Introduction
Carbamylation is a posttranslational modification of
proteins induced by cyanate in human blood plasma (1). The active form of cyanate, isocyanic
acid, reacts irreversibly with the amino- or thiol groups of amino
acids in proteins. The resulting in vivo carbamylation
changes the structure of proteins, and modifies the activity of
enzymes, cofactors, hormones and antibodies (2).
Several carbamylated proteins have been reported.
Carbamylated erythropoietin protects the kidneys from
cyclosporine-induced nephropathy (3) and astrocyte swelling induced by
ischemia and reperfusion-like injury (4). Carbamylated albumin decreased
O2-production in polymorphonuclear neutrophils (5). Our group previously reported that
carbamylated low-density lipoprotein (cLDL) increases reactive
oxygen species (ROS) and apoptosis via the lectin-like oxidized LDL
receptor-mediated pathway in human umbilical vein endothelial cells
(6).
Carbamylation of low-density lipoprotein (LDL) has
thus far been investigated predominantly in the context of uremia
(7,8) as urea spontaneously converts to
cyanate and ammonia in aqueous solutions (1). However, a recent study demonstrated
that thiocyanate, elevated by smoking and diet, is changed into
cyanate in human blood plasma (9).
The reaction is catalyzed by myeloperoxidase (MPO), a heme protein
derived from leukocytes. MPO-catalyzed carbamylation attenuates LDL
receptor recognition of LDL and raises scavenger receptor
recognition of LDL leading to cholesterol accumulation in
macrophages and foam-cell formation. Increased levels of MPO are
also associated with type 1 and 2 diabetes mellitus (DM) (10,11).
Skeletal muscle is key in glucose homeostasis
(12). The removal of excess
glucose from the circulation involves the stimulation of glucose
transport into skeletal muscle tissue, and glucose intolerance in
type 2 diabetes mellitus (T2DM) is associated with defects in this
glucose transport process, which is regulated predominantly by
insulin. Glucose transporter 4 (GLUT4) deficiency is involved in
impaired glucose uptake in skeletal muscle (13,14).
In this study, it was examined whether cLDL has
biologic effects that are relevant to DM. Thus, the present study
was designed to determine the effects of cLDL using cultured L6
skeletal muscle cells.
Materials and methods
Cell culture
L6 myoblasts (American Type Culture Collection,
Manassas, VA, USA) were maintained in Dulbecco’s modified Eagle’s
medium (DMEM) supplemented with 10% (v/v) fetal bovine serum and 1%
streptomycin and penicillin (all from; Gibco Life Technologies,
Carlsbad, CA, USA) under an atmosphere of 5% CO2 and 95%
humidified air at 37°C. The cells were grown for 4 days to form
myotubes in DMEM supplemented with 2% horse serum (Gibco Life
Technologies). The medium was renewed every 2 days.
Carbamylation of LD
Plasma samples from 3 healthy subjects and 5
patients with chronic renal failure (CRF) who underwent routine
dialysis at the Dongsan Medical Center (Daegu, Republic of Korea)
between 2001 and 2004 were obtained. LDL was prepared as previously
described [Horkko et al 1992]. Briefly, LDL was isolated by
ultracentrifugation at 4°C at 250,000 × g for 22 h, yielding LDL at
a final density of 1.019–1.063 g/ml in sodium chloride. The
isolated LDL was dialyzed, for 24 h against 1 mM EDTA, pH 8.0. The
dialyzed LDL was termed native LDL (nLDL). The nLDL concentration
was determined by the Bradford protein assay (BioRad, Hercules, CA,
USA). Potassium cyanate was added to the nLDL at 20 mg per mg of
nLDL protein and the mixture was incubated at 37°C for 4 h.
Excessive reagents were removed by dialysis for 36 h in sterile
conditions at 4°C against 0.15 M NaCl and 0.01% EDTA, pH 7.0. The
modification of the net charge of cLDL was assessed by 0.5% agarose
gel electrophoresis, as previously described (15).
Glucose uptake assay
The L6 myotubes were cultured on 6-well culture
plates, washed with Krebs-Ringer phosphate-HEPES (KRBH) buffer
[containing 10 mM Na2HPO4, 1 mM
MgSO4, 1 mM CaCl2 136 mM NaCl, 4.7 mM KCl, 10
mM HEPES, pH 7.4; and 0.2% bovine serum albumin (BSA)] and treated
with 20 ng/ml insulin or 100 μg/ml cLDL. Glucose uptake was
measured by adding 0.5 μCi/ml 2-[3H]-deoxy-d-glucose
followed by incubation for 20 min at 37°C. The reaction was
terminated by washing the cells three times with ice-cold
phosphate-buffered saline (PBS). Cells were subsequently lysed with
1 M NaOH solution. Cell associated radioactivity was measured in a
liquid scintillation counter (Beckman Coulter, Brea, CA, USA).
Immunofluorescence microscopy
L6 myotubes were grown on glass coverslips in a
12-well plate and were fixed with 4% paraformaldehyde in PBS for 10
min and permeabilized with 0.25% Triton X-100 (Sigma-Aldrich, St.
Louis, MO, USA) in PBS for 5 min. Cells were blocked with 2% BSA in
PBS at room temperature (RT) for 1 h, prior to incubation with
primary antibody at 4°C overnight. The primary antibodies were as
follows: Polyclonal rabbit anti-GLUT4 (sc-7938; Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA), and monoclonal mouse
anti-β-actin (A5441; Sigma-Aldrich). Fluorescein-conjugated goat
anti-rabbit IgG-HRP (sc-2004; Santa Cruz Biotechnology, Inc.) was
used as the secondary antibody. Nuclear staining was performed with
4′,6-Diamidino-2-phenylindole dihydrochloride (DAPI; Invitrogen
Life Technologies, Carlsbad, CA, USA) at RT for 15 min. The slides
were mounted and analyzed by fluorescent microscopy (Zeiss Axiovert
200M; Zeiss, Jena, Germany).
Preparation of plasma membrane from L6
myotubes
L6 myotubes were harvested with 0.25 M sucrose and
homogenated with 26-gauge needle. The homogenate was centrifuged at
600 x g for 10 min at 4°C and the supernatant was centrifuged at
10,000 × g for 20 min at 4°C. The supernatant was centrifuged at
140,000 × g for 60 min at 4°C. The supernatant was collected and
stored as a cytosolic fraction at −70°C until analyses. The
precipitate obtained was suspended again in 0.25 M sucrose and
stored as a membrane fraction.
Western blot analysis
L6 myotubes were washed twice with cold PBS and then
lysed in ice-cold radioimmune precipitation assay (RIPA) buffer (pH
7.4; 50 mM Tris-HCl, 25 mM EDTA, 650 mM NaCl and 5% Triton X-100)
containing protease inhibitors cocktail solution (Promega
Corporation, Madison, WI, USA). The lysates were centrifuged for 20
min at 12,000 x g at 4°C and the supernatant was collected. The
proteins were separated by SDS-PAGE and transferred to
nitrocellulose membrane (GE Healthcare Life Sciences, Chalfont,
UK). The membrane was blocked in 5% skimmed milk in Tris-buffered
saline plus 0.1% Tween 20 (TBS-T) prior to incubation for 1 h at RT
with primary antibodies. The primary antibodies were as follows:
Polyclonal rabbit anti-GLUT4 (sc-7938; Santa Cruz Biotechnology,
Inc.), polyclonal rabbit anti-iNOS (06-573; EMD Millipore,
Billerica, MA, USA), monoclonal mouse anti-nitrotyrosine (sc-32757;
Santa Cruz Biotechnology, Inc.), polyclonal rabbit anti-insulin
receptor substrate-1 (IRS-1; sc-559; Santa Cruz Biotechnology,
Inc.) and monoclonal mouse anti-β-actin (A5441; Sigma-Aldrich). The
membrane was then washed in TBS-T and incubated with horseradish
peroxidase-conjugated goat anti-rabbit or anti-mouse IgG-HRP
secondary antibodies (Santa Cruz Biotechnology, Inc.). Protein
bands were detected using enhanced chemiluminescensce reagents (GE
Healthcare Life Sciences, Piscataway, NJ, USA).
Nitric oxide (NO) production
NO production was determined spectrophotometrically
using Griess reagent [1% (w/v) sulfanilamide and 0.1% (w/v)
naphthylethylenediamine dihydrochloride in 2.5% phosphoric acid].
The Griess reagent was added to samples of the incubation medium,
and the absorption was read at 540 nm (VICTOR3; PerkinElmer,
Waltham, MA, USA).
Immunoprecipitation
Cell lysates were immunoprecipitated with an
anti-nitrotyrosine antibody coupled to protein A/G plus agarose
overnight at 4°C. The immune complex was washed three times in PBS,
resuspended in RIPA buffer, and boiled for 5 min. Proteins were
resolved on SDS-PAGE and processed for western blot analysis.
Statistical analysis
Data are shown in terms of relative values and
presented as the mean ± standard deviation. Multi-group comparisons
were conducted by Student’s t-test with SPSS, version 18.0 (SPSS,
Inc., Chicago, IL, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
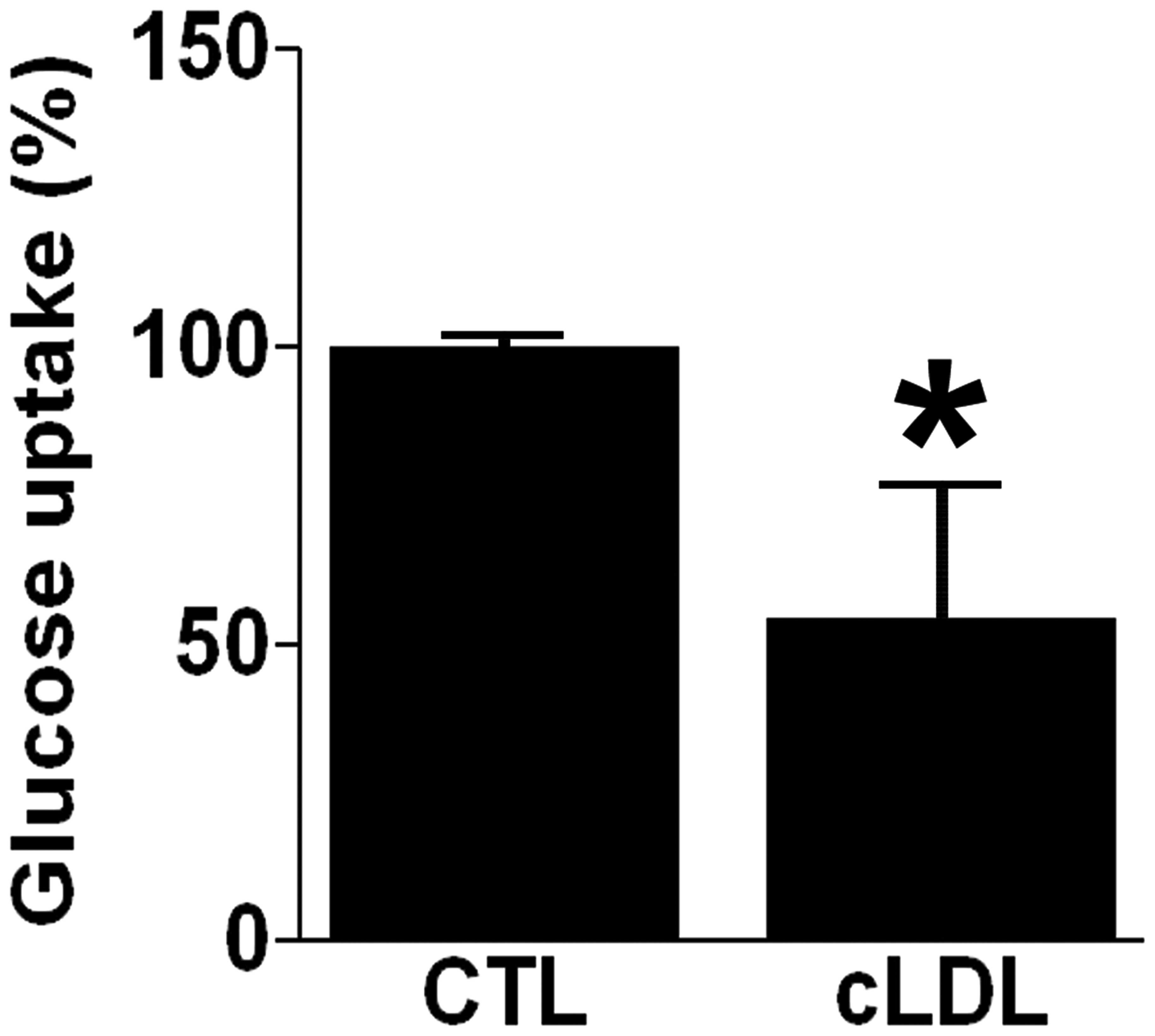
cLDL decreases glucose uptake
Glucose uptake was determined in differentiated L6
myotubes after incubation with 100 μg/ml cLDL. It was
demonstrated that glucose uptake was decreased in cLDL-treated L6
myotubes by 54±22% relative to control (P<0.05, Fig. 1). The effect of oxidized LDL
(oxLDL) on glucose uptake was also observed and it was found that
oxLDL did not affect glucose uptake in differentiated L6 myotubes
(data not shown).
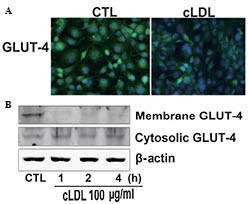
cLDL stimulates GLUT-4 translocation
Cells were incubated with cLDL for the indicated
time and investigated for GLUT-4 translocation using
immunofluorescence and immunoblot analysis. It was observed that
cLDL treatment decreased the fluorescence intensity of GLUT-4 in L6
myotubes compared with that of the control (Fig. 2A). It was also observed that cLDL
treatment attenuated the expression of GLUT-4 in the plasma
membrane of myotube cells while increased the expression of GLUT-4
in the cytosol of myotube cells (Fig.
2B).
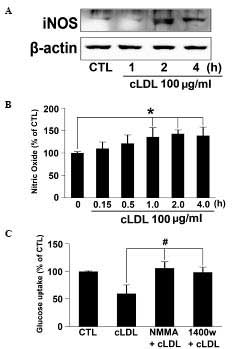
cLDL increases NO mediated tyrosine
nitration of IRS-1
L6 myotubes were incubated with cLDL (100
μg/ml) for the indicated times and iNOS expression was
determined via western blot analysis. Expression of iNOS in cells
treated with cLDL were noticeably increased at 1 h and maintained
at 4 h (Fig. 3A). NO production in
the media of cLDL-treated L6 myotubes also increased in a time
dependent manner (Fig. 3B).
Significantly increased quantities of NO at 1, 2, and 4 h of cLDL
incubation (P<0.05) were observed. In order to determine whether
cLDL attenuates glucose uptake via NO production, NMMA and 1400 W,
specific inhibitors of NOS were employed, and it was observed that
treatment with NMMA or 1400 W reversed the inhibitory effect of
cLDL on glucose uptake in L6 cells (Fig. 3C).
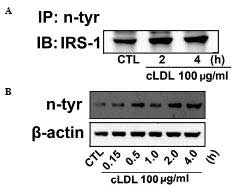
To gain further understanding molecular mechanism
underlying the effect of cLDL-induced NO production on the tyrosine
nitration of IRS-1 was determined by immunoprecipitation and
western blot analysis was examined. An increase in tyrosine
nitration of IRS-1 in cLDL treated L6 cells (Fig. 4A) was observed. In addition, cLDL
treatment was shown to increase levels of protein tyrosine
nitration in a time-dependent manner (Fig. 4B).
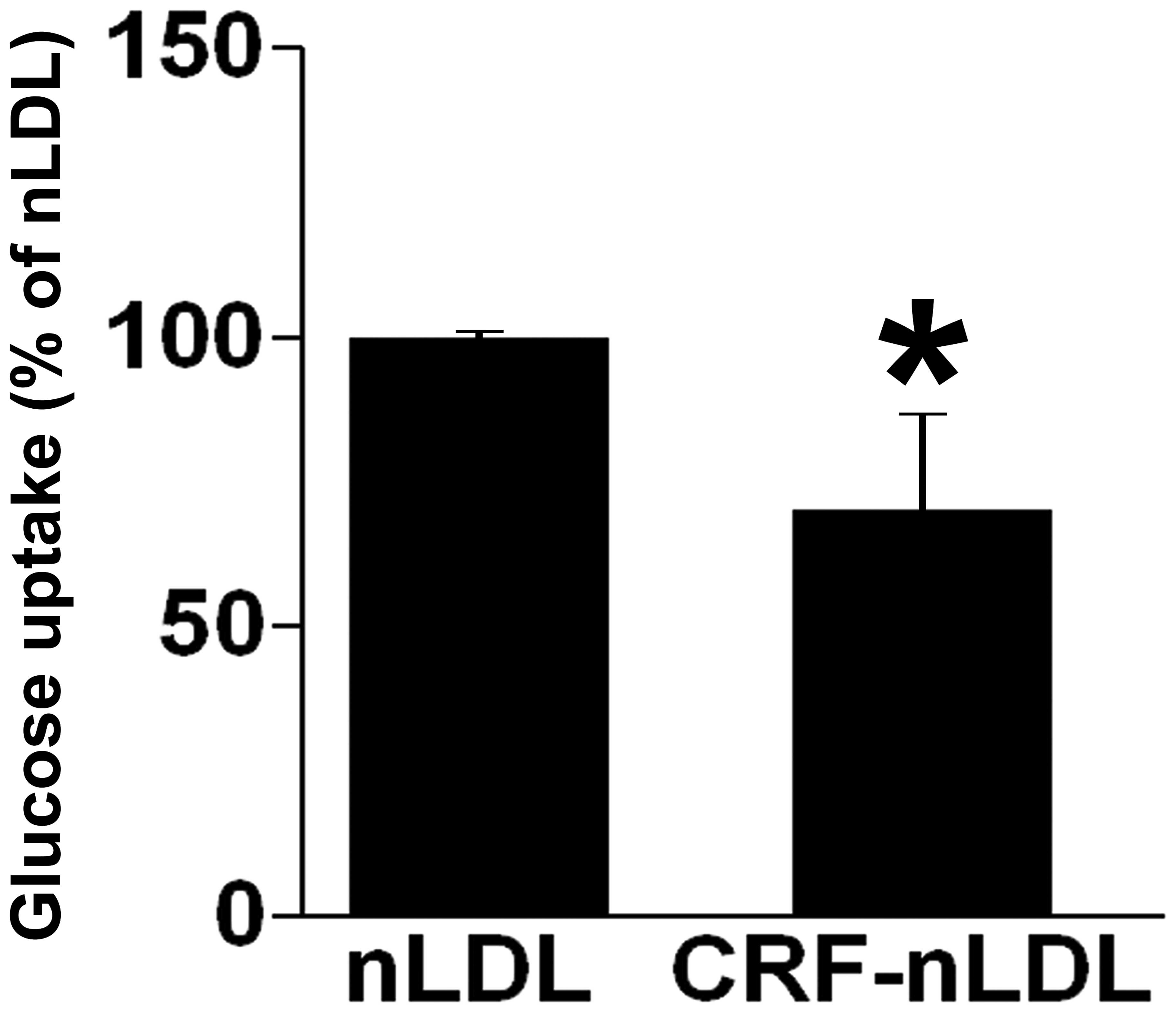
LDLs derived from patients with CRF
decrease glucose uptake
The effects of LDLs derived from patients with CRF
on glucose uptake were next investigated in L6 cells compared with
native LDLs (nLDL) derived from healthy subjects. Exposure of L6
myotubes to CRF-nLDL for 4 h markedly reduced glucose uptake by
70±16% compared with nLDL (Fig.
5).
Discussion
While oxLDL is the modified LDL that has been most
extensively investigated (16),
little is known regarding the functions of cLDL, the most abundant
isoform of modified LDL in the plasma (17). In the current study, it was
hypothesized that cLDL may be involved in glucose metabolism in
muscle cells based on our previous study, which demonstrated that
cLDL increases ROS in endothelial cells (6). Indeed, it was shown that cLDL
attenuates glucose uptake in skeletal muscle cells and the
inhibitory effect of cLDL on glucose uptake is mediated via an NO
mediated pathway.
Carbamylation of protein has been considered
quantitatively important only in the presence of chronic renal
disease as urea is hypothesized to be the endogenous source of
cyanate (8). Thus, uremia-induced
carbamylation of protein presents an important risk factor for the
development of cardiovascular disease. Studies proved that cLDL is
also involved in the pathogenesis of atherosclerosis (9,18,19).
Exposure of endothelial cells to cLDL induces expression of
adhesion molecules and stimulates vascular muscle cell
proliferation (20–22). Our group also previously reported
that cLDL induced increases in ROS and apoptosis in endothelial
cells (6).
A recent study determined that MPO-mediated protein
carbamylation is a uremia-independent mechanism for the synthesis
of cLDL (9). MPO mediates the
production of cyanate from the substrates thiocyanate and
H2O2. Another recent study reported increased
plasma levels of cLDL and MPO in patients with T2DM even in the
absence of renal impairment (23).
In keeping with these findings, results of the current study
indicated that cLDL attenuated glucose uptake in skeletal muscle
cells.
In this study, the effects of cLDL on glucose
transport were explored in skeletal muscle cells. It was
demonstrated that cLDL alone markedly attenuated glucose uptake in
myotube cells. Since skeletal muscle is responsible for the
majority of glucose disposal, the inhibitory effect of cLDL on
glucose transport suggests a possible role for cLDL in the
development of T2DM. GLUT4 translocation from the cytosol to the
membrane is the rate-limiting step for glucose transport and is
associated with insulin resistance (12,13,24).
It was observed that cLDL decreased GLUT4 expression in the
membrane while increased it in the cytosol.
A previous study demonstrated that iNOS induction is
linked to impaired insulin-stimulated glucose uptake in skeletal
muscles (25). Genetic deletion of
iNOS protects against high-fat diet-induced insulin resistance
(26). iNOS-derived NO induces
tyrosine nitration of IRS-1 and is associated with insulin
resistance (27). In this study,
cLDL treatment markedly induced the expression of iNOS in L6 cells
and increased NO production. Co-treatment of 1400 W or NMMA,
selective iNOS inhibitors, reversed cLDL-attenuated glucose
transport. These results indicate that the inhibitory effect of
cLDL on glucose transport is mediated via the iNOS pathway. To
confirm the effect of cLDL on glucose transport, myotube cells were
treated with nLDL from healthy subjects or CRF-nLDL and found that
LDL derived from patients with CRF decreased glucose uptake in L6
cells compared with that from healthy subjects.
In conclusion, the present results suggest that cLDL
is involved in the development of T2DM via induction of NO in
muscle cells. These results also provide insights into the as yet
unidentified functions of cLDL in relation to T2DM.
Acknowledgments
This study was supported by the Kidney Institute
Research Grant of Keimyung University in 2010 and the National
Research Foundation of Korea (NRF) funded by the Korean Government
(MSIP) (grant no. 2014R1A5A2010008).
References
|
1
|
Ha E: Cyanate attenuates insulin secretion
in cultured pancreatic β cells. Mol Med Rep. 5:1461–1464.
2012.PubMed/NCBI
|
|
2
|
Ok E, Basnakian AG, Apostolov EO, et al:
Carbamylated low-density lipoprotein induces death of endothelial
cells: a link to atherosclerosis in patients with kidney disease.
Kidney Int. 68:173–178. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Abe T, Isaka Y, Imamura R, et al:
Carbamylated erythropoietin ameliorates cyclosporine nephropathy
without stimulating eryth-ropoiesis. Cell Transplant. 21:571–580.
2012. View Article : Google Scholar
|
|
4
|
Tang Z, Sun X, Shi Q, et al: Beneficial
effects of carbamylated erythropoietin against oxygen-glucose
deprivation/reperfusion-induced astrocyte swelling: proposed
molecular mechanisms of action. Neurosci Lett. 530:23–28. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jaisson S, Delevallée-Forte C, Touré F, et
al: Carbamylated albumin is a potent inhibitor of polymorphonuclear
neutrophil respiratory burst. FEBS Lett. 581:1509–1513. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Son JN, Lho Y, Shin S, et al: Carbamylated
low-density lipoprotein increases reactive oxygen species (ROS) and
apoptosis via lectin-like oxidized LDL receptor (LOX-1) mediated
pathway in human umbilical vein endothelial cells. Int J Cardiol.
146:428–430. 2011. View Article : Google Scholar
|
|
7
|
Hörkkö S, Huttunen K, Kervinen K, et al:
Decreased clearance of uraemic and mildly carbamylated low-density
lipoprotein. Eur J Clin Invest. 24:105–113. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kraus LM and Kraus AP Jr: Carbamoylation
of amino acids and proteins in uremia. Kidney Int Suppl.
78:S102–S107. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang Z, Nicholls SJ, Rodriguez ER, et al:
Protein carbamylation links inflammation, smoking, uremia and
atherogenesis. Nat Med. 13:1176–1184. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Heilman K, Zilmer M, Zilmer K, et al:
Arterial stiffness, carotid artery intima-media thickness and
plasma myeloperoxidase level in children with type 1 diabetes.
Diabetes Res Clin Pract. 84:168–173. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wiersma JJ, Meuwese MC, van Miert JN, et
al: Diabetes mellitus type 2 is associated with higher levels of
myeloperoxidase. Med Sci Monit. 14:CR406–CR410. 2008.PubMed/NCBI
|
|
12
|
Moller DE: New drug targets for type 2
diabetes and the metabolic syndrome. Nature. 414:821–827. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yea K, Kim J, Yoon JH, et al:
Lysophosphatidylcholine activates adipocyte glucose uptake and
lowers blood glucose levels in murine models of diabetes. J Biol
Chem. 284:33833–33840. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fam BC, Rose LJ, Sgambellone R, et al:
Normal muscle glucose uptake in mice deficient in muscle GLUT4. J
Endocrinol. 214:313–327. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ha E, Bang JH, Son JN, et al: Carbamylated
albumin stimulates microRNA-146, which is increased in human renal
cell carcinoma. Mol Med Rep. 3:275–279. 2010.
|
|
16
|
Lautamäki R, Rönnemaa T, Huupponen R, et
al: Low serum adiponectin is associated with high circulating
oxidized low-density lipoprotein in patients with type 2 diabetes
mellitus and coronary artery disease. Metabolism. 56:881–886. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Apostolov EO, Shah SV, Ok E, et al:
Quantification of carbamylated LDL in human sera by a new sandwich
ELISA. Clin Chem. 51:719–728. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Apostolov EO, Ray D, Savenka AV, et al:
Chronic uremia stimulates LDL carbamylation and atherosclerosis. J
Am Soc Nephrol. 21:1852–1857. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Apostolov EO, Basnakian AG, Ok E and Shah
SV: Carbamylated low-density lipoprotein: nontraditional risk
factor for cardiovascular events in patients with chronic kidney
disease. J Ren Nutr. 22:134–138. 2012. View Article : Google Scholar
|
|
20
|
Apostolov EO, Shah SV, Ok E and Basnakian
AG: Carbamylated low-density lipoprotein induces monocyte adhesion
to endothelial cells through intercellular adhesion molecule-1 and
vascular cell adhesion molecule-1. Arterioscler Thromb Vasc Biol.
27:826–832. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Asci G, Basci A, Shah SV, et al:
Carbamylated low-density lipoprotein induces proliferation and
increases adhesion molecule expression of human coronary artery
smooth muscle cells. Nephrology (Carlton). 13:480–486. 2008.
View Article : Google Scholar
|
|
22
|
Apostolov EO, Shah SV, Ray D and Basnakian
AG: Scavenger receptors of endothelial cells mediate the uptake and
cellular proatherogenic effects of carbamylated LDL. Arterioscler
Thromb Vasc Biol. 29:1622–1630. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shiu SW, Xiao SM, Wong Y, et al:
Carbamylation of LDL and its relationship with myeloperoxidase in
type 2 diabetes mellitus. Clin Sci (Lond). 126:175–181. 2014.
View Article : Google Scholar
|
|
24
|
Wallberg-Henriksson H and Zierath JR:
GLUT4: a key player regulating glucose homeostasis? Insights from
transgenic and knockout mice (review). Mol Membr Biol. 18:205–211.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kapur S, Bedard S, Marcotte B, et al:
Expression of nitric oxide synthase in skeletal muscle: a novel
role for nitric oxide as a modulator of insulin action. Diabetes.
46:1691–1700. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Perreault M and Marette A: Targeted
disruption of inducible nitric oxide synthase protects against
obesity-linked insulin resistance in muscle. Nat Med. 7:1138–1143.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Charbonneau A and Marette A: Inducible
nitric oxide synthase induction underlies lipid-induced hepatic
insulin resistance in mice: potential role of tyrosine nitration of
insulin signaling proteins. Diabetes. 59:861–871. 2010. View Article : Google Scholar : PubMed/NCBI
|