x(t1),…,x(tm))
and all n×m matrices with
x′i(tj)=[xi(tj+1)−xi(tj)]/[tj+1−tj]
for i=1,…,n;
j=1,…m.X(t)=(x1(t),…,xn(t)T∑Rn,
a=(a1…,an)T∑Rn,xi(t)
is the expression level (mRNA concentrations) of gene i at
time instance t.y=(yij) is an nxn
matrix, where yij is zero if
ej=0. U is a unitary m×n
matrix of left eigenvectors, ∧=diag
(e1,…,en) is a diagonal n×n
matrix containing the n eigenvalues and VT
is the transpose of a unitary n×n matrix of right
eigenvectors.
The parameters were λ, 0.0 and threshold,
1×10−9.
Functional annotation clustering
The database for annotation, visualization and
integrated discovery (DAVID; http://www.david.niaid.nih.gov) was used. The DAVID
gene function clustering tool provides representative annotation
and gene ontology (GO) term enrichment analysis, which separates
genes into different enrichment score groups according to the gene
annotation collective frequency occurrence (7,8).
Molecule annotation system 3.0 (MAS
3.0)
MAS (http://bioinfo.capitalbio.com/mas3/) is an analysis
platform, which adds biological function annotation to high
throughput microarray data. By integrating the relevant annotation
information from a number of public information databases, MAS
synthesizes biological data, including genes, proteins, functions,
expression, protein interactions, signaling pathways, diseases and
methylation. MAS enables the incorporation of genetic data from
Genebank, European molecular biology laboratory, SwissPort, GO,
KEGG, BioCarta, gene map annotator and pathway profiler (GenMapp),
mirBase, expected progeny differences, HRPD, MIND, BIND, Intact,
TRANSAC, UniGene, single nucleotide polymorphism database, OMIM,
InterPro, HUGO, mouse genome informatics and rat genome
database.
Results
Gene enrichment analyses
ATF3 was one of fifty genes which were significantly
differentially expressed between Huh7 and HCV-Huh7 cell lines (fold
change=8.782885; Table I). Gene GO
term enrichment analysis for ATF3 demonstrated that the molecular
function of ATF3 is associated with transcription corepressor
activity, protein binding, sequence-specific DNA binding and
protein dimerization activity. ATF3 biological processes are
associated with DNA-dependent transcription regulation. ATF3
cellular component is localized in the nucleus and nucleolus.
GenMAPP analysis demonstrated that ATF3 is associated with smooth
contraction, hypertrophy model, NetPath 5 and Hs transforming
growth factor β1 (TGF-β) NetPath 7, and that it exhibits
transcription cofactor activity. Disease analysis demonstrated that
ATF3 is associated with shock, leukemia, colorectal cancer,
neoplasm metastasis, nervous system disease, stomach cancer,
necrosis, leukemia T cell, lymphoma non-hodgkins, malignant
neoplasm of the breast, hyperalgesia, episodic ataxia type 2 and
hereditary ataxia overview.
 | Table IFifty genes exhibiting significant
differential expression between Huh7 and HCV-Huh7 cell lines
identified using SAM. |
Table I
Fifty genes exhibiting significant
differential expression between Huh7 and HCV-Huh7 cell lines
identified using SAM.
| Gene ID | Gene | Fold change |
|---|
| 201010_s_at | TXNIP | 21.518297 |
| 203438_at | STC2 | 20.873947 |
| 210587_at | Inhibin β E
chain | 19.26132 |
| 201008_s_at | TXNIP | 15.231962 |
| 203439_s_at | STC2 | 14.20261 |
| 201009_s_at | TXNIP | 13.7505045 |
| 238029_s_at | SLC 16, member
14 | 11.429825 |
| 205047_s_at | Asparagine
synthetase | 11.315236 |
| 231202_at | Aldehyde
dehydrogenase family 1 member | 11.254294 |
| 225283_at | Arrestin
domain-containing protein 4 | 10.549104 |
| 228653_at | SAMD5 | 9.061874 |
| 202672_s_at | Activating
transcription factor 3 | 8.782885 |
| 201300_s_at | Prion protein | 7.5039783 |
| 212909_at | LY6/PLAUR domain
containin 1 | 7.415823 |
| 218332_at | Brain expressed,
X-linked 1 | 7.3808937 |
| 203372_s_at | SOCS2 | 7.347132 |
| 219195_at | Peroxisome
proliferator-activated receptor γ coactivator 1-α | 6.9846096 |
| 212810_s_at | SLC 1 member 4 | 6.877748 |
| 210426_x_at | RORA | 6.6006436 |
| 209183_s_at | Chromosome 10 open
reading frame 10 | 6.530727 |
| 226682_at | RORA | 6.4361844 |
| 202393_s_at | Kruppel-like factor
10 | 6.4241524 |
| 214285_at | Fatty acid binding
protein 3 | 6.3771186 |
| 203882_at | Interferon
regulatory factor 9 | 6.129926 |
| 221523_s_at | Ras-related
glutamic-pyruvate transaminase binding D | 6.011061 |
| 212295_s_at | SLC 7 (cationic
amino acid transporter, Y+ system) member 1 | 5.976633 |
| 1554008_at | Oncostatin M
receptor | 5.8743997 |
| 218851_s_at | SFT2 domain
containing 3, WD repeat domain 33 | 5.831587 |
| 223681_s_at | InaD-like | 5.778195 |
| 203373_at | SOCS2 | 5.741121 |
| 209651_at | Transforming growth
factor β 1 induced transcript 1 | 5.6655536 |
| 201063_at | Reticulocalbin 1,
EF-hand calcium binding domain | 5.624495 |
| 1569433_at | SAMD5 | 5.4978223 |
| 206382_s_at | Brain-derived
neurotrophic factor | 5.459223 |
| 221530_s_at | Basic
helix-loop-helix family, member e41 | 5.424661 |
| 228708_at | Ras-related protein
Rab-27B | 5.3274593 |
| 209185_s_at | IRS 2 | 5.273203 |
| 209610_s_at | SLC 1 member 4 | 5.230212 |
| 214755_at |
UDP-N-acteylglucosamine pyrophosphorylase
1-like 1 | 5.206161 |
| 202847_at | Phosphoenolpyruvate
carboxykinase 2 | 5.199079 |
| 227037_at | Phospholipase D
family, member 6 | 5.078215 |
| 225539_at | Zinc finger protein
295 | 5.0584846 |
| 210479_s_at | RORA | 5.0370793 |
| 212290_at | SLC 7 (cationic
amino acid transporter, Y+ system) member 1 | 5.0187483 |
| 228519_x_at | Cold inducible RNA
binding protein | 4.95052 |
| 219584_at | Phospholipase A1
member A | 4.935018 |
| 242979_at | IRS 1 | 4.919549 |
| 202949_s_at | Four and a half LIM
domains 2 | 4.856401 |
| 1568813_at | LOC100506392 | 4.792747 |
| 212811_x_at | SLC 1 member 4 | 4.7892227 |
Identification of genes upstream and
downstream of ATF3
Using GRNInfer, a cell network was constructed,
which included gene clusters upstream and downstream of ATF3 in
Huh7 and HCV-Huh7 cell lines. According to DAVID software analysis,
12 gene clusters were identified downstream of ATF3 in HCV-Huh7
cells (10 activation and 2 inhibition; Table II). Using an MAS 3.0 software GO
analysis, ATF3 annotation regulation networks were enriched. The
upstream pathway of ATF3 in Huh7 cells included the activation of
stanniocalcin 2 cationic amino acid transporter, Y+ system member 1
(SLC7A1) and insulin receptor substrate 2 (IRS2), involving 19 GO
terms and three KEGG pathways, and inhibition of four and a half
LIM domains 2 (FHL2), involving five GO terms. The downstream
pathway of ATF3 in Huh7 cells included inhibition of zinc finger
protein 295 (ZNF295), involving two GO terms. The upstream pathway
of ATF3 in HCV-Huh7 cells included the activation of inhibin β E
chain (INHBE), asparagine synthetase (ASNS) and SLC7A1, involving
ten GO terms and four KEGG pathways, and the inhibition of
reticulocalbin 1, EF-hand calcium binding domain (RCN1), involving
one GO term. The downstream pathway of in HCV-Huh7 cells included
the activation of the following genes: Brain-derived neurotrophic
factor, cold inducible RNA binding protein, FHL2, fatty acid
binding protein 3, interferon regulatory factor 9, InaD-like
(INADL), IRS2, IRS1, kruppel-like factor 10, LOC100506392,
LY6/PLAUR domain containin 1, prion protein, peroxisome
proliferator-activated receptor γ coactivator 1-α, RAR-related
orphan receptor A, phospholipase A1 member A, SFT2 domain
containing 3, SMAD family member 5, TGFβ 1 induced transcript 1,
UDP-N-acteylglucosamine pyrophosphorylase 1-like 1, ZNF295 and
SLC7A1, involving 128 GO terms and 13 KEGG pathways. The downstream
pathway in HCV-Huh7 cells included the inhibition of the following
genes: SLC7A1, arrestin domain-containing protein 4, ASNS,
chromosome 10 open reading frame 10, INHBE, phosphoenolpyruvate
carboxykinase 2, PCK2, RCN1, stanniocalcin 2, suppressor of
cytokine signaling 2, SLC1A4 and thioredoxin interacting protein,
involving 37 GO terms and 13 KEGG pathways.
 | Table IIFunctional annotation clusters
(activation and inhibition) downstream of activating transcription
factor 3 in hepatitis C virus-infected Huh7 cells. |
Table II
Functional annotation clusters
(activation and inhibition) downstream of activating transcription
factor 3 in hepatitis C virus-infected Huh7 cells.
| A, Activation |
|---|
|
|---|
| Cluster | Annotation | Enrichment |
|---|
| 1 | Positive regulation
of fatty acid oxidation; positive regulation of glucose metabolic
process; positive regulation of carbohydrate metabolic process;
regulation of carbohydrate biosynthetic process; positive
regulation of fatty acid metabolic process; regulation of fatty
acid oxidation; positive regulation of macromolecule biosynthetic
process; regulation of glucose metabolic process; positive
regulation of cellular biosynthetic process; regulation of cellular
carbohydrate metabolic process; positive regulation of biosynthetic
process; regulation of fatty acid metabolic process; regulation of
cellular ketone metabolic process; positive regulation of
macromolecule metabolic process; adipocytokine signaling pathway;
regulation of lipid metabolic process; Insulin signaling pathway;
chemical homeostasis; homeostatic process. | 2.93 |
| 2 | Regulation of cell
proliferation; response to insulin stimulus; enzyme linked receptor
protein signaling pathway; response to hormone stimulus; response
to organic substance; response to endogenous stimulus; response to
peptide hormone stimulus; cell surface receptor linked signal
transduction. | 1.98 |
| 3 | Regulation of cell
proliferation; negative regulation of cell proliferation; positive
regulation of cell differentiation; positive regulation of
developmental process. | 1.94 |
| 4 | Androgen receptor
binding; positive regulation of macromolecule biosynthetic process;
androgen receptor signaling pathway; steroid hormone receptor
binding; positive regulation of cellular biosynthetic process;
activator; transcription factor; steroid hormone receptor signaling
pathway; positive regulation of macromolecule metabolic process;
intracellular receptor-mediated signaling pathway; nuclear hormone
receptor binding; hormone receptor binding; positive regulation of
transcription, DNA-dependent; positive regulation of RNA metabolic
process; transcription regulator activity; transcription
coactivator activity; positive regulation of transcription;
transcription; positive regulation of gene expression;
transcription from RNA polymerase II promoter; positive regulation
of nucleobase, nucleoside, nucleotide and nucleic acid metabolic
process positive regulation of nitrogen compound metabolic process;
nucleus; transcription regulation; transcription, DNA-dependent;
RNA biosynthetic process; regulation of RNA metabolic process;
intracellular signaling cascade; transcription cofactor activity;
transcription activator activity; transcription factor binding;
phosphoprotein; transition metal ion binding; zinc-finger; zinc ion
binding; DNA binding; cation binding; ion binding. | 1.57 |
| 5 | Intracellular
signaling cascade; cell junction; plasma membrane part. 1.07 | |
| 6 | Chemical
homeostasis; homeostatic process; protein complex biogenesis;
protein complex assembly; macromolecular complex assembly;
macromolecular. complex subunit organization. | 1.04 |
| 7 | Activator; nuclear
lumen; intracellular organelle lumen; organelle lumen;
membrane-enclosed lumen. | 0.81 |
| 8 | Regulation of
apoptosis; regulation of programmed cell death. 0.61 | |
| 9 | Mutagenesis site;
alternative splicing; splice variant. 0.35 | |
| 10 | Plasma membrane;
glycosylation site: N-linked; signal; glycoprotein; signal peptide;
disulfide bond; disulfide bond; intrinsic to membrane;
membrane. | 0.27 |
|
| B, Inhibition |
|
| Cluster | Annotation | Enrichment |
|
| 1 | Response to
endogenous stimulus; response to organic substance; regulation of
apoptosis; regulation of programmed cell death; regulation of cell
death. | 1.47 |
| 2 | Glycosylation site:
N-linked; glycoprotein; signal; signal peptide. | 0.54 |
Functional annotation clustering
analyses
Ten activation and two inhibition annotation
clusters were enriched downstream of ATF3, in HCV-Huh7 cells. The
enrichment scores of the activation clusters ranged from 0.27–2.93,
and the two inhibition clusters were 0.54 and 1.47. Among the
activation clusters, the highest enrichment score (cluster 1; 2.93)
was associated with positive metabolism regulation, such as fatty
acid oxidation, glucose metabolism, carbohydrate metabolism,
cellular carbohydrate metabolism, fatty acid metabolism,
macromolecule biosynthesis, cellular biosynthesis, lipid
metabolism, fatty acid metabolic regulation, macromolecule
metabolism and androgen receptor binding. The activation clusters
exhibiting enrichment scores 0.27–1.98 were associated with
regulating cell proliferation, transcription, DNA-dependent
developmental processes, gene expression, responses to stimuli of
insulin, endogenous organic substances, peptide hormones, androgen
receptor binding, androgen receptor signaling pathways, steroid
hormone receptor binding, nuclear hormone receptor binding,
intracellular signaling cascades, zinc-finger transcription factors
and metal-binding. Therefore, it is hypothesized that ATF3 is
inactive in Huh7 cells and is activated upon HCV infection, thereby
regulating a number of cytokine functions, metabolic pathways and
signal transduction. The present study identified 4 genes that were
upstream of ATF3 and 33 that were downstream of ATF3 in HCV-Huh7
cells. Therefore, ATF3 may be an important regulatory factor
involved in the pathological reactions of Huh7 cells undergoing the
initial stages of infection with HCV.
Fig. 1 demonstrates
the ATF3 network construction and analysis processes.
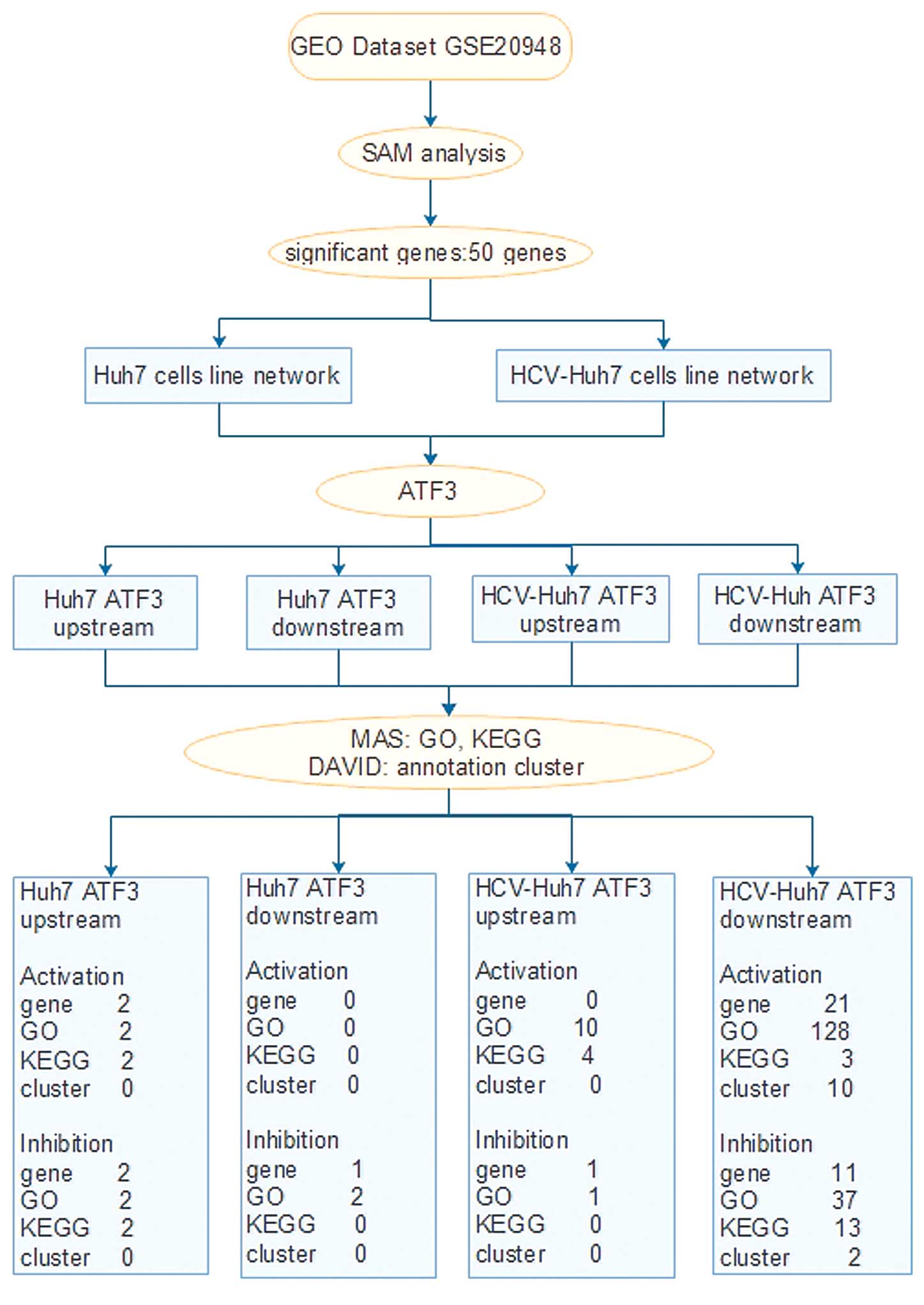 | Figure 1ATF3 network construction and
analysis processes. ATF3, activating transcription factor 3; GO,
gene ontology; TXNIP, thioredoxin interacting protein; STC2,
stanniocalcin 2; SAMD5, sterile α motif domain containing 5; SOCS2,
suppressor of cytokine signaling 2; SLC, solute carrier family;
RORA, RAR-related orphan receptor A; IRS, insulin receptor
substrate; DAVID, database for annotation, visualization, and
integrated discovery; KEGG, kyoto encyclopedia of genes and
genomes; SAM, significant analysis of microarrays; GEO, gene
expression omnibus; HCV, hepatitis C virus; HCV-Huh7 cells,
HCV-infected Huh7 cells; Huh7 cells, hepatoma cells. |
Discussion
In order to understand the involvement of ATF3 in
HCV-infected Huh7 cells, an ATF3 GO network was established for
Huh7 and HCV-Huh7 cell lines. HCV infection leads to complex
biological responses and, therefore, the subsequent genetic
interactions in the infected cells are typically non-linear.
However, gene networks may only be predicted based on linear
equations, due to the limited understanding of biological processes
required for more complex statistical analyses (9–12).
In the present study, GRNinfer, a linear programming and
decomposition software, was used in order to analyze and compare
ATF3 networks in Huh7 and HCV-Huh7 cell lines. Constructed networks
included upstream and downstream gene clusters of ATF3 in Huh7 and
HCV-Huh7 cell lines (Fig. 2).
Furthermore, using DAVID and MAS 3.0 software, the KEGG pathways
and GO terms of four gene clusters were enriched. The results of
the present study demonstrated that downstream pathways of ATF3 in
HCV-Huh7 cells were significantly enhanced.
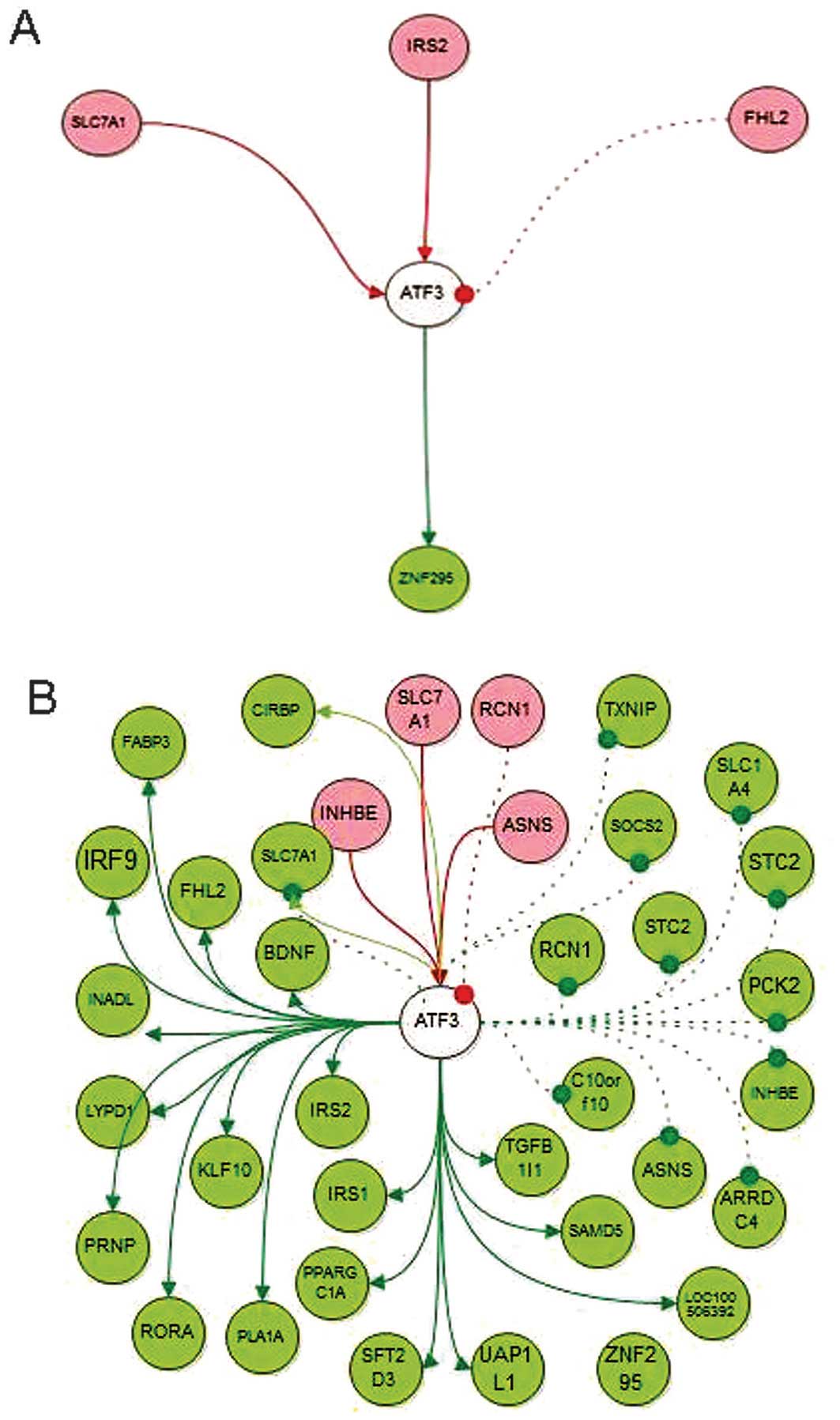 | Figure 2ATF3 network in healthy HUH7 and
HCV-infected Huh7 cell lines. (A) ATF3 network in Huh7 cells; (B)
ATF3 network in HCV-Huh7 cell lines. Red circle with gene name
indicates the genes upstream of ATF3; Green circle with gene name
indicates the genes downstream of ATF3; Red solid line with red
arrow indicates the activation of ATF3 by the gene upstream to
ATF3; Red dashed line with red dot indicates the inactivation of
ATF3 by the gene upstream ; Green solid line with green arrow
indicates the activation role of the gene in the downstream by
ATF3; Green dashed line with green dot indicate the inhibition of
the gene in the downstream by ATF3. ATF3, activating transcription
factor 3; HCV, hepatitis C virus; TXNIP, thioredoxin interacting
protein; STC2, stanniocalcin 2; SAMD5, sterile α motif domain
containing 5; SOCS2, suppressor of cytokine signaling 2; SLC1A4,
solute carrier family member 4; RORA, RAR-related orphan receptor
A; IRS, insulin receptor substrate. INHBE, inhibin β E; ASND,
asparagine synthetase; ARRDC4, arrestin domain-containing protein
4; LYPD1, LY6/PLAUR domain containin 1; PPARGC1A, peroxisome
proliferator-activated receptor γ coactivator 1-α; KLF10,
kruppel-like factor 10; FABP3, fatty acid binding protein 3; IRF9,
interferon regulatory factor 9; SLC7A1, SLC 7 (cationic amino acid
transporter, Y+ system) member 1; C10orf10, chromosome 10 open
reading frame 10; SFT2D3, SFT2 domain containing 3, INADL,
inaD-like; TGTB1I1, transforming growth factor β 1 induced
transcript 1; RCN1, reticulocalbin 1, EF-hand calcium binding
domain; BDNF, brain-derived neurotrophic factor; UAP1L1,
UDP-N-acteylglucosamine pyrophosphorylase 1-like 1; PCK2,
phosphoenolpyruvate carboxykinase 2; ZNF295, zinc finger protein
295; CIRBP, cold inducible RNA binding protein; PLA1A,
phospholipase A1 member A; FHL2, four and a half LIM domains 2;
PRNP, prion protein. |
The results of the present study suggested that ATF3
may be inactive in Huh7 cells and activated following HCV infection
of Huh7 cells. These results are in accordance with those observed
in previous studies. ATF3 expression levels were found to be low in
other cell lines or tissue (13)
and were shown to rise when cells were subjected to a number of
different stresses, including ischemia and trauma, as well as
exposure to toxic chemicals. ATF3 mRNA expression was not detected,
or remained at low levels, in a number of cell types (14). The present study demonstrated that
4 genes were upstream or downstream of ATF3 in Huh7 cells, whereas
36 genes were upstream or downstream of ATF3 in HCV-Huh7 cells.
Therefore, ATF3 may be inactive in healthy Huh7 cells while it is
activated in HCV-infected Huh7 cells (Fig. 2). These observations require
further investigation.
Four genes were identified upstream, while 33 genes
were downstream of ATF3 in HCV-Huh7 cells. Therefore, ATF3 may be
associated with HCV pathology in Huh7 cells. A previous study
suggested that ATF3 may represent an immediate early responsive
gene, which is predominantly activated at a transcriptional level
(15).
Extensive studies have shown that ATF3 is involved
in immune regulation (16–23), endocrine regulation (24–27),
tumorigenicity (28–32), apoptosis, cell cycling (33–37)
and inflammation (17,34,38–43).
This suggests that ATF3 is associated with host defensive responses
to inflammation, viruses and cancer. These processes require the
interactions of certain cellular pathways, which influence disease
outcomes. ATF3 is an adaptive-response gene, which is involved in a
number of cellular processes, and accommodates cellular changes by
transducing signals from various receptors via activating or
repression of gene expression. ATF3 may also regulate host defense
mechanisms (13). According to
DAVID functional annotation clustering analysis, no annotation
clusters were identified downstream of ATF3 in Huh7 cells nor were
there clusters identified upstream in Huh7 and HCV-Huh7 cells.
However, ten activation annotation clusters were enriched
downstream of ATF3 in HCV-Huh7 cells that exhibited enrichment
scores from 0.27–2.93 and two inhibition annotation clusters were
enriched downstream of ATF3 in HCV-Huh7 cells that exhibited
enrichment scores of 0.54 and 1.47. In addition, analyses using MAS
3.0 GO and KEGG, suggested that 128 GO terms and 13 KEGG pathways
are involved downstream of ATF3 in HCV-Huh7 cells and 37 GO terms
and 13 KEGG pathways were inhibited. It is hypothesized that ATF3,
as an adaptive-response gene, may be involved in the
pathophysiological responses of Huh7 cells to HCV infection.
In addition to liver injury and hepatocellular
carcinoma, HCV infection is associated with fatty degeneration of
the liver, suggesting that hepatitis C is a metabolic disease
(44,45). Considerable evidence suggests that
HCV infection is associated with metabolic syndromes, which
referred to constellation of problems, including insulin
resistance, obesity, hypertension and hyperlipaemia. Fatty
degeneration of the liver and insulin resistance may be important
in HCV-induced metabolic syndrome and a molecular mechanism
underlying this process is a virus-induced metabolic disorder of
fat in the liver (46). The liver
is important for the control of lipogenesis, gluconeogenesis and
cholesterol metabolism. A number of investigations into
pathological examination have highlighted the importance of
metabolic function in liver diseases. The process of lipid
metabolism is important in the proliferation of HCV infection
(47).
As a cyclic AMP response element-binding protein
family member, ATF3 participates in a range of cellular processes.
Recent studies have shown that changes in ATF3 activity is
associated with energy balance. Homeostasis imbalance may lead to
organ growth failure, retardation and metabolic disorder. A loss of
ATF3 in Drosophila was found to result in chronic
inflammation and primary starvation responses, including lipid
overloading in the fat bodies of the larval epithelium, which may
lead to energy imbalance and death (48). Kim et al (49) showed that ATF3 was involved in a
cholesterol-sensing network.
In the present study, DAVID cluster analysis found
that the cluster with the highest enrichment score (2.93) was
downstream of ATF3 in HCV-Huh7 cells (Table II). This suggests that ATF3 not
only activates lipid metabolism, but also positively regulates
fatty acid metabolic, macromolecular biosynthetic and cellular
biosynthetic processes. The results of the present study showed
that ATF3 may influence metabolic processes in HCV-Huh7 cells. The
current study provides a novel perspective for exploring the
association of ATF3 in HCV infection of Huh7 cells. However,
further research is required in order to explore how ATF3 is
associated with metabolic processes and the regulatory functions of
ATF3.
In conclusion, an ATF3 network was constructed for
Huh7 and HCV-Huh7 cell lines and gene functional annotation and
enrichment cluster analyses were performed. The results of the
present study suggested that ATF3 is inactive in healthy Huh7 cells
and activated following HCV infection. ATF3 may contribute to the
initial pathological responses to HCV infection in Huh7 cells. ATF3
may be associated with a number of processes, in particular lipid
metabolism, during acute HCV infection of Huh7 cells.
Acknowledgments
This study was supported by the National Natural
Science Foundation in China (grant no. 81170393/H0316).
References
|
1
|
Shepard CW, Finelli L and Alter MJ: Global
epidemiology of hepatitis C virus infection. Lancet Infect Dis.
5:558–567. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Seeff LB, Hollinger FB, Alter HJ, Wright
EC, Cain CM, Buskell ZJ, Ishak KG, Iber FL, Toro D, Samanta A, et
al: Long-term mortality and morbidity of transfusion-associated
non-A, non-B, and type C hepatitis: A National Heart, Lung, and
Blood Institute collaborative study. Hepatology. 33:455–463. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Guo QM: DNA microarray and cancer. Curr
Opin Oncol. 15:36–43. 2003. View Article : Google Scholar
|
|
4
|
Ding C and Cantor CR: Quantitative
analysis of nucleic acids - the last few years of progress. J
Biochem Mol Biol. 37:1–10. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Xiong J, Lu Y, Feng J, Yuan D, Tian M,
Chang Y, Fu C, Wang G, Zeng H and Miao W: Tetrahymena functional
genomics database (TetraFGD): An integrated resource for
Tetrahymena functional genomics. Database (Oxford).
2013:bat0082013. View Article : Google Scholar
|
|
6
|
Wang Y, Joshi T, Zhang XS, Xu D and Chen
L: Inferring gene regulatory networks from multiple microarray
datasets. Bioinformatics. 22:2413–2420. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Huang DW, Sherman BT, Tan Q, Collins JR,
Alvord WG, Roayaei J, Stephens R, Baseler MW, Lane HC and Lempicki
RA: The DAVID Gene Functional Classification Tool: A novel
biological module-centric algorithm to functionally analyze large
gene lists. Genome Biol. 8:R1832007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dennis G Jr, Sherman BT, Hosack DA, Yang
J, Gao W, Lane HC and Lempicki RA: DAVID: Database for Annotation,
Visualization, and Integrated Discovery. Genome Biol. 4:32003.
View Article : Google Scholar
|
|
9
|
Haeseleer F, Sokal I, Li N, Pettenati M,
Rao N, Bronson D, Wechter R, Baehr W and Palczewski K: Molecular
characterization of a third member of the guanylyl
cyclase-activating protein subfamily. J Biol Chem. 274:6526–6535.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gustafsson M, Hornquist M and Lombardi A:
Constructing and analyzing a large-scale gene-to-gene regulatory
network - lasso-constrained inference and biological validation.
IEEE/ACM Trans Comput Biol Bioinform. 2:254–261. 2005. View Article : Google Scholar
|
|
11
|
Wang L, Huang J, Jiang M and Sun L: MYBPC1
computational phosphoprotein network construction and analysis
between frontal cortex of HIV encephalitis (HIVE) and HIVE-control
patients. Cell Mol Neurobiol. 31:233–241. 2011. View Article : Google Scholar
|
|
12
|
Wang L, Sun L, Huang J and Jiang M:
Cyclin-dependent kinase inhibitor 3 (CDKN3) novel cell cycle
computational network between human non-malignancy associated
hepatitis/cirrhosis and hepatocellular carcinoma (HCC)
transformation. Cell Prolif. 44:291–299. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Thompson MR, Xu D and Williams BR: ATF3
transcription factor and its emerging roles in immunity and cancer.
J Mol Med (Berl). 87:1053–1060. 2009. View Article : Google Scholar
|
|
14
|
Hai T, Wolfgang CD, Marsee DK, Allen AE
and Sivaprasad U: ATF3 and stress responses. Gene Expr. 7:321–335.
1999.PubMed/NCBI
|
|
15
|
Cai Y, Zhang C, Nawa T, Aso T, Tanaka M,
Oshiro S, Ichijo H and Kitajima S: Homocysteine-responsive ATF3
gene expression in human vascular endothelial cells: Activation of
c-Jun NH(2)-terminal kinase and promoter response element. Blood.
96:2140–2148. 2000.PubMed/NCBI
|
|
16
|
Kawai T and Akira S: TLR signaling. Cell
Death Differ. 13:816–825. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gilchrist M, Henderson WR Jr, Clark AE,
Simmons RM, Ye X, Smith KD and Aderem A: Activating transcription
factor 3 is a negative regulator of allergic pulmonary
inflammation. J Exp Med. 205:2349–2357. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Swann JB, Vesely MD, Silva A, Sharkey J,
Akira S, Schreiber RD and Smyth MJ: Demonstration of
inflammation-induced cancer and cancer immunoediting during primary
tumorigenesis. Proc Natl Acad Sci USA. 105:652–656. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rakoff-Nahoum S and Medzhitov R:
Regulation of spontaneous intestinal tumorigenesis through the
adaptor protein MyD88. Science. 317:124–127. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Leitner WW, Hwang LN, deVeer MJ, Zhou A,
Silverman RH, Williams BR, Dubensky TW, Ying H and Restifo NP:
Alphavirus-based DNA vaccine breaks immunological tolerance by
activating innate antiviral pathways. Nat Med. 9:33–39. 2003.
View Article : Google Scholar
|
|
21
|
Scheule RK: The role of CpG motifs in
immunostimulation and gene therapy. Adv Drug Deliv Rev. 44:119–134.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Whitmore MM, DeVeer MJ, Edling A, Oates
RK, Simons B, Lindner D and Williams BR: Synergistic activation of
innate immunity by double-stranded RNA and CpG DNA promotes
enhanced antitumor activity. Cancer Res. 64:5850–5860. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Litvak V, Ramsey SA, Rust AG, Zak DE,
Kennedy KA, Lampano AE, Nykter M, Shmulevich I and Aderem A:
Function of C/EBPdelta in a regulatory circuit that discriminates
between transient and persistent TLR4-induced signals. Nat Immunol.
10:437–443. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xie J and Roberson MS: 3′, 5′-cyclic
adenosine 5′-mono-phosphate response element-dependent
transcriptional regulation of the secretogranin II gene promoter
depends on gonadotropin-releasing hormone-induced mitogen-activated
protein kinase activation and the transactivator activating
transcription factor 3. Endocrinology. 149:783–792. 2008.
View Article : Google Scholar
|
|
25
|
Salisbury TB, Binder AK, Grammer JC and
Nilson JH: GnRH-regulated expression of Jun and JUN target genes in
gonadotropes requires a functional interaction between TCF/LEF
family members and beta-catenin. Mol Endocrinol. 23:402–411. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mayer SI, Dexheimer V, Nishida E, Kitajima
S and Thiel G: Expression of the transcriptional repressor ATF3 in
gonadotrophs is regulated by Egr-1, CREB, and ATF2 after
gonadotropin-releasing hormone receptor stimulation. Endocrinology.
149:6311–6325. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Pelzer AE, Bektic J, Haag P, Berger AP,
Pycha A, Schäfer G, Rogatsch H, Horninger W, Bartsch G and Klocker
H: The expression of transcription factor activating transcription
factor 3 in the human prostate and its regulation by androgen in
prostate cancer. J Urol. 175:1517–1522. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wu X, Nguyen BC, Dziunycz P, Chang S,
Brooks Y, Lefort K, Hofbauer GF and Dotto GP: Opposing roles for
calcineurin and ATF3 in squamous skin cancer. Nature. 465:368–372.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yin X, Dewille JW and Hai T: A potential
dichotomous role of ATF3, an adaptive-response gene, in cancer
development. Oncogene. 27:2118–2127. 2008. View Article : Google Scholar
|
|
30
|
Kawai M, Jin M, Nishimura J, Dewa Y,
Saegusa Y, Matsumoto S, Taniai E, Shibutani M and Mitsumori K:
Hepatocarcinogenic susceptibility of fenofibrate and its possible
mechanism of carcinogenicity in a two-stage hepatocarcinogenesis
model of rasH2 mice. Toxicol Pathol. 36:950–957. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Xie JJ, Xu LY, Xie YM, Zhang HH, Cai WJ,
Zhou F, Shen ZY and Li EM: Roles of ezrin in the growth and
invasiveness of esophageal squamous carcinoma cells. Int J Cancer.
124:2549–2558. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hamdi M, Popeijus HE, Carlotti F, Janssen
JM, van der Burgt C, Cornelissen-Steijger P, van de Water B, Hoeben
RC, Matsuo K and van Dam H: ATF3 and Fra1 have opposite functions
in JNK- and ERK-dependent DNA damage responses. DNA Repair (Amst).
7:487–496. 2008. View Article : Google Scholar
|
|
33
|
Huang X, Li X and Guo B: KLF6 induces
apoptosis in prostate cancer cells through up-regulation of ATF3. J
Biol Chem. 283:29795–29801. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Rosenberger CM, Clark AE, Treuting PM,
Johnson CD and Aderem A: ATF3 regulates MCMV infection in mice by
modulating IFN-gamma expression in natural killer cells. Proc Natl
Acad Sci USA. 105:2544–2549. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wang H, Mo P, Ren S and Yan C: Activating
transcription factor 3 activates p53 by preventing E6-associated
protein from binding to E6. J Biol Chem. 285:13201–13210. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Tian Z, An N, Zhou B, Xiao P, Kohane IS
and Wu E: Cytotoxic diarylheptanoid induces cell cycle arrest and
apoptosis via increasing ATF3 and stabilizing p53 in SH-SY5Y cells.
Cancer Chemother Pharmacol. 63:1131–1139. 2009. View Article : Google Scholar
|
|
37
|
Kashiwakura Y, Ochiai K, Watanabe M,
Abarzua F, Sakaguchi M, Takaoka M, Tanimoto R, Nasu Y, Huh NH and
Kumon H: Down-regulation of inhibition of differentiation-1 via
activation of activating transcription factor 3 and Smad regulates
REIC/Dickkopf-3-induced apoptosis. Cancer Res. 68:8333–8341. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Gilchrist M, Thorsson V, Li B, Rust AG,
Korb M, Roach JC, Kennedy K, Hai T, Bolouri H and Aderem A: Systems
biology approaches identify ATF3 as a negative regulator of
Toll-like receptor 4. Nature. 441:173–178. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Whitmore MM, Iparraguirre A, Kubelka L,
Weninger W, Hai T and Williams BR: Negative regulation of
TLR-signaling pathways by activating transcription factor-3. J
Immunol. 179:3622–3630. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Gilchrist M, Henderson WR Jr, Morotti A,
Johnson CD, Nachman A, Schmitz F, Smith KD and Aderem A: A key role
for ATF3 in regulating mast cell survival and mediator release.
Blood. 115:4734–4741. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Shukla A, MacPherson MB, Hillegass J,
Ramos-Nino ME, Alexeeva V, Vacek PM, Bond JP, Pass HI, Steele C and
Mossman BT: Alterations in gene expression in human mesothelial
cells correlate with mineral pathogenicity. Am J Respir Cell Mol
Biol. 41:114–123. 2009. View Article : Google Scholar :
|
|
42
|
Khuu CH, Barrozo RM, Hai T and Weinstein
SL: Activating transcription factor 3 (ATF3) represses the
expression of CCL4 in murine macrophages. Mol Immunol.
44:1598–1605. 2007. View Article : Google Scholar
|
|
43
|
Herzig S, Long F, Jhala US, Hedrick S,
Quinn R, Bauer A, Rudolph D, Schutz G, Yoon C, Puigserver P, et al:
CREB regulates hepatic gluconeogenesis through the coactivator
PGC-1. Nature. 413:179–183. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Lonardo A, Adinolfi LE, Loria P, Carulli
N, Ruggiero G, et al: Steatosis and hepatitis C virus: mechanisms
and significance for hepatic and extrahepatic disease.
Gastroenterology. 126:586–597. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Koike K: Hepatitis C as a metabolic
disease: Implication for the pathogenesis of NASH. Hepatol Res.
33:145–150. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Kawaguchi Y and Mizuta T: Interaction
between hepatitis C virus and metabolic factors. World J
Gastroenterol. 20:2888–2901. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Syed GH, Amako Y and Siddiqui A: Hepatitis
C virus hijacks host lipid metabolism. Trends Endocrinol Metab.
21:33–40. 2010. View Article : Google Scholar :
|
|
48
|
Rynes J, Donohoe CD, Frommolt P, Brodesser
S, Jindra M and Uhlirova M: Activating transcription factor 3
regulates immune and metabolic homeostasis. Mol Cell Biol.
32:3949–3962. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Kim J, Di Vizio D, Kim TK, Kim J, Kim M,
Pelton K, Clinton SK, Hai T, Hwang D, Solomon KR, et al: The
response of the prostate to circulating cholesterol: Activating
transcription factor 3 (ATF3) as a prominent node in a
cholesterol-sensing network. PLoS One. 7:e394482012. View Article : Google Scholar : PubMed/NCBI
|
















