Introduction
Stroke is the one of leading causes of morbidity and
mortality worldwide (1,2). Ischemic stroke is a sudden
interruption of blood supply to the brain caused by the blockage of
an artery, which may result in brain damage and neurologic
dysfunction (3). The suppression
of brain damage resulting from ischemic stroke is essential in
order to prevent a decrease in the quality of life for patients.
However, a therapeutic strategy for cerebral ischemia/reperfusion
injury has yet to be established (4–6).
Neuroinflammation following ischemia is characterized by the rapid
activation of resident microglia and the infiltration of
inflammatory cells. Neuroinflammation causes an increase in the
expression of proinflammatory cyto-kines and reactive oxygen
species, and may lead to blood-brain barrier disruption, brain
edema, and cell necrosis and apoptosis. Recent studies have
demonstrated that reactive microglia express inflammatory mediators
that may increase the risk of stroke in patients with permanent
middle cerebral artery occlusion (MCAO) and transient ischemia
(7–9). The maintenance of microglial function
during focal stroke may be more important than that of neurons. The
mechanisms of microglial activation involve nuclear factor-κB
(NF-κB) and mitogen-activated protein kinases (MAPK) signaling
pathways. The inflammatory responses to brain injury are associated
with the pathogenesis of stroke (10–12).
The selective inhibition of inflammatory cytokine activity is
important for the development of effective treatments for brain
ischemia and reperfusion injury (13).
Guolou Guizhi decoction (GLGZD), a traditional
Chinese medicine, has been widely used in China for the treatment
of stroke-induced spasticity (14,15).
A previous study has demonstrated that GLGZD treatment may inhibit
spasticity resulting from ischemia by modulating glutamate levels
in an experimental rat model (16). Furthermore, previous studies have
suggested that GLGZD may exhibit anti-neuroinflammatory effects by
suppressing microglial activation in a lipopolysac-charide
(LPS)-induced microglial cell culture experimental model (17,18).
In light of the results of previous studies, it is hypothesized
that GLGZD may be used for the treatment of cerebral ischemia
injury via a number of pathological pathways. The present study
investigated the underlying regulatory effects of GLGZD in an
experimental rat model. In the present study, transient focal
cerebral ischemia was induced in rats using a MCAO model and one
group of the rats received GLGZD treatment. Inflammatory cytokine
expression levels, microglial activation and neutrophil
infiltration were measured, which are associated with the NF-κB
signaling pathway and are indicative of a neuroinflammatory
response. The aim of the present study was to investigate whether
GLGZD exerted an anti-inflammatory and protective effect via the
NF-κB signaling pathway, following cerebral ischemic injury.
Materials and methods
Animals
Male Sprague-Dawley rats (age, 6 weeks; weight,
200–250 g), were obtained from Shanghai SLAC Laboratory Animal Co.,
Ltd. (Shanghai, China). Rats were housed at constant temperature
and relative humidity and exposed to a 12 h light and darkness
cycle. They were treated according to the animal facility
guidelines of Fujian University of Traditional Chinese Medicine
(Fuzhou, China). The animals were fed standard rodent food and pure
water, ad libitum. Experimental procedures were conducted
strictly in accordance with international ethical guidelines and
the National Institutes of Health Guide concerning the Care and Use
of Laboratory Animals. This protocol was approved by the
Institutional Animal Care and Use Committee of Fujian University of
Traditional Chinese Medicine.
Preparation of herbal extracts
Medicinal plants were obtained from Guo Yi Tang
Chinese Herbal Medicine Store (Fujian, China) for the preparation
of the GLGZD extract. The preparation included a mixture of six
crude plant extract ingredients: Trichosanthis radix, Ramulus
cinnamomi, Paeonia lactiflora, Glycyrrhiza radix, Zingiber
officinale Roscoe and Fructus jujubae in a ratio of
3:3:3:2:3:3. The mixture was incubated in double distilled water
for 30 min, and then heated to 100°C and refluxed twice for 2 h.
Subsequently, the mixture was filtered and concentrated using a
rotary evaporator (RE-2000; Shanghai Yarong Biochemistry Instrument
Factory, Shanghai, China) to a final concentration of 1.16 g/ml
(16,17).
Rat model and experimental grouping
The MCAO rat model was established according to the
methods of a previous study (16).
Briefly, the rats were anesthetized using 10% chloral hydrate
(Sinopharm Chemical Reagent Co., Ltd., Shanghai, China),
subsequently the left common carotid artery (CCA), the left
external carotid artery and the internal carotid artery (ICA) were
isolated and exposed. A monofilament nylon suture coated with
poly-L-lysine (Beijing Sunbio Biotech Co., Ltd., Beijing, China)
was inserted though the CCA into the ICA (~8–20 mm beyond the
carotid artery bifurcation) until resistance was felt. The neck
incision was then closed. Blood flow to the brain was blocked for 2
h in order to induce ischemia, subsequently the suture was
withdrawn slowly by ~10 mm in order to permit perfusion. Rats that
exhibited hemiparesis or an increase in body temperature were used
for the experiments. Rats were then randomly divided into three
groups (n=15 per group): Sham group, rats received sham surgery
(not MCAO); MCAO model group, rats were subjected to MCAO with no
GLGZD treatment. GLGZD group, rats were subjected to MCAO and
treated daily with GLGZD (1.16 g/ml) for seven days.
Behavioral examination
Following 2 h and 7 days of perfusion, blinded
observer evaluations of neurological deficits were conducted for
eight rats per group. The criteria for the neurological severity
score was graded from 0–4 (19):
0, rat movement without any neurological deficit; 1, complete
failure to move the right forepaw; 2, repeated circling to the
right when crawling; 3, falling to the right; 4, complete loss of
the ability to walk. Rats with score 0 or 4 were excluded from the
subsequent stages of the investigation (20).
Assessment of cerebral infarct
volume
Following the evaluation of neurological deficits,
the rats were anesthetized using an intraperitoneal injection of
10% chloral hydrate (100 g/0.3 ml) and then decapitated. Brains
were removed and placed on ice for isolation of the cerebral
cortex, subsequently 2-mm coronal sections were prepared. The
sections were stained using tetrazolium chloride (TTC; 20 g/l,
Sigma-Aldrich, St. Louis, MO, USA) with phosphate-buffered saline
(PBS), for 30 min at 37°C, in order to measure brain cell death.
TTC is converted into a red dye when taken up by living cells.
Therefore, ischemic brain cells appeared white and non-ischemic
brain cells appeared red. Images of the sections were captured
using a digital camera (SX20; Canon Inc., Tokyo, Japan). Infarct
volume was measured using Image analysis software (Image J 1.37,
National Institutes of Health, Betheseda, MA, USA) and calculated
as the percentage of infarcted volume of the total cortex
volume.
Tissue collection
At the end of treatment, rats (n=6 per group) were
sacrificed and brains were rapidly removed for TTC staining. Then,
nine other rats in each group were anesthetized using an
intraperitoneal injection of 10% chloral hydrate (100 g/0.3 ml) and
then perfused transcardially with saline (250 ml) and 4%
paraformaldehyde (250 ml), followed by rapid removal of the brain.
The cortex was then dissected for immunohistochemistry, RNA
isolation and protein extraction. Blood was collected via cardiac
puncture using a heparinized syringe (Nanjing Chemical Reagent Co.,
Ltd., Nanjing, China) and centrifuged at 1,625 × g for 20 min in
order to obtain the plasma that was subsequently stored at
−80°C.
Enzyme-immunosorbent assay (ELISA)
cytokine analysis
Cytokine production in the plasma samples [tumor
necrosis factor-α (TNF-α), interleukin 1β (IL-1β), interleukin 6
(IL-6) and monocyte chemotactic protein 1 (MCP-1)] were measured
using ELISA kits (R&D Systems, Inc., Minneapolis, MN, USA),
according to the manufacturer’s instructions. Microwell absorbance
was measured at 450 nm using a microplate reader (BioTek 8008, Bad
Friedrichshall, Germany).
Hematoxylin & eosin (H&E)
staining
H&E histology was conducted in order to examine
the histopathological alterations in ischemic brain samples. Brains
were dissected and fixed in 4% paraformaldehyde at 4°C for 72 h,
and then dehydrated and embedded in paraffin blocks. Coronal
sections (3-mm) were cut backward from the optic chiasma. Sections
were deparaffinized and hydrated with decreasing concentrations of
alcohol, stained with H&E, and photographed under a microscope
(DFC310 FX; Leica, Wetzlar, Germany).
Immunohistochemistry
Brains were dissected and fixed in 4%
paraformaldehyde at room temperature. Tissue blocks were then
dehydrated, embedded in paraffin and cut into 5-μm coronal
sections. The paraffin sections were gently washed with PBS for 15
min followed by blocking with normal horse serum (containing 0.3%
H2O2; Santa Cruz Biotechnology, Inc., Dallas,
TX, USA) in PBS for 30 min. The sections were then incubated
overnight at 4°C with anti-neuronal nuclei (anti-NeuN; cat. no.
bs-1613R), anti-macrophage galactose-specific lectin-2 (anti-Mac2;
cat. no. bs-9505R) and anti-myeloperoxidase (anti-MPO; cat. no.
bs-4943R) primary monoclonal antibodies (Beijing Biosynthesis
Biotechnology Co., Ltd., Beijing, China). Sections were then rinsed
with 0.1% PBS Tween-20® and incubated with a secondary
antibody (anti-rabbit IgG; cat. no. SP-9001; Beijing ZSGB
Biotechnology Co., Ltd., Beijing, China), for 1 h at room
temperature. Subsequently, they were treated with a DAB peroxidase
substrate kit (Maixin Bio, Fuzhou, China) at 4°C in order to
visualize the immunoreaction. Brain sections were observed and
photographed under a microscope, and the percentage of positively
stained cells was measured using Image J software for a
semi-quantitative evaluation.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
RNA was isolated from ipsilateral cortical tissue
(n=3 rats per group) using TRIzol® (Invitrogen Life
Technologies, Carlsbad, CA, USA). RNA was then reverse-transcribed
(PrimeScript™ II 1st Strand cDNA Synthesis kit; Takara Bio, Inc.,
Otsu, Japan) from 2 μg of total RNA, in order to generate
cDNA, and amplified with an SYBR Green I quantitative PCR kit
(Takara Bio, Inc.) using Applied Biosystems Prism 7500 (7500
software v2.0.5; Applied Biosystems Life Technologies, Carlsbad,
CA, USA). Quantitative PCR was performed using the following
primers (Takara Bio, Inc.): Forward: 5′-CACCACGCTCTTCTGTCTACTG-3′
and reverse: 5′-GTACTTGGGCAGATTGACCTC-3′ for TNF-α; forward:
5′-GTAATGATCGTCAACGGGGGAGGAC-3′ and reverse:
5′-CCAGCAAGCCTTGCAACCTTAACCTTAACCA-3′ for IL-1β; forward:
5′-CCACCACTACAGCAAGGG-3′ and reverse: 5′-GAACTGGGCAGACTCAAA-3′ for
IL-6; forward: 5′-TCGGAACCAAATGAGATCAGAAC-3′ and reverse:
5′-GAGGTGGTTGTGGAAAAGGTAGTG-3′ for MCP-1; and forward:
5′-TGGAGTCTACTGGCGTCTT-3′ and reverse: 5′-TGTCATATTTCTCGTGGTTCA-3′
for GAPDH, which was used as an internal control. Results were
normalized to GAPDH expression and the fold change in relative mRNA
levels of the gene of interest was determined using the
2−∆∆Ct method.
Western blot analysis
Brain samples were dissected from the ipsilateral
cortex and extractions were conducted using a lysis buffer (Beijing
Solarbio Science & Technology Co., Ltd., Beijing, China)
containing the protease inhibitor phenylmethanesulfonylfluoride.
Protein concentrations were determined using the bicinchoninic acid
method Beijing Solarbio Science & Technology Co., Ltd.).
Samples (50 μg) were denatured at 100°C for 5 min and
separated by 10% SDS-PAGE. Proteins were then transferred to
polyvinylidene fluoride membranes (EMD Millipore, Billerica, MA,
USA), blocked with 5% non-fat milk and detected using the following
primary antibodies: Mouse monoclonal p65 (cat. no. sc-8008), rabbit
polyclonal phosphor-p65 (p-p65; cat. no. sc-33020), rabbit
polyclonal inhibitor κB-α (IκB-α; cat. no. sc-847), mouse monclonal
phosphor-IκB-α (p-IκB-α; cat. no. sc-8404) or goat polyclonal
β-Actin (cat. no. sc-1616) (1:1,000) (Santa Cruz Biotechnology,
Inc.) at 4°C, overnight. Membranes were incubated with horseradish
peroxidase-conjugated secondary antibodies (anti-rabbit IgG, cat.
no. ZB-2301; and anti-mouse IgG, cat. no. ZB-2305; Beijing ZSGB
Biotechnology Co., Ltd.) for 1 h at room temperature. Protein
immunoblots were detected using enhanced chemiluminescence
(RPN2132; GE Healthcare Bio-Sciences, Pittsburgh, PA, USA) for 1
min, chemiluminescent bands were exposed to a Kodak film (Eastman
Kodak, Rochester, NY, USA) in a dark room and the densitometry of
the gel bands was measured using Image J software.
Statistical analysis
All results are represented as the mean ± standard
error of the mean. Data were analyzed using one-way analysis of
variance using SPSS 15.0 (SPSS, Inc., Chicago, IL, USA). P<0.05
was considered to indicate a statistically significant
difference.
Results
GLGZD reduces cerebral infarction in
rats
Brain infarct volume was determined in order to
investigate the therapeutic effects of GLGZD on ischemic injury in
rats that had experienced MCAO. According to the TTC staining,
brain ischemia was not observed in rats in the sham group (Fig. 1). Infarct volume was significantly
greater in rats in the MCAO group compared with those in the sham
group. Infarct volume was significantly smaller in the GLGZD
treatment group compared with those in the MCAO group (Fig. 1).
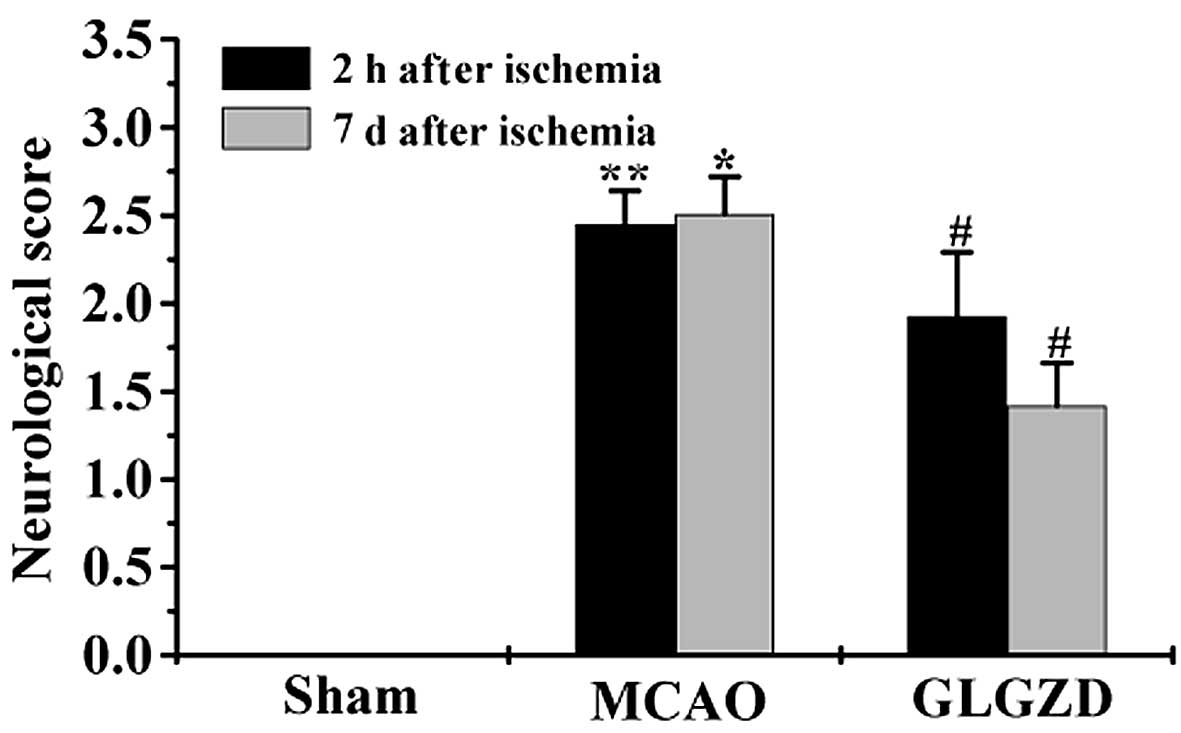
GLGZD treatment reduces neurological
deficit in rats in the MCAO group
In order to confirm the therapeutic effects of GLGZD
on ischemia-induced spasticity, neurological deficit was examined.
Rats in the MCAO group demonstrated motor functional disability,
resulting in a higher neurological behavior score compared with the
sham group (Fig. 2). GLGZD
treatment led to a decrease in the neurological deficit score in
rats following seven days of treatment. The results of the present
study demonstrated that GLGZD treatment may reduce spasticity in
rats following ischemic injury.
GLGZD suppresses inflammatory cytokine
expression in rats in the MCAO group
Protein and mRNA expression levels of the following
cytokines: TNF-α, IL-1β, IL-6 and MCP-1, which are involved in
neuroinflammation post-ischemia, were measured using ELISA and
RT-qPCR. The results suggested that the cytokine expression levels
were significantly greater in plasma samples from rats in the MCAO
group, compared with those in the sham group. By contrast, GLGZD
treatment exhibited significant inhibitory effects on cytokine
expression compared with the MCAO group (Fig. 3A). These results were in accordance
with the results of protein expression analyses. Cytokine mRNA
levels were upregulated in rats in the MCAO group compared with
those in the sham group, and GLGZD treatment led to significantly
lower cytokine mRNA expression levels compared with the MCAO group
(Fig. 3B).
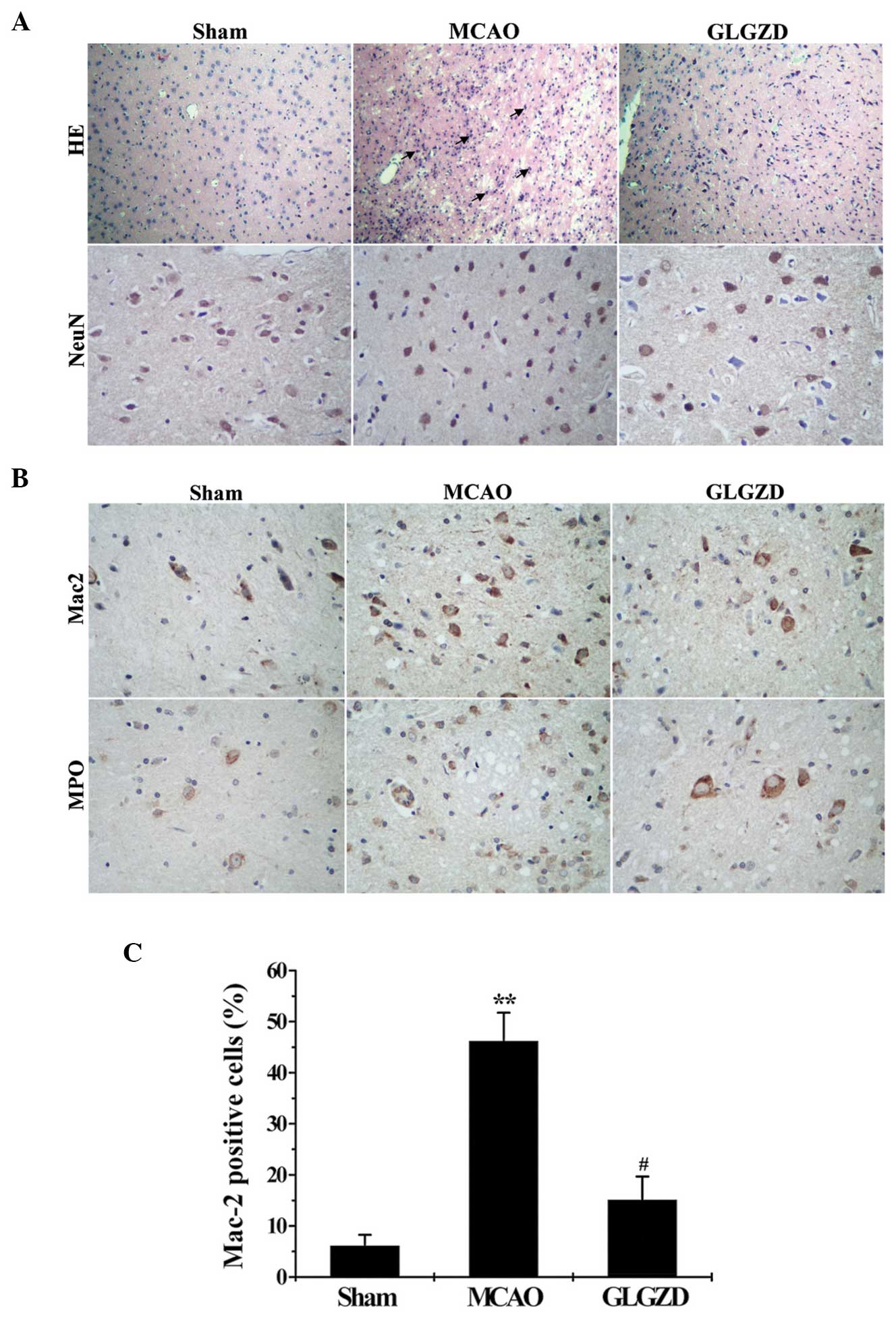
GLGZD attenuates the inflammatory
reaction and neuronal injury in rats in the MCAO group
Microglial activation, neutrophil infiltration and
neuronal damage in the brain tissues were evaluated by analyzing
cerebral histology using H&E and immunohistochemical staining
with anti-NeuN, anti-Mac-2 and anti-MPO antibodies. H&E and
NeuN staining were performed in order to detect neuronal loss and
nuclear shrinkage. According to H&E staining, the nuclei of
neurons in the cerebral hemisphere of rats in the sham group were
healthy, round and aligned. By contrast, the nuclei of neurons in
the MCAO model group were pyknotic and few healthy neurons were
observed in the core ischemic zone. Furthermore, the results of
NeuN staining demonstrated marked neuronal shrinkage and reduction
in rats in the MCAO group compared with the sham group. By
contrast, GLGZD treatment led to lower levels of neuron death
compared with the MCAO group (Fig.
4A). In order to demonstrate the anti-inflammatory effects of
GLGZD treatment in rats that underwent MCAO, representative images
of Mac-2 (an indicator of activated microglia) and MPO (an
indicator of neutrophil infiltration) are shown in Fig. 4B. Low levels of activated microglia
and neutrophil infiltration were observed in the cerebral cortex of
rats in the sham group, compared with those in the MCAO group, as
demonstrated by MPO and Mac-2. By contrast, lower levels of
microglial activation and neutrophil infiltration were observed in
the cerebral cortex of MCAO rats in the GLGZD group compared with
those in the MCAO group (Fig.
4C).
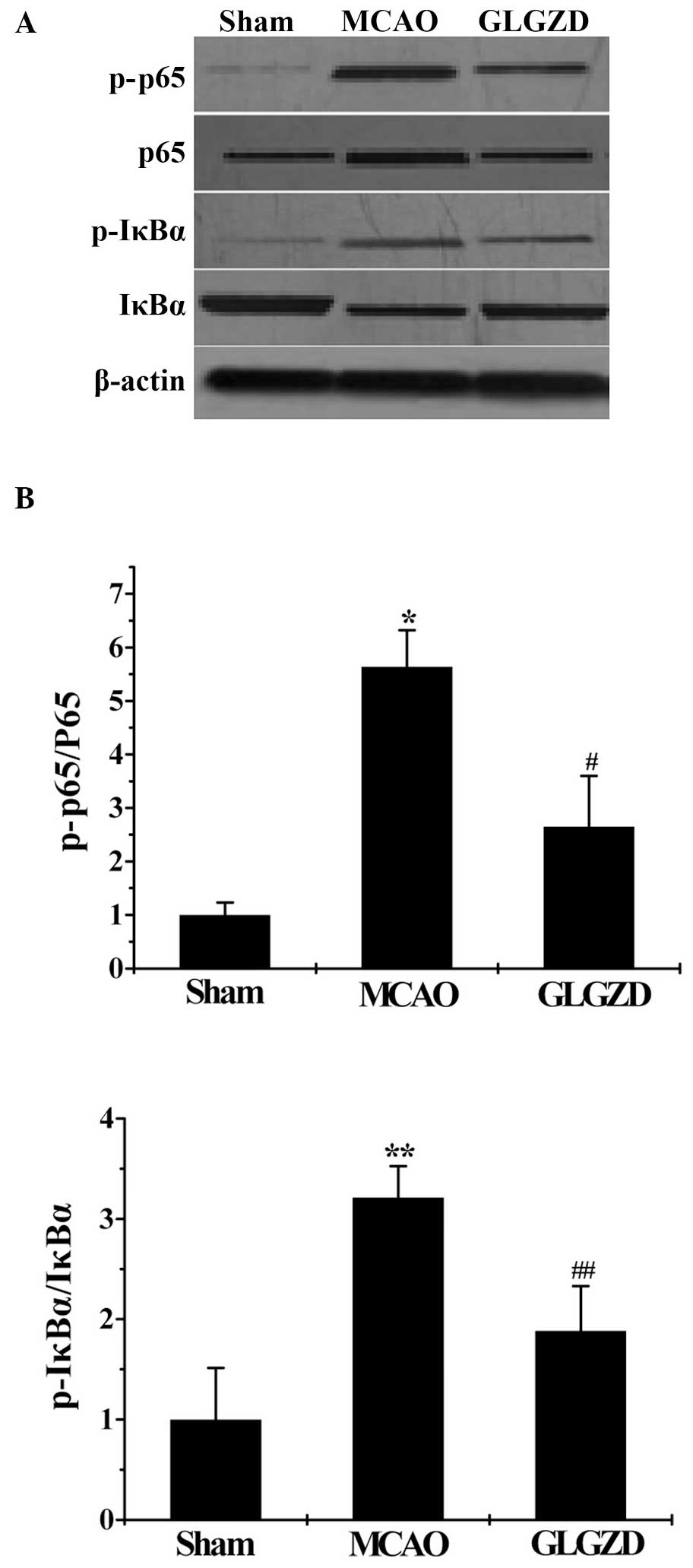
GLGZD induces the reduction of
inflammatory mediators associated with NF-κB signaling in rats in
the MCAO group
In order to further investigate whether NF-κB
signaling is associated with the anti-inflammatory effects of
GLGZD, western blot analyses were performed. p-p65 expression was
lower in the sham group compared with the MCAO group, and was
significantly higher in the MCAO group compared with the GLGZD
group. As shown in Fig. 5, IκBa
phosphorylation was significantly higher in the MCAO group compared
with the sham group, whereas treatment with GLGZD resulted in a
decrease in IκBa phosphorylation, compared with the MCAO group.
Furthermore, IκBα degradation was markedly blocked through
suppressing the phosphorylated forms of IκBα in rats following
treatment with GLGZD.
Discussion
Previous studies have demonstrated that the duration
of isch-emic stroke is associated with motor function disorders,
such as spasticity (21,22). A number of pathological events are
associated with brain damage, including ischemic injury-induced
neuroinflammation, which leads to tissue damage as indicated by the
results of the present study. Microglia are the resident innate
immune cells of the central nervous system (CNS) (23) and they are involved in host defense
of the CNS. Inflammation in ischemic stroke is characterized by the
rapid activation of resident microglia and the infiltration of
inflammatory cells, including MPO+ neutrophils and
leukocytes. Following cerebral ischemia, neutrophils invade the
cerebral parenchyma through the brain endothelium, and subsequently
induce the inflammatory process. A number of inflammatory
mediators, such as TNF-α, IL-1β, IL-6 and MCP-1, which are
indicators of neuroinflammation, are secreted by activated
microglia (24). Inflammatory
responses are associated with the NF-κB signaling pathway
activation, which is involved in microglial activation (25). Upon NF-κB signaling activation,
IκBα phosphorylation leads to IκBα degradation, causing NF-κB to
translocate from the cytoplasm to the nucleus, resulting in the
expression of the target genes (26). A number of studies have reported
that the inhibition of inflammatory processes, for example via the
production of inflammatory mediators, may reduce infarct area in
MCAO models (27,28).
GLGZD is a traditional Chinese medicine, consisting
of a combination of six herbs, including Trichosanthis
radix, Ramulus cinnamomi, Paeonia lactiflora,
Glycyrrhiza radix, Zingiber officinale Roscoe and
Fructus jujubae. GLGZD, an alternative therapy that may
complement conventional medicine, has long been used in China to
clinically treat post-stroke disabilities, such as muscular
spasticity (14,15). Studies have demonstrated that GLGZD
treatment may contribute to the anti-inflammatory effects in
LPS-induced microglial activation. To the best of our knowledge,
the underlying mechanisms involved in of GLGZD neuroprotection are
yet to be elucidated. Based on previous in vitro results, it
is hypothesized that GLGZD may protect the brain from further
neuronal damage in vivo by inhibiting microglial activation
and inflammatory action (17,18).
In the present study, an MCAO model was established
in order to measure the anti-inflammatory effects of GLGZD on
cerebral infarction and neurological deficit in rats with cerebral
ischemia (29).
The effects of seven days of GLGZD treatment on
ischemic-induced infarction and the level of neurological deficit
in rats was investigated. GLGZD treatment significantly reduced the
infarction volume and improved the neurological function in rats
compared with rats without GLGZD treatment. Transcriptional and
translational levels of inflammatory cytokines were measured using
RT-qPCR and ELISA. Seven days of GLGZD treatment resulted in a
reduction in the expression of neuroinflammation-associated
mediators compared with the MCAO group. Cytokine and chemokine
expression levels are associated with inflammatory cascade
signaling, such as the NF-κB signaling pathway. GLGZD treatment led
to a reduction in p-p65 (NF-κB subunit) and p-IκBα expression
levels, and an increase in p65 expression compared with the MCAO
group.
In order to further examine neuronal morphologic
changes and inflammatory responses in ischemic brain samples,
neuron morphology, microglia activation and neutrophil infiltration
were observed, using H&E and immunohistochemical staining for
NeuN, Mac-2 and MPO, seven days after treatment. Neuron loss and
injury were lower in the GLGZD group compared with the MCAO group.
In addition, the activation of Mac-2 and MPO observed in rats in
the MCAO group was inhibited following GLGZD treatment, which
suggests that GLGZD may exhibit an inhibitory effect on microglia
activation and neutrophil infiltration.
In conclusion, the results of the present study
demonstrate that GLGZD exhibits a therapeutic effect on ischemic
injury in rats. Molecular mechanisms underlying these effects
include the reduction of cytokine expression and inactivation of
NF-κB signaling pathway. The present study provides novel insights
into the molecular mechanisms underlying the neuroprotective
effects of GLGZD and its potential as a novel therapeutic target
for ischemic stroke.
Acknowledgments
The present study was supported by Natural Science
Foundation of China (grant no. 81403265), The Guidance Project of
the Fujian Provincial Department of Science & Technology (grant
no. 2012D012) and the Key Project of Department of Health of Fujian
Province (grant no. zlckf01).
Abbreviations:
|
GLGZD
|
gualou guizhi decoction
|
|
MCAO
|
middle cerebral artery occlusion
|
|
TNF-α
|
tumor necrosis factor-α
|
|
IL-1β
|
interleukin 1β
|
|
MCP-1
|
monocyte chemotactic protein 1
|
|
Mac-2
|
macrophage galactose-specific
lectin-2
|
|
MPO
|
myeloperoxidase
|
|
NeuN
|
neuronal nuclei
|
|
NF-κB
|
nuclear factor κ-B
|
|
IκBα
|
inhibitor κB-α
|
|
ANOVA
|
analysis of variance
|
References
|
1
|
Lloyd-Jones D, Adams R, Carnethon M, De
Simone G, Ferguson TB, Flegal K, Ford E, Furie K, Go A, Greenlund
K, et al: American Heart Association Statistics Committee and
Stroke Statistics Subcommittee: Heart disease and stroke statistics
- 2009 update: A report from the American heart association
statistics committee and stroke statistics subcommittee.
Circulation. 119:e21–e181. 2009. View Article : Google Scholar
|
|
2
|
Durai Pandian J, Padma V, Vijaya P, Sylaja
PN and Murthy JM: Stroke and thrombolysis in developing countries.
Int J Stroke. 2:17–26. 2007. View Article : Google Scholar
|
|
3
|
Sims NR and Muyderman H: Mitochondria,
oxidative metabolism and cell death in stroke. Biochim Biophys
Acta. 1802:80–91. 2010. View Article : Google Scholar
|
|
4
|
Hishida R, Kamatani D, Kitaura H, Kudoh M
and Shibuki K: Functional local connections with differential
activity-dependence and critical periods surrounding the primary
auditory cortex in rat cerebral slices. Neuroimage. 34:679–693.
2007. View Article : Google Scholar
|
|
5
|
Ginsberg LD: Impact of drug tolerability
on the selection of antidepressant treatment in patients with major
depressive disorder. CNS Spectr. 14(Suppl 12): 8–14. 2009.
|
|
6
|
Tuma RF and Steffens S: Targeting the
endocannabinod system to limit myocardial and cerebral ischemic and
reperfusion injury. Curr Pharm Biotechnol. 13:46–58. 2012.
View Article : Google Scholar
|
|
7
|
Kim HJ and Chuang DM: HDAC inhibitors
mitigate ischemia-induced oligodendrocyte damage: Potential roles
of oligodendrogenesis, VEGF, and anti-inflammation. Am J Transl
Res. 6:206–223. 2014.PubMed/NCBI
|
|
8
|
Xie L, Sun F, Wang J, Mao X, Xie L, Yang
SH, Su DM, Simpkins JW, Greenberg DA and Jin K: mTOR signaling
inhibition modulates macrophage/microglia-mediated
neuro-inflammation and secondary injury via regulatory T cells
after focal ischemia. J Immunol. 192:6009–6019. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sheridan GK and Murphy KJ: Neuron-glia
crosstalk in health and disease: Fractalkine and CX3CR1 take centre
stage. Open Biol. 3:1301812013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Vartanian KB, Stevens SL, Marsh BJ,
Williams-Karnesky R, Lessov NS and Stenzel-Poore MP: LPS
preconditioning redirects TLR signaling following stroke: TRIF-IRF3
plays a seminal role in mediating tolerance to ischemic injury. J
Neuroinflammation. 8:1402011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sladojevic N, Stamatovic SM, Keep RF,
Grailer JJ, Sarma JV, Ward PA and Andjelkovic AV: Inhibition of
junctional adhesion molecule-A/LFA interaction attenuates leukocyte
trafficking and inflammation in brain ischemia/reperfusion injury.
Neurobiol Dis. 67:57–70. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen S, Yin ZJ, Jiang C, Ma ZQ, Fu Q, Qu R
and Ma SP: Asiaticoside attenuates memory impairment induced by
transient cerebral ischemia-reperfusion in mice through
anti-inflammatory mechanism. Pharmacol Biochem Behav. 122:7–15.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu Y, Lian Z, Zhu H, Wang Y, Yu S, Chen
T, Qu J, Li J, Ma S and Chen X: A systematic, integrated study on
the neuroprotective effects of hydroxysafflor yellow A revealed by
(1)H NMR-based metabonomics and the NF-κB pathway. Evid Based
Complement Alternat Med. 2013:1473622013. View Article : Google Scholar
|
|
14
|
Zhang L and Ai H: Effects of Gua Lou Gui
Zhi decoction on c-fos and c-jun on epileptic rats. Sichuan. J
Tradit Chin Med. 23:21–22. 2005.In Chinese.
|
|
15
|
Yang C, Chen L and Tao J: New usage of a
classical formula-Gua Lou Gui Zhi decoction. Liaoning J Tradit Chin
Med. 39:1599–1600. 2012.In Chinese.
|
|
16
|
Huang J, Tao J, Xue X, Yang S, Han P, Lin
Z, Xu W, Lin J, Peng J and Chen L: Gua Lou Gui Zhi decoction exerts
neuroprotective effects on post-stroke spasticity via the
modulation of glutamate levels and AMPA receptor expression. Int J
Mol Med. 31:841–848. 2013.PubMed/NCBI
|
|
17
|
Hu H, Li Z, Zhu X, Lin R, Lin J, Peng J,
Tao J and Chen L: Gua Lou Gui Zhi decoction suppresses LPS-induced
activation of the TLR4/NF-κB pathway in BV-2 murine microglial
cells. Int J Mol Med. 31:1327–1332. 2013.PubMed/NCBI
|
|
18
|
Hu H, Li Z, Zhu X, Lin R, Peng J, Tao J
and Chen L: GuaLou GuiZhi decoction inhibits LPS-induced microglial
cell motility through the MAPK signaling pathway. Int J Mol Med.
32:1281–1286. 2013.PubMed/NCBI
|
|
19
|
Xue X, You Y, Tao J, Ye X, Huang J, Yang
S, Lin Z, Hong Z, Peng J and Chen L: Electro-acupuncture at points
of Zusanli and Quchi exerts anti-apoptotic effect through the
modulation of PI3K/Akt signaling pathway. Neurosci Lett. 558:14–19.
2014. View Article : Google Scholar
|
|
20
|
Longa EZ, Weinstein PR, Carlson S and
Cummins R: Reversible middle cerebral artery occlusion without
craniectomy in rats. Stroke. 20:84–91. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jiang B, Wang WZ, Chen H, Hong Z, Yang QD,
Wu SP, Du XL and Bao QJ: Incidence and trends of stroke and its
subtypes in China: Results from three large cities. Stroke.
37:63–68. 2006. View Article : Google Scholar
|
|
22
|
Guo JM, Liu AJ and Su DF: Genetics of
stroke. Acta Pharmacol Sin. 31:1055–1064. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang L, Dong LY, Li YJ, Hong Z and Wei
WS: The microRNA miR-181c controls microglia-mediated neuronal
apoptosis by suppressing tumor necrosis factor. J
Neuroinflammation. 9:2112012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lu X, Ma L, Ruan L, Kong Y, Mou H, Zhang
Z, Wang Z, Wang JM and Le Y: Resveratrol differentially modulates
inflammatory responses of microglia and astrocytes. J
Neuroinflammation. 7:462010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kim JB, Yu YM, Kim SW and Lee JK:
Anti-inflammatory mechanism is involved in ethyl pyruvate-mediated
efficacious neuroprotection in the postischemic brain. Brain Res.
1060:188–192. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Perkins ND: Integrating cell-signalling
pathways with NF-kappaB and IKK function. Nat Rev Mol Cell Biol.
8:49–62. 2007. View
Article : Google Scholar
|
|
27
|
Guo RB, Wang GF, Zhao AP, Gu J, Sun XL and
Hu G: Paeoniflorin protects against ischemia-induced brain damages
in rats via inhibiting MAPKs/NF-κB-mediated inflammatory responses.
PLoS One. 7:e497012012. View Article : Google Scholar
|
|
28
|
Dejda A, Seaborn T, Bourgault S, Touzani
O, Fournier A, Vaudry H and Vaudry D: PACAP and a novel stable
analog protect rat brain from ischemia: Insight into the mechanisms
of action. Peptides. 32:1207–1216. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gibson CL: Cerebral ischemic stroke: Is
gender important? J Cereb Blood Flow Metab. 33:1355–1361. 2013.
View Article : Google Scholar : PubMed/NCBI
|