Introduction
Paclitaxel (PTX) is a first-line chemotherapeutic
agent used to treat patients with ovarian cancer. PTX is a novel
microtubule-damaging agent that stabilizes the structure of
tubulin, by promoting its polymerization and suppressing its
depolymerization (1). Therefore,
PTX effectively inhibits cellular mitosis (2). Curcuma zedoaria (Berg.) Rosc.
is a traditional medicine that is used to treat flatulence,
dyspepsia, menstrual disorders, cough and fever (3). Furthermore, previous studies have
demonstrated its anticancer action (4–6). A
major component of Curcuma zedoaria (Berg.) Rosc. is
essential oil (3,7–9). PTX
and Curcuma zedoaria (Berg.) Rosc. essential oil (CZEO) are
considered to be potential anticancer drugs. These compounds
directly target nuclear DNA, in order to suppress or inhibit tumor
cell growth. Furthermore, tumor immunogenicity can be increased by
either treatment, resulting in the induction of a stronger
cytotoxic response to tumor cells (10).
Apoptosis, also known as programmed cell death, is
an active intracellular death program that has a key role in the
maintenance of organisms (11).
Caspases, a family of cysteine proteases, are the key executors of
apoptosis (12). Caspase-3 is
situated at pivotal junctions in apoptosis pathways and its
activation leads to a series of cellular events (13). Poly adenosine diphosphate-ribose
polymerase (PARP), a nuclear protein involved in the DNA damage
response, is a well-known substrate for caspase-3 cleavage during
apoptosis (14).
In the present study, in order to explore the
antitumor effects of the combined treatment of PTX and CZEO, an
in vitro experiment was conducted using the SKOV3 human
ovarian cancer cell line. The effects of the treatment on cell
growth were determined, and the underlying mechanisms were
investigated.
Materials and methods
Chemicals and reagents
Cell Counting kit-8 (CCK-8) was purchased from
Dojindo Molecular Technologies, Inc. (Kumamoto, Japan). RPMI-1640
media was purchased from Thermo Fisher Scientific (Beijing, China),
and fetal bovine serum (FBS) was obtained from Gibco Life
Technologies (Carlsbad, CA, USA). Propidium iodide (PI) was
purchased from Sigma-Aldrich (St. Louis, MO, USA).
Curcuma zedoaria (Berg.) Rosc. essential
oil
Zedoray Turmeric Oil Injection, containing 0.1 g/10
ml CZEO, was purchased from Xuzhou Lai’en Pharmaceutical Co., Ltd
(Xuzhou, China). The main components of CZEO are neocurdione,
curdione, germacrone, curzerene, furanodiene, γ-elemene and
8,9-dehydro-9-formyl-cycloisolongifolene (15).
Cell lines and cell culture
The SKOV3 human ovarian cancer cells were purchased
from the Cell Bank of the Cinese Academy of Sciences (Shanghai,
China). The cells were cultured in RPMI-1640 supplemented with 10%
heat-inactivated FBS, 100 U/ml penicillin and 100 μg/ml
streptomycin (Gibco Life Technologies), at 37°C in a humidified
atmosphere containing 5% CO2.
Cell proliferation assay
The SKOV3 cells were dispersed in culture medium
supplemented with 10% FBS and were seeded in a 96-well plate at a
density of 2×104 cells/ml. The cells were then treated
with PTX (10 nM; Xi’an Sanjiang Bio-Engineering Co. Ltd, Xi’an,
China), CZEO (62.5 μg/ml) or a combination of the two for 48
h, following 24 h growth. Cell in the control group were treated
with 0.2 ml phosphate-buffered saline (PBS). A total of 10
μl CCK-8 was added to each well, and the cells were then
cultured in an incubator for a further 3 h. The optical density
(OD) of the cells was measured at 490 nm using a microplate
spectrophotometer (Spectramax 190; Molecular Devices Corp.,
Sunnyvale, CA, USA). Each concentration corresponds to three
parallel wells for detection. The cell viability was calculated as
follows: Cell viability=(ODtreated
group-ODblank)/(ODcontrol-ODblank)×100.
The interaction between PTK and CZEO was analyzed using CalcuSyn
2.0 software (Biosoft, Cambridge, UK), using the Chou and Talalay
method (16). The combination
index (CI) was determined on the basis of the isobologram analysis:
CI<1, synergistic effect; CI=1, additive effect; and CI>1,
antagonistic effect.
Hoechst 33342 staining
The SKOV3 cells (5×105 cells/ml) were
seeded in six-well plates, and cultured overnight. Following
treatment with PTX (10 nM), CZEO (62.5 μg/ml) or a
combination of the two for 24–48 h in a 37°C incubator containing
5% CO2, the cells were incubated with Hoechst 33342 (5
μl; Sigma-Aldrich) for 30 min. Hoechst 33342-stained cell
nuclei were observed using an inverted fluorescence microscope
(BX60; Olympus Optical Co., Tokyo, Japan), and images were captured
with a confocal microscope (LSM510; Carl Zeiss AG, Oberkochen,
Germany).
Detection of apoptosis by flow
cytometry
The SKOV3 cells (5×105 cells/ml) were
seeded in six-well plates and cultured for 12 h. Following
treatment of the cells with PTX, CZEO, or a combination of the two
for 48 h in a 37°C incubator containing 5% CO2, the
cells were collected and washed twice with cold PBS in order to
remove the medium. The cells were then resuspended in 100 μl
of 1X binding buffer (Invitrogen Life Technologies, Carlsbad, CA,
USA), prior to the addition of 5 μl Annexin-V (Invitrogen
Life Technologies) and 1 μl propidium iodide (PI;
Sigma-Aldrich) and incubation in the dark on ice. Finally, 400
μl of 1X binding buffer was added to the cells and they were
analyzed by flow cytometry using a BD FACSAria cell sorter
(Becton-Dickinson, San Jose, CA, USA).
Cell cycle analysis by flow
cytometry
The SKOV3 cells were seeded in six-well plates and
treated as mentioned in the previous section. The cells were then
harvested, washed twice with PBS and resuspended in 0.3 ml PBS.
RNase A (Roche, Indianapolis, IN, USA) was added, in order to
digest the cells for 30 min at 37°C, after which the cells were
collected and washed twice with PBS. The reaction was terminated by
placing the mixture on ice. PI was added to the cells in the dark,
in order to prepare the samples for flow cytometry.
Western blotting of key signal proteins
caspase-3 and PARP for apoptosis
The SKOV3 cells (5×105 cells/ml) were
seeded in 60 mm culture dishes in the presence of PTX, CZEO, or a
combination of the two for 48 h, and cultured in a 37°C incubator
containing 5% CO2. The cells were collected according to
the standard western blotting procedure. The protein samples were
used straight after protein concentration determination, or were
stored at −20°C until further use.
Protein concentration of the samples was determined
using bicinchoninic acid protein reagent. (Pierce Biotechnology,
Inc., Rockford, IL, USA). The protein samples were separated by SDS
electrophoresis on polyacrylamide gels and transferred to a
nitrocellulose membrane (Millipore, Billerica, MA, USA). The
membranes were then blocked in 5% bovine serum albumin
(Sigma-Aldrich) for 3 h at room temperature, followed by incubation
for 2 h at room temperature with primary antibodies (rabbit
polyclonal anti-PARP and anti-caspase-3; 1:1,000; Cell Signaling
Technology, Inc., Beverly, MA, USA). The membranes were washed
(3×10 min) in tris-buffered saline with Tween® (TBST)
and were then incubated with a horseradish peroxidase-conjugated
sheep anti-rabbit secondary antibody (1:2,000; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA) for 1 h at room temperature,
followed by further washing (3×10 min) with TBST. Immunoblot
signals were detected by Odyssey Infrared Imaging v1.2 system
(LI-COR Biosciences, Lincoln, NE, USA).
Statistical analysis
All of the data are expressed as the mean ± standard
error of the mean. Student’s t-test was used to determine the
statistical significance between two observations and a one-way
analysis of variance followed by the Bonferroni test were used for
the multiple comparisons. Statistical analyses were performed using
SPSS version 11.0 (SPSS Inc., Chicago, IL, USA). P≤0.05 was
considered to indicate a statistically significant difference.
Results
Treatment with a combination of CZEO and
PTX significantly suppresses proliferation of SKOV3 cells
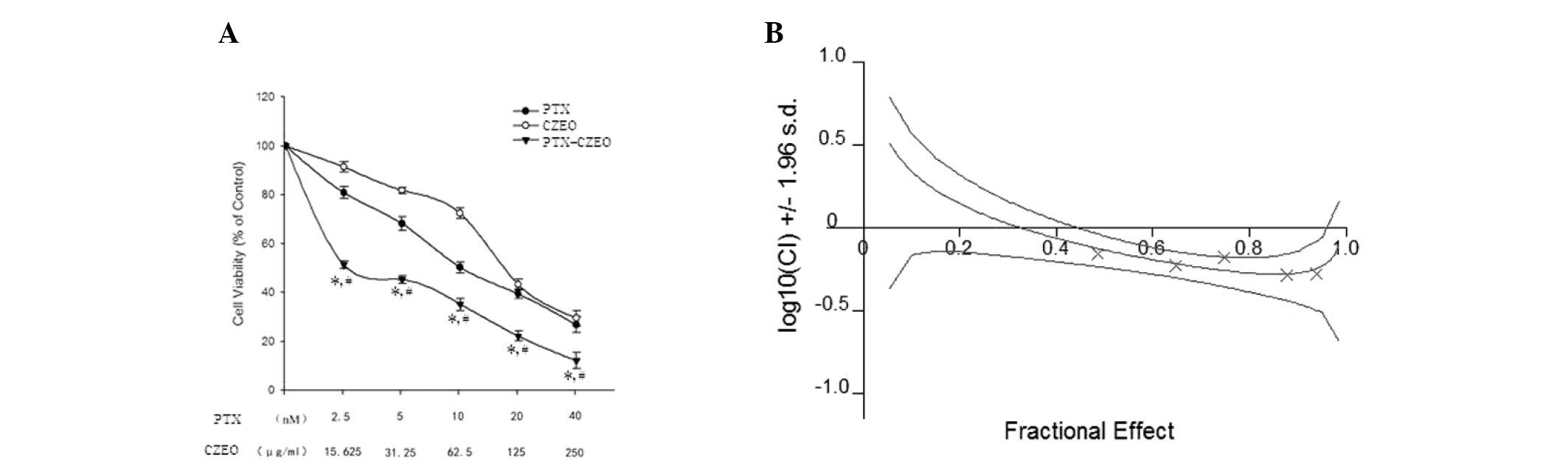
Treatment with CZEO or PTX significantly inhibited
the viability of SKOV3 cells in a dose-dependent manner, at
concentrations between 2.5 and 40 nM PTX, and between 15.625 and
250 μg/ml CZEO (Fig. 1A).
Furthermore, treatment with a combination of CZEO and PTX enhanced
the suppressive effect on the SKOV3 cells (Fig. 1B; confidence interval, 0.526 to
0.705).
Treatment with a combination of CZEO and
PTX enhances the apoptosis of SKOV3 cells and induces cell cycle
arrest at G2/M phase
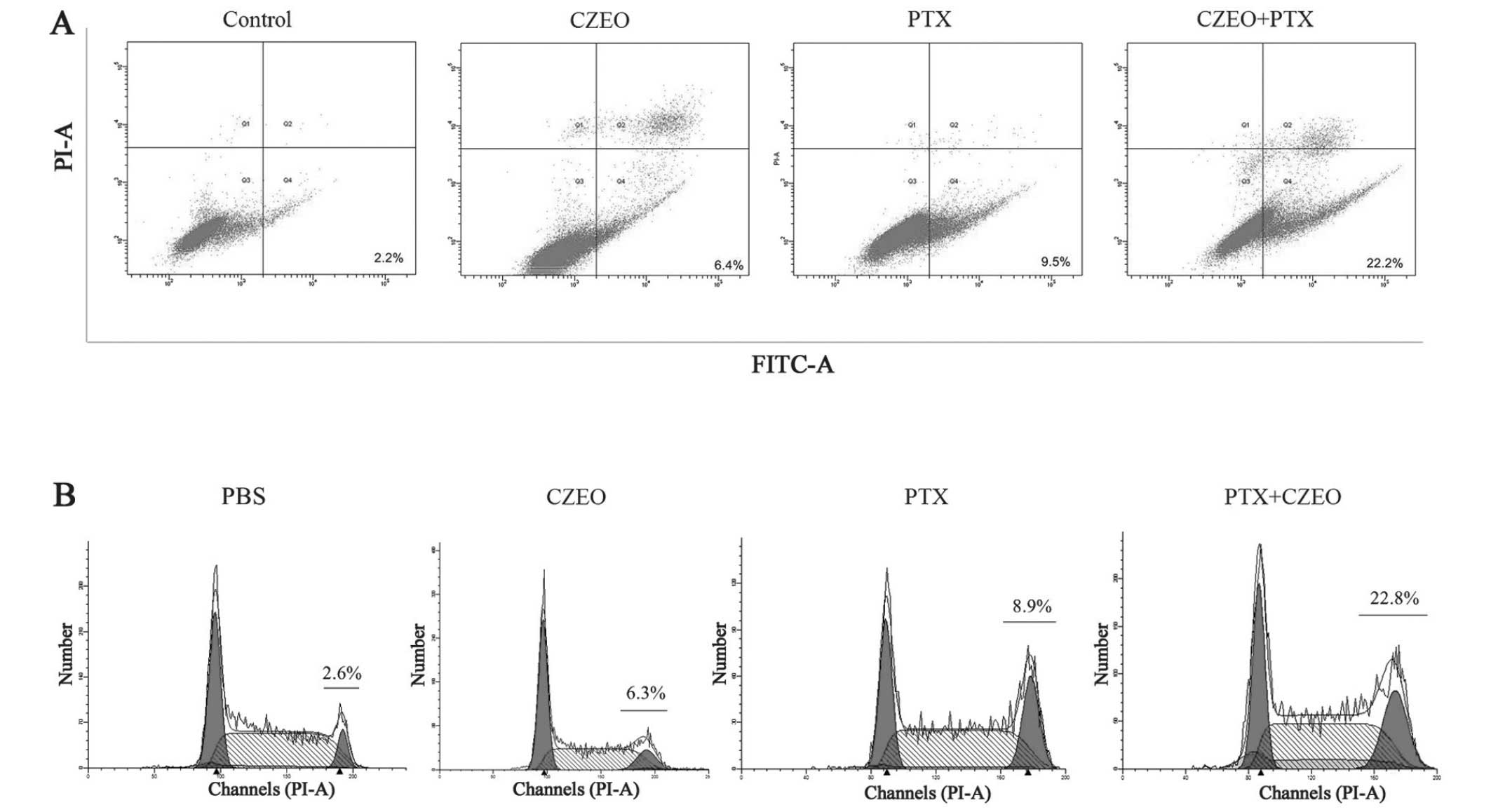
Flow cytometry was used to detect apoptosis,
following treatment of the SKOV3 cells with CZEO (62.5
μg/ml), PTX (10 nM), or a combination of the two for 48 h.
The cells were collected for dual staining using Annexin V/PI and
then underwent flow cytometry. The cells stained with Annexin V but
not PI were considered to be in the early-apoptotic phase.
Treatment with a combination of CZEO and PTX significantly enhanced
the rate of apoptosis (Fig. 2A).
The rate of apoptosis of the cells treated with CZEO (62.5
μg/ml), PTX (10 nM) and a combination of the two was 6.4%,
9.5% and 22.2%, respectively.
Flow cytometry was used to perform a cell cycle
analysis, following treatment of the SKOV3 cells with CZEO (62.5
μg/ml), PTX (10 nM), or a combination of the two for 24 h.
The number of cells arrested at G2/M phase were
significantly increased (Fig. 2B),
which may be partially attributed to the anti-proliferative effects
of CZEO and PTX.
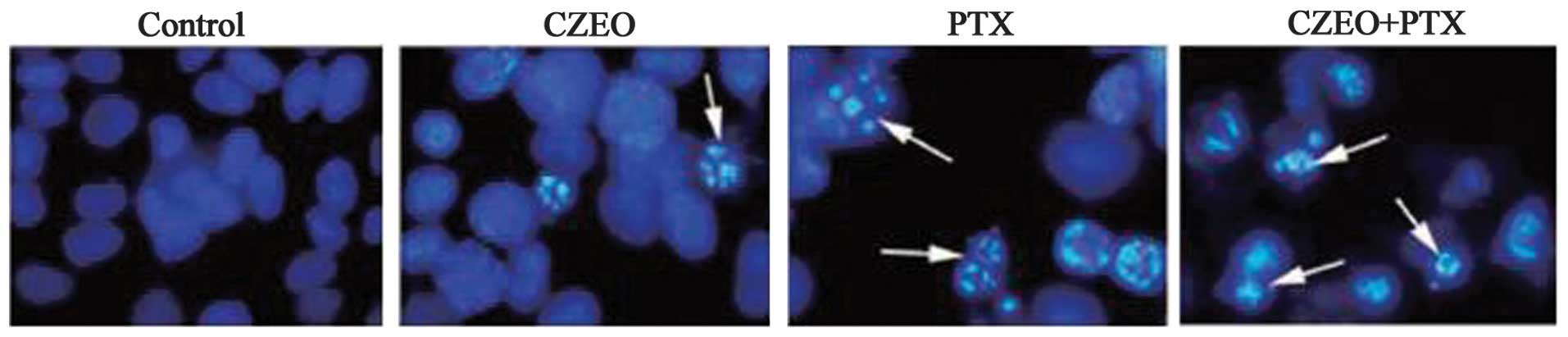
Morphological changes
The SKOV3 cells exhibited morphological changes
characteristic of apoptosis following treatment with CZEO (62.5
μg/ml), PTX (10 nM), or a combination of the two. A
microscopic observation demonstrated that the cells treated with
CZEO (62.5 μg/ml), PTX (10 nM), or a combination of the two
for 48 h had significantly reduced growth and exhibited
morphological changes characteristic of apoptosis, including
widespread chromatin condensation, exocytosis, and condensation and
fragmentation of nuclei (Fig.
3).
Hoechst 33342 staining was also performed to detect
apoptosis following treatment with CZEO (62.5 μg/ml), PTX
(10 nM) or a combination of the two for 48 h. The untreated control
cells exhibited normal chromatin without condensation or
fragmentation, with no bright staining in the nuclei, thus
indicating the absence of dying cells. Whereas, the treated cells
exhibited significantly condensed and fragmented chromatin,
disintegration of nuclei and formation of apoptotic bodies.
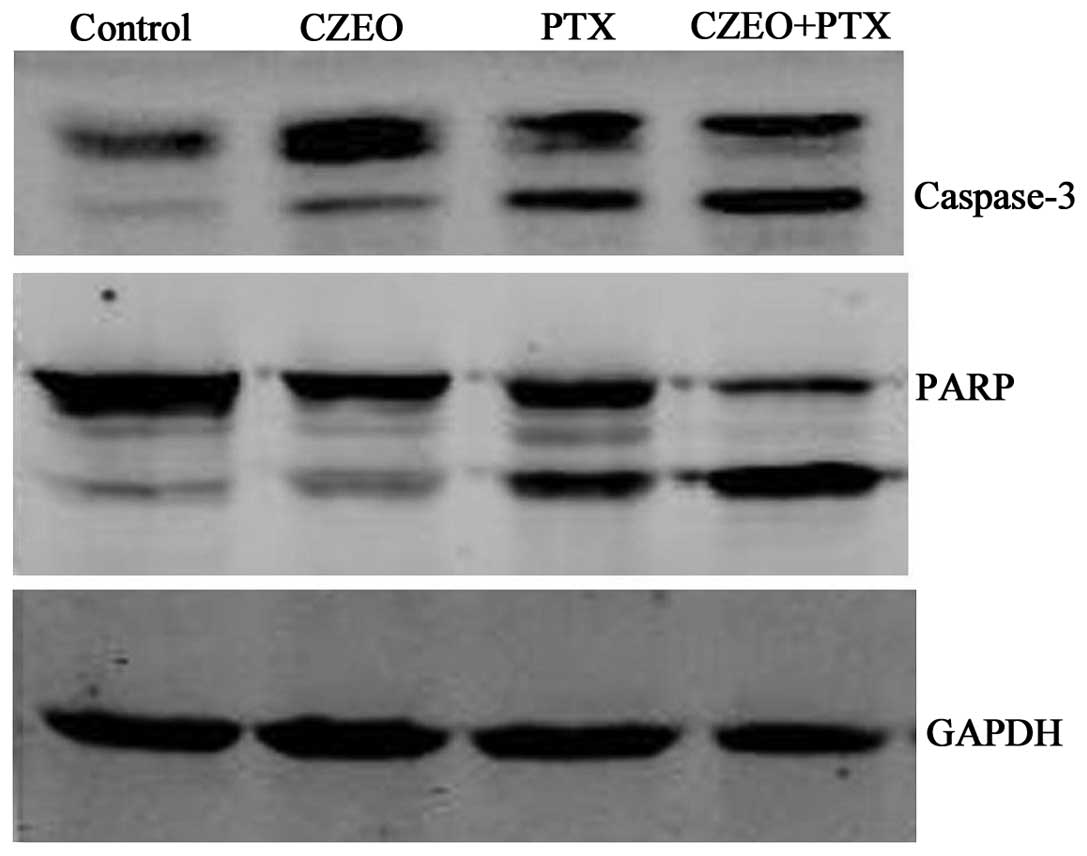
Relative expression levels of apoptotic
pathway proteins detected by western blotting
Following treatment with CZEO (62.5 μg/ml),
PTX (10 nM), or a combination of the two for 24 h, the cells were
harvested for western blotting, in order to detect the expression
levels of proteins involved in the apoptotic pathway. Treatment
with a combination of CZEO and PTX activated caspase-3, to form a
cleaved product of the substrate PARP (Fig. 4).
Discussion
Ovarian cancer is one of the three most common
malignant cancers that occur within the female reproductive tract,
which poses a severe threat to the health of the female population.
The incidence of ovarian cancer has increased annually during the
past two decades (17). The early
stages of ovarian cancer can be effectively treated with
chemotherapy; however, ~70% of ovarian cancers are diagnosed at a
late stage (18), which severely
effects the life quality and mortality rates of patients, since the
cancer cells develop resistance to drugs, such as PTX (19). Cyto-reductive surgery is the
first-line therapy for patients with all stages of ovarian cancer,
which is used in combination with chemotherapy or radiotherapy for
patients with late stage or recurrent ovarian cancer, so as to
improve their quality of life and chances of survival (20).
PTX is a potent drug of natural origin, which is
isolated from the bark of the Pacific yew (Taxus brevifolia)
(21), and is considered the
first-line antitumor drug for ovarian cancer. However, the success
of PTX chemotherapy in treating ovarian cancer is limited, due to
its extreme toxicity (22,23). Therefore, developing a novel
therapeutic strategy with higher therapeutic efficacy and lower
toxicity is required.
Previous studies regarding combination chemotherapy
have focused on identifying natural compounds that may increase the
therapeutic index. Cang et al (24) reported that phenethyl
isothiocyanate enhanced the apoptosis and α-tubulin
hyperacetylation abilities of PTX in MCF7 and MDA-MB-231 breast
cancer cell lines. Yang et al (25) previously demonstrated that luteolin
could enhance PTX-induced apoptosis in MDA-MB-231 human breast
cancer cells, by blocking signal transducer and activator of
transcription 3. Hossein et al (19) showed that PectaSol-C modified
citrus pectin could sensitize ovarian cancer cells to PTX by
inducing apoptosis, which may lead to an accumulation of cells in
the subG1 and G1 phases, and cleavage of
caspase-3 (24–27). Numerous studies have reported that
CZEO is a promising antitumor drug, which has a direct cytotoxic
effect that inhibits tumor cell growth and proliferation, disrupts
nuclear metabolism, inhibits angiogenesis and impairs the membrane
potential, all of which can be lethal to cancer cells (3,15,28).
To the best of our knowledge, the present study was the first to
demonstrate the effect of the combination of CZEO and PTX in
suppressing cancer cell growth.
The cell viability assay demonstrated that the tumor
survival rate of the cells decreased following treatment with CZEO,
in a dose-dependent manner, which is concordant with the findings
of Chen et al (3) and Chen
et al (15). Furthermore,
the inhibitory effect was increased by treatment with the
combination of CZEO and PTX. In addition, the combination also
enhanced the rate of apoptosis, which was demonstrated by the
observation of morphological changes. These results indicate that
it may be possible to reduce the side effects of PTX whilst
enhancing its clinical efficacy by using it in combination with
CZEO.
PTX can arrest the cell cycle at the G2/M
phase and induce caspase-3 enzymatic activity (29–32).
In the present study, treatment with the combination of CZEO and
PTX increased the accumulation of cells in the G2/M
phase, and the expression levels of caspase-3. These results
indicate that the synergistic antitumor effects of CZEO and PTX are
achieved by inducing apoptosis and arresting the cell cycle at
G2/M phase.
In conclusion, the present study demonstrated that
CZEO can sensitize ovarian cancer cells to PTX, through inducing
apoptosis, which was the result of the accumulation of cells in the
G2/M phase, and cleavage of caspase-3. These results
suggest that PTX supplemented with CZEO may be an effective
treatment strategy to decrease the dose and toxicity of PTX.
Further studies are required to clarify the signaling pathways and
key molecules underlying the effects of a combination of CZEO and
PTX in human ovarian cancer.
Acknowledgments
The present study was supported by the Zhejiang
Traditional Chinese Medicine Grant, China (grant no. 2010ZA
064).
References
|
1
|
Blagosklonny MV and Fojo T: Molecular
effects of paclitaxel: myths and reality (a critical review). Int J
Cancer. 83:151–156. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Neijt JP, Engelholm SA, Tuxen MK, et al:
Exploratory phase III study of paclitaxel and cisplatin versus
paclitaxel and carboplatin in advanced ovarian cancer. J Clin
Oncol. 18:3084–3092. 2000.PubMed/NCBI
|
|
3
|
Chen W, Lu Y, Gao M, Wu J, Wang A and Shi
R: Anti-angiogenesis effect of essential oil from Curcuma zedoaria
in vitro and in vivo. J Ethnopharmacol. 133:220–226. 2011.
View Article : Google Scholar
|
|
4
|
Lobo R, Prabhu KS and Shirwaikar A and
Shirwaikar A: Curcuma zedoaria Rosc. (white turmeric): a review of
its chemical, pharmacological and ethnomedicinal properties. J
Pharm Pharmacol. 61:13–21. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lakshmi S, Padmaja G and Remani P:
Antitumour effects of isocurcumenol isolated from Curcuma zedoaria
rhizomes on human and murine cancer cells. International Journal of
Medicinal Chemistry. 2011:20112011. View Article : Google Scholar
|
|
6
|
Kim KI, Kim JW, Hong BS, et al: Antitumor,
genotoxicity and anticlastogenic activities of polysaccharide from
Curcuma zedoaria. Mol Cells. 10:392–398. 2000.PubMed/NCBI
|
|
7
|
Seo WG, Hwang JC, Kang SK, et al:
Suppressive effect of Zedoariae rhizoma on pulmonary metastasis of
B16 melanoma cells. J Ethnopharmacol. 101:249–257. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shin Y and Lee Y: Cytotoxic activity from
Curcuma zedoaria through mitochondrial activation on ovarian cancer
cells. Toxicol Res. 29:257–261. 2013. View Article : Google Scholar
|
|
9
|
Wu WY, Luo YJ and Cheng JH: Zedoary
turmeric oil inhibits tranplantal hepatoma in rat via hepatic
artery perfusion. Shi Jie Hua Ren Xiao Hua Za Zhi. 11:260–263.
1998.
|
|
10
|
Giampietri A, Bonmassar A, Puccetti P, et
al: Drug-mediated increase of tumor immunogenicity in vivo for a
new approach to experimental cancer immunotherapy. Cancer Res.
41:681–687. 1981.PubMed/NCBI
|
|
11
|
Elmore S: Apoptosis: a review of
programmed cell death. Toxicol Pathol. 35:495–516. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jiang C, Wang Z, Ganther H, et al:
Caspases as key executors of methyl selenium-induced apoptosis
(anoikis) of DU-145 prostate cancer cells. Cancer Res.
61:3062–3070. 2001.PubMed/NCBI
|
|
13
|
Lakhani SA, Masud A, Kuida K, et al:
Caspases 3 and 7: key mediators of mitochondrial events of
apoptosis. Science. 311:847–851. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chowdhry MF, Vohra HA and Galiñanes M:
Diabetes increases apoptosis and necrosis in both ischemic and
nonischemic human myocardium: Role of caspases and poly–adenosine
diphosphate–ribose polymerase. J Thorac Cardiovasc Surg.
134:124–131. 2007. View Article : Google Scholar
|
|
15
|
Chen CC, Chen Y, Hsi YT, et al: Chemical
constituents and anticancer activity of Curcuma zedoaria roscoe
essential oil against non-small cell lung carcinoma cells in vitro
and in vivo. J Agric Food Chem. 61:11418–11427. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chou TC and Talalay P: Quantitative
analysis of dose-effect relationships: the combined effects of
multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 22:27–55.
1984. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Smith RA, Brooks D, Cokkinides V, et al:
Cancer screening in the United States, 2013: a review of current
American Cancer Society guidelines, current issues in cancer
screening, and new guidance on cervical cancer screening and lung
cancer screening. CA Cancer J Clin. 63:87–105. 2013. View Article : Google Scholar
|
|
18
|
McGuire V, Jesser CA and Whittemore AS:
Survival among U.S. woman with invasive epithelical ovarian cancer.
Gynecol Oncol. 84:399–403. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hossein G, Keshavarz M, Ahmadi S and
Naderi N: Synergistic effects of PectaSol-C modified citrus pectin
an inhibitor of Galectin-3 and paclitaxel on apoptosis of human
SKOV-3 ovarian cancer cells. Asian Pac J Cancer Prev. 14:7561–7568.
2013. View Article : Google Scholar
|
|
20
|
Kushner DM, Connor JP, Sanchez F, et al
Wisconsin Oncology Network: Weekly docetaxel and carboplatin for
recurrent ovarian and peritoneal cancer: a phase II trial. Gynecol
Oncol. 105:358–364. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wani MC, Taylor HL, Wall ME, Coggon P and
McPhail AT: Plant antitumor agents. VI. The isolation and structure
of taxol, a novel antileukemic and antitumor agent from Taxus
brevifolia. J Am Chem Soc. 93:2325–2357. 1971. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Maier-Lenz H, Hauns B, Haering B, et al:
Phase I study of paclitaxel administered as a 1-hour infusion:
toxicity and pharmacokinetics. Semin Oncol. 24:S16–S19. 1997.
|
|
23
|
Scripture CD, Figg WD and Sparreboom A:
Peripheral neuropathy induced by paclitaxel: recent insights and
future perspectives. Curr Neuropharmacol. 4:165–172. 2006.
View Article : Google Scholar
|
|
24
|
Cang S, Ma Y, Chiao JW and Liu D:
Phenethyl isothiocyanate and paclitaxel synergistically enhanced
apoptosis and alpha-tubulin hyperacetylation in breast cancer
cells. Exp Hematol Oncol. 3:52014. View Article : Google Scholar :
|
|
25
|
Yang MY, Wang CJ, Chen NF, Ho WH, Lu FJ
and Tseng TH: Luteolin enhances paclitaxel-induced apoptosis in
human breast cancer MDA-MB-231 cells by blocking STAT3. Chem Biol
Interact. 213:60–68. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kang H, Lee SH, Price JE and Kim LS:
Curcumin suppresses the paclitaxel-induced nuclear factor-kappaB in
breast cancer cells and potentiates the growth inhibitory effect of
paclitaxel in a breast cancer nude mice model. Breast J.
15:223–229. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kuo HC, Lee HJ, Hu CC, Shun HI and Tseng
TH: Enhancement of esculetin on Taxol-induced apoptosis in human
hepatoma HepG2 cells. Toxicol Appl Pharmacol. 210:55–62. 2006.
View Article : Google Scholar
|
|
28
|
Long KJ, Han FJ, Wu XK and Wang XY:
Effects of Zedoary turmeric oil injection on SKOV3 cell
proliferation and pathological morphological changes in ovarian
cancer. World Journal of Integrated Traditional and Western
Medicine. 6:660–662. 2011.
|
|
29
|
George J, Banik NL and Ray SK: Molecular
Mechanisms of Taxol for Induction of Cell Death in Glioblastomas.
Glioblastoma: Molecular Mechanisms of Pathogenesis and Current
Therapeutic Strategies. Ray SK: Springer; New York: pp. 283–298.
2010, View Article : Google Scholar
|
|
30
|
Gonçalves A, Braguer D, Carles G, André N,
Prevôt C and Briand C: Caspase-8 activation independent of
CD95/CD95-L interaction during paclitaxel-induced apoptosis in
human colon cancer cells (HT29-D4). Biochem Pharmacol.
60:1579–1584. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Perkins C, Kim CN, Fang G and Bhalla KN:
Overexpression of Apaf-1 promotes apoptosis of untreated and
paclitaxel- or etoposide-treated HL-60 cells. Cancer Res.
58:4561–4566. 1998.PubMed/NCBI
|
|
32
|
Weigel TL, Lotze MT, Kim PK, Amoscato AA,
Luketich JD and Odoux C: Paclitaxel-induced apoptosis in non-small
cell lung cancer cell lines is associated with increased caspase-3
activity. J Thorac Cardiovasc Surg. 119:795–803. 2000. View Article : Google Scholar : PubMed/NCBI
|