Introduction
Each year, approximately 230,000 American males are
diagnosed with prostate cancer (PCa) and nearly 30,000 die from
this disease (1,2). However, for the majority of patients,
the disease is detected at the local or regional stages, meaning
that long-term prognosis is typically good (3). Radical prostatectomy is the selected
treatment for 50% of patients with PCa, of which approximately 40%
exhibit aggressive clinicopathological features, such as a high
Gleason score, invasion of the seminal vesicles or lymph node
involvement. These features are associated with an increased risk
of metastatic disease (4–6). Approximately 15% of patients with PCa
are at risk of death, many receive potentially unnecessary
additional postoperative interventions, such as adjuvant radiation
(7). Therefore, they often
experience treatment-associated morbidity (8). Furthermore, PCa-associated mortality
has been observed in patients who do not exhibit adverse clinical
features. Current methods for predicting the risk of metastasis and
mortality in patients with PCa are insufficient (9). Therefore, specific genetic markers
are required in order to develop prognostic indicators for patients
with PCa.
Golgi phosphoprotein 3 (GOLPH3), a member of the
trans-golgi matrix family, has recently been demonstrated to act as
an oncogene in carcinoma of the lung, ovary, breast, colon and
prostate, and in melanoma, rhabdomyosarcoma, and glioma (10–13).
GOLPH3 overexpression has been reported to promote cell
proliferation and tumorigenesis via activation of mammalian target
of rapamycin signaling, which enhances protein kinase B activity
and decreases transcriptional activity of the forkhead box protein
O gene (10,14,15).
However, to the best of our knowledge, few studies have
investigated the association between GOLPH3 expression and the
survival of patients with PCa. In the present study,
immunohistochemical staining of PCa and control prostate tissues
was performed, in order to evaluate the expression of GOLPH3, and
to analyze the association between GOLPH3 expression and
clinicopathological factors in patients with PCa.
Materials and methods
Patients and prostate specimens
PCa samples (117) were obtained from patients with
an average age of 62.4 years and a range of 48–77 years between
1999 and 2012 at the Department of Urology, Jinan Central Hospital
of Shandong University (Jinan, China). Patients had undergone
radical prostatectomy between January 1999–2012. The control
resections were obtained from patients with benign prostatic
hyperplasia (BPH), comprising 50 age-matched patients examined
during the same period. Samples were resected from areas of
invasive adenocarcinoma, which had been pathologically identified
according to a hematoxylin and eosin staining pattern. Tumor grade
and clinical stage of the samples were assessed according to the
2002 TNM classification and the Gleason system (16).
Follow-up
Serum prostate-specific antigen (PSA) levels were
evaluated postoperatively every three months during the first year,
every six months from the second to the fifth year, and then
annually from the sixth year. Follow-up data were obtained by
consulting medical records held by the hospital and the
departmental database of patients with PCa, and by contacting the
patients or their family members. Biochemical recurrence was
defined as a sustained elevation of the total serum PSA level
(>0.2 ng/ml) on ≥2 occasions. The biochemical recurrence date
was recorded as the time that the first value was >0.2 ng/ml.
Follow-up time ranged from 6 to 171 months. Patients provided
informed consent. The present study was approved by the Jinan
Central Hospital of Shandong University Ethical Committee (Jinan,
China).
Immunohistochemistry analysis
Tissue sections (5 μm) were deparaffinized,
hydrated and incubated in water with 3% H2O2
(Sinopharm Chemical Reagent Co., Ltd., Beijing, China) for 30 min
in order to destroy endogenous peroxidases. Antigen retrieval was
performed by immersing sections in 10 mM citrate buffer (pH 6.0;
Sinopharm Chemical Reagent Co., Ltd.) and heating in a microwave
for 30 min at 95°C. Non-specific binding to sections was blocked
with normal goat serum [5% (Jackson ImmunoResearch Labs, Inc., West
Grove, PA, USA) in phosphate-buffered saline (Sinopharm Chemical
Reagent Co., Ltd.)] for 1 h. Subsequently, the cells were incubated
with a polyclonal rabbit GOLPH3 antibody (1:100; ab91492; Abcam,
Cambridge, MA, USA) overnight, at 4°C. For the negative control, 5%
normal goat serum without a primary antibody was used. Staining was
detected using a polyclonal secondary horseradish
peroxidase-conjugated rabbit IgG antibody (1:2,000; ab6721; Abcam)
followed by hematoxylin counter-staining. Positive staining was
defined as brown oxidized 3,3′-diaminobenzidine in cellular
compartments, without background signal. The stained sections were
evaluated by two pathologists, unaware of patient clinical
information, using light microscopy (SZ51; Olympus, Tokyo, Japan).
Immunostaining scores for GOLPH3 were determined using a numeric
intensity score of 0–3: 0, no staining; 1 +, weak staining; 2 +,
moderate staining and 3 +, strong staining. Staining was also
dichotomized into negative and positive. Negative was scored if 0
or 1+ and positive was scored if 2+ or 3+.
Cell culture and small interfering RNA
(siRNA) transfection
PC-3 and LNCaP human PCa cell lines were purchased
from the American Type Culture Collection (ATCC; Manassas, VA,
USA). Cells were cultured in an RPMI1640 medium, supplemented with
10% fetal bovine serum (HyClone Laboratories, Inc., Logan, UT, USA)
in 5% CO2, at 37°C. GOLPH3-siRNA and non-specific
control siRNA (Invitrogen Life Technologies, Carlsbad, CA, USA)
were transfected into PC-3 and LNCaP cells using Lipofectamine
2000® (Invitrogen Life Technologies) according to the
manufacturer’s instructions.
Western blot analysis
Total protein was extracted from cultured cells.
Equal amounts of protein (60 μg) were subjected to
electrophoresis using a 10% SDS-polyacrylamide gel (Jackson
ImmunoResearch Labs, Inc.) then transferred to polyvinylidene
difluoride membranes (EMD Millipore, Billerica, MA, USA).
Non-specific binding to membranes was blocked using 5% non-fat milk
prior to incubation with polyclonal rabbit anti-GOLPH3 (1:1,000;
ab91492; CA, Abcam) or monoclonal rabbit anti-GAPDH antibodies
(1:2,000; ab181602; Abcam) overnight, at 4°C. The membranes were
washed and incubated with specific peroxidase-conjugated secondary
antibodies. Specific proteins were detected using an enhanced
chemiluminescence system (Thermo Fisher Scientific, Inc., Waltham,
MA, USA).
Cell vitality, migration and invasion
assays
The effect of transfection on cell growth was
determined by seeding 4,000 cells/well into a 96-well plate and
counting cell numbers 5 days later using
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide
reagent (MTT; Sigma-Aldrich, St. Louis, MO, USA), according to the
manufacturer’s instructions. The cell migratory and invasive
capability levels were measured using Transwell assays. Transfected
cells (1×104; in fetal bovine serum-free medium) were
plated in the upper chambers of a Transwell plate (Corning Life
Sciences, Union City, CA, USA). Lower chambers were filled with a
medium supplemented with 10% fetal bovine serum. Cell invasion
assays were performed similar to the cell growth assays. However,
the Transwell membranes were coated with Matrigel®
(Sinopharm Chemical Reagent Co., Ltd.), prior to adding the cells.
At 24 h, cells were removed from the upper chambers by swabbing,
and those that had moved into the lower chambers were fixed with 4%
paraformaldehyde and then stained with 0.1% crystal violet (both
from Sinopharm Chemical Reagent Co., Ltd.). The number of cells in
five independent fields of view for each well was recorded and
photographed.
Statistical analysis
A χ2 test was performed in order to
analyze the association between GOLPH3 expression and
clinicopathological features of the patients with PCa. Survival
data were evaluated using the Kaplan-Meier method and the log-rank
test. Clinical parameters were analyzed using univariate and
multivariate Cox proportional hazards models in SPSS version 16.0
(SPSS, Inc., Chicago, IL, USA). P<0.05 was considered to
indicate a statistically significant difference.
Results
Associations between GOLPH3 expression
and clinical parameters in patients with PCa
GOLPH3 protein expression was assessed
immunohistochemically in 117 tissues from patients with PCa and in
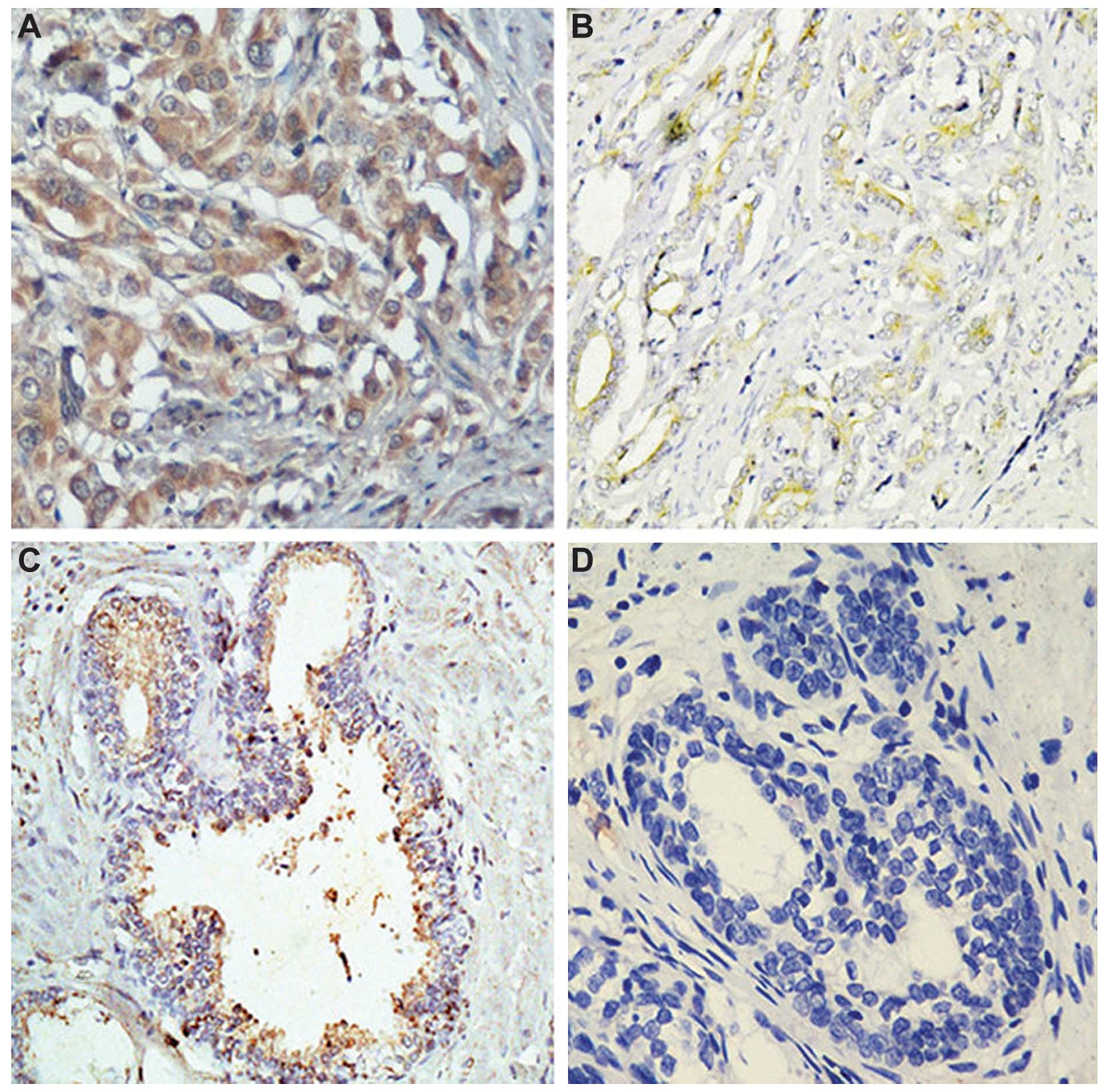
50 BPH tissues from age-matched controls. As shown in Table I, 54 of the 117 PCa tissues (56%)
were classified as GOLPH3-positive (Fig. 1).
 | Table IClinicopathological features of
prostate cancer patients according to GOLPH3 status. |
Table I
Clinicopathological features of
prostate cancer patients according to GOLPH3 status.
| Features | GOLPH3 positive | GOLPH3 negative | P-value |
|---|
| Patient (n) | 54 | 63 | |
| Serum PSA level
(ng/ml) | | | 0.779 |
| <10 | 25 | 29 | |
| ≥10 | 29 | 34 | |
| Gleason score | | | 0.031 |
| ≤6 | 19 | 38 | |
| ≥7 | 35 | 25 | |
| T stage | | | 0.020 |
| T1-T2 | 35 | 54 | |
| T3-T4 | 19 | 9 | |
| Lymph node
status | | | 0.013 |
| (−) | 33 | 53 | |
| (+) | 21 | 10 | |
| Surgical margin
status | | | 0.812 |
| (−) | 35 | 43 | |
| (+) | 19 | 20 | |
Analyses of associations between GOLPH3 expression
and clinical and prognostic parameters, shown in Table I, demonstrated that GOLPH3
expression was positively correlated with Gleason score (P=0.031),
T stage (P=0.020) and lymph node status (P=0.013).
Association between GOLPH3 expression and
patient survival
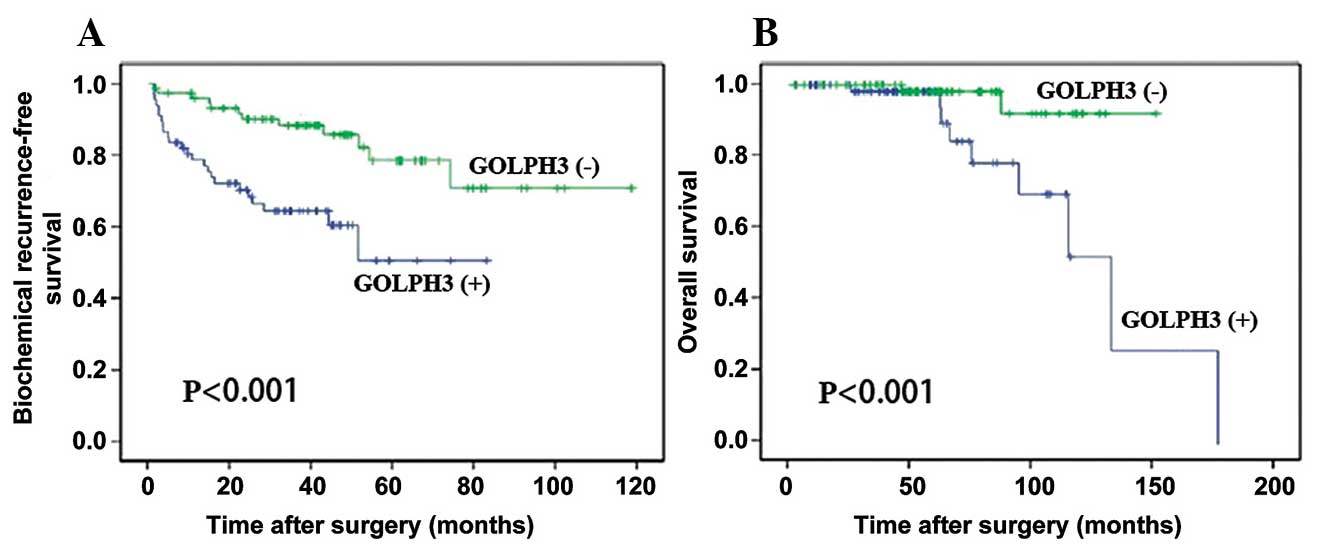
Results of the Kaplan-Meier and log rank tests
suggested that GOLPH3-positive patients exhibited a shorter
biochemical recurrence-free survival time compared with
GOLPH3-negative patients (Fig.
2A). GOLPH3-positive patients also demonstrated significantly
shorter overall survival rates (Fig.
2B).
Univariate and multivariate analyses suggested that
positive GOLPH3 expression was significantly correlated with poorer
biochemical recurrence-free survival [hazard ratio (HR), 2.943; 95%
confidence interval (CI), 1.190–5.521; P=0.028, Table II], and overall survival (HR,
4.371; 95% CI, 2.045–7.109; P=0.014, Table III). These results suggested that
GOLPH3 expression may be useful as a prognostic indicator for
patients with PCa.
 | Table IICorrelations of clinical variables and
GOLPH3 expression with biochemical recurrence-free survival. |
Table II
Correlations of clinical variables and
GOLPH3 expression with biochemical recurrence-free survival.
| Variable | Univariate analysis
| Multivariate analysis
|
|---|
| HR (95% CI) | P-value | HR (95% CI) | P -value |
|---|
| Preoperative PSA | | | | |
| <10 vs. ≥10 | 0.696
(0.462–1.189) | 0.496 | | |
| Gleason score | | | | |
| ≤6 vs. ≥7 | 1.917
(1.014–3.512) | 0.021 | 1.037
(0.521–1.689) | 0.219 |
| T stage | | | | |
| ≤2 vs. ≥3 | 2.192
(1.334–4.328) | 0.019 | 1.663
(0.752–2.436) | 0.097 |
| Lymph node
status | | | | |
| + vs. − | 1.029
(0.570–1.968) | 0.837 | | |
| Surgical margin
status | | | | |
| + vs. − | 1.251
(0.706–2.429) | 0.296 | | |
| GOLPH3
expression | | | | |
| + vs. − | 4.257
(1.985–7.235) | <0.001 | 2.943
(1.190–5.521) | 0.028 |
 | Table IIICorrelations of clinical variables and
GOLPH3 expression with overall survival. |
Table III
Correlations of clinical variables and
GOLPH3 expression with overall survival.
| Variable | Univariate analysis
| Multivariate analysis
|
|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Preoperative PSA | | | | |
| <10 vs. ≥10 | 0.814
(0.486–2.121) | 0.642 | | |
| Gleason score | | | | |
| ≤6 vs. ≥7 | 2.702
(1.209–4.910) | 0.025 | 1.207
(0.603–2.917) | 0.389 |
| T stage | | | | |
| ≤2 vs. ≥3 |
2.154(1.002–3.506) | 0.041 | 1.497
(0.477–2.098) | 0.745 |
| Lymph node
status | | | | |
| + vs. − | 1.019
(0.414–2.708) | 0.401 | | |
| Surgical margin
status | | | | |
| + vs. − | 1.544
(0.673–3.097) | 0.120 | | |
| GOLPH3
expression | | | | |
| + vs. − | 4.598
(2.042–12.109) | 0.001 | 4.371
(2.045–7.109) | 0.014 |
Silencing of GOLPH3 reduces
proliferation, migration and invasion of PCa cells in vitro
Western blot analysis demonstrated that transfection
with GOLPH3-siRNA knocked down GOLPH3 protein expression (Fig. 3A), and MTT assays suggested that
the increase in PC-3 and LNCaP cell numbers over five days were
significantly reduced following transfection with GOLPH3-siRNA
(Fig. 3B and C). Cell migration
assays (Fig. 4A) demonstrated that
GOLPH3-siRNA transfection significantly reduced the migratory
capability of PC-3 and LNCaP cell lines, compared with that in
cells transfected with the control siRNA. Furthermore, cell
invasion was significantly lower in GOLPH3-siRNA-transfected cell,
compared with that in the control siRNA-transfected cells (Fig. 4B). These results indicated that
GOLPH3 expression may induce PCa cell proliferation, migration and
invasion.
Discussion
To the best of our knowledge, the association
between GOLPH3 and PCa progression has yet to be investigated. In
the present study, positive GOLPH3 expression was observed in 56%
of PCa tissues compared with 6% of BPH tissues. In addition,
positive GOLPH3 was significantly correlated with impaired
biochemical recurrence-free survival and overall survival. Further
analysis indicated that GOLPH3 expression is a potential
independent factor indicating a poor prognosis in patients with
PCa.
The gene encoding GOLPH3 in humans is located on
chromosome 5p13 and is expressed in several solid tumor types,
including carcinoma of the lung, ovary, breast and skin (10). However, the association between
GOLPH3 expression and PCa remains largely unknown. Recent studies
have indicated that overexpression of GOLPH3 promotes tumorigenesis
and progression in a number of types of malignancies, and is
associated with poor survival in patients with cancer. GOLPH3
expression was shown to be present in >50% of the 76 patients
with glioma who were studied, and GOLPH3 expression levels were
associated with the severity of the tumor, with higher GOLPH3
expression levels observed in higher grade astrocytomas (12). GOLPH3 overexpression is associated
with poor prognosis in cN0 oral tongue cancer patients and may
represent a novel and useful prognostic indicator for cN0 oral
tongue cancer (17). The results
of a study using cultured glioblastoma multiforme cell lines,
suggested that the down-regulation of GOLPH3, using an siRNA, led
to the suppression of cell proliferation and clonogenic growth.
These observations are in accordance with the findings of an
association between high levels of GOLPH3 expression and a poor
prognosis in patients with glioblastoma multiforme (18). Markedly higher levels of mRNA and
protein GOLPH3 expression were observed in esophageal squamous cell
cancer (ESCC) cell lines and tissues, compared with control cells
(19). Expression of GOLPH3 in
patients with ESCC was found to be positively associated with
clinical stage, TNM classification and histological
differentiation. Furthermore, expression of GOLPH3 is an
independent prognostic factor for patients with ESCC (20). In gastric cancer, expression levels
of GOLPH3 were found to be positively associated with tumor size,
histological grade, depth of invasion, lymph node metastasis,
distant metastasis and TNM stage. In a multivariate analysis the
level of GOLPH3 expression was an independent prognostic factor for
patients with gastric cancer following radical resection (21). In accordance with these findings,
the results of the present study suggested that increased positive
GOLPH3 staining was observed in PCa tissues compared with that in
non-PCa tissues. Furthermore, a positive GOLPH3 staining result was
positively associated with Gleason score, T stage and lymph node
status. Patients with positive GOLPH3 expression exhibited shorter
biochemical recurrence-free, and overall survival. Furthermore,
multivariate analyses suggested that GOLPH3 expression was an
independent indicator of both biochemical recurrence-free and
overall survival. Silencing of GOLPH3 expression inhibited the
proliferation, migration and invasion capabilities of PC-3 and
LNCaP cell lines.
In conclusion, the results of the present study
suggested that GOLPH3 is involved in the proliferation, migration
and invasion of PCa cells. Therefore, GOLPH3 may provide a novel
prognostic marker for patients with PCa who have undergone radical
prostatectomy.
References
|
1
|
Denmeade SR and Isaacs JT: Development of
prostate cancer treatment: the good news. Prostate. 58:211–224.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sarkar D, Lebedeva IV, Su ZZ, et al:
Eradication of therapy-resistant human prostate tumors using a
cancer terminator virus. Cancer Res. 67:5434–5442. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Siegel R, DeSantis C, Virgo K, Stein K,
Mariotto A, Smith T, Cooper D, Gansler T, Lerro C, et al: Cancer
treatment and survivorship statistics, 2012. CA Cancer J Clin.
62:220–241. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hull GW, Rabbani F, Abbas F, Wheeler TM,
Kattan MW and Scardino P: Cancer control with radical prostatectomy
alone in 1,000 consecutive patients. J Urol. 167:528–534. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Patel AR and Stephenson AJ: Radiation
therapy for prostate cancer after prostatectomy: adjuvant or
salvage? Nat Rev Urol. 8:385–392. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mishra MV, Champ CE, Den RB, Scher ED,
Shen X, Trabulsi EJ, Lallas CD, Knudsen KE, Dicker AP and Showalter
TN: Postprostatectomy radiation therapy: an evidence-based review.
Future Oncol. 7:1429–1440. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chang AJ, Autio KA, Roach M III and Scher
HI: High-risk prostate cancer-classification and therapy. Nat Rev
Clin Oncol. 11:308–323. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Swanson GP and Basler JW: Prognostic
factors for failure after prostatectomy. J Cancer. 2:1–19.
2010.
|
|
9
|
Crawford ED, Bennett CL, Andriole GL,
Garnick MB and Petrylak DP: The utility of prostate-specific
antigen in the management of advanced prostate cancer. BJU Int.
112:548–560. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Scott KL, Kabbarah O, Liang MC, Ivanova E,
Anagnostou V, et al: GOLPH3 modulates mTOR signalling and rapamycin
sensitivity in cancer. Nature. 459:1085–1090. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kunigou O, Nagao H, Kawabata N, Ishidou Y,
Nagano S, et al: Role of GOLPH3 and GOLPH3L in the proliferation of
human rhabdomyosarcoma. Oncol Rep. 26:1337–1342. 2011.PubMed/NCBI
|
|
12
|
Li XY, Liu W, Chen SF, Zhang LQ, Li XG and
Wang LX: Expression of the Golgi phosphoprotein-3 gene in human
gliomas: a pilot study. J Neurooncol. 105:159–163. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Romanuik TL, Wang G, Holt RA, Jones SJ,
Marra MA and Sadar MD: Identification of novel androgen-responsive
genes by sequencing of LongSAGE libraries. BMC Genomics.
10:4762009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Abraham RT: GOLPH3 links the Golgi network
to mTOR signaling and human cancer. Pigment Cell Melanoma Res.
22:378–379. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zeng Z, Lin H, Zhao X, Liu G, Wang X, Xu
R, Chen K, Li J and Song L: Overexpression of GOLPH3 promotes
proliferation and tumorigenicity in breast cancer via suppression
of the FOXO1 transcription factor. Clin Cancer Res. 18:4059–4069.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Steuber T, Erbersdobler A, Graefen M,
Haese A, Huland H and Karakiewicz PI: Comparative assessment of the
1992 and 2002 pathologic T3 substages for the prediction of
biochemical recurrence after radical prostatectomy. Cancer.
106:775–782. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li H, Guo L, Chen SW, Zhao XH, Zhuang SM,
Wang LP, Song LB and Song M: GOLPH3 overexpression correlates with
tumor progression and poor prognosis in patients with clinically N0
oral tongue cancer. J Transl Med. 10:1682012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhou J, Xu T, Qin R, et al: Overexpression
of Golgi phosphoprotein 3 (GOLPH3) in glioblastoma multiforme is
associated with worse prognosis. J Neurooncol. 110:195–203. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hua X, Yu L, Pan W, Huang X, Liao Z, Xian
Q, Fang L and Shen H: Increased expression of Golgi phosphoprotein
3 is associated with tumor aggressiveness and poor prognosis of
prostate cancer. Diagn Pathol. 7:1272012. View Article : Google Scholar
|
|
20
|
Wang JH, Chen XT, Wen ZS, Zheng M, Deng
JM, Wang MZ, Lin HX, Chen K, Li J, et al: High expression of GOLPH3
in esophageal squamous cell carcinoma correlates with poor
prognosis. PLoS One. 7:e456222012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hu BS, Hu H, Zhu CY, Gu YL and Li JP:
Overexpression of GOLPH3 is associated with poor clinical outcome
in gastric cancer. Tumor Biol. 34:515–520. 2013. View Article : Google Scholar
|