Introduction
Melanin is the main pigment responsible for skin,
eye and hair pigmentation in animals. It is produced in
melanocytes, in lysosome-derived organelles, termed melanosomes.
Melanin is synthesized from tyrosine by the enzyme tyrosinase
(monophenol, L-dopa: oxygen oxidoreductase, EC 1.14.18.1). The gene
solute carrier family 45 member 2 (SLC45A2) encodes the
membrane-associated transporter protein (MATP) that is hypothesized
to be localized to the melanosome membrane and to regulate
tyrosinase activity by controlling melanosome pH (1). MATP is an integral membrane protein
that contains twelve predicted membrane spans and belongs to the
glycoside-pentoside-hexuronide (GPH):cation symporter family, which
is related to the major facilitator superfamily (MFS) (2). Although no transport function has so
far been identified for MATP, plant sucrose transporters, which
also belong to the GPH family, have been characterized in detail.
Plant sucrose transporters are localized to the plasma membrane or
the vacuolar (lysosomal) membrane and catalyze the symport of
H+ and sucrose, as well as that of other glucosides
(3–5).
Loss of function mutations in SLC45A2 result in a
lack of melanin pigmentation. In humans, mutations in SLC45A2 are
the underlying cause of oculocutaneous albinism 4 (OCA4) (6). Similarly, SLC45A2 mutations in other
animals result in hypopigmentation (7,8).
Evidence that MATP regulates the pH of the melanosome lumen
includes the ability of bafilomycin A1 (BafA1), an inhibitor of
V-type ATPases, to reverse the albino phenotype of zebrafish
carrying a mutation in SLC45A2 (1). Therefore, the transport activity of
MATP may involve H+ cotransport similar to that of plant
sucrose transporters. V-type ATPases acidify the lumen of
membrane-bound organelles, such as lysosomes, in animal cells or
vacuoles in plants. Sucrose transporters in plants utilize this
proton gradient to transport sucrose into the cytoplasm (4,9). In
melanosomes, MATP may employ the same mechanism, cotransporting an
unknown substrate with a proton in order to raise the pH of the
melanosome lumen. The activity of tyrosinase is strongly dependent
on the lumenal pH of the melanosome; it is most active at neutral
pH and is inhibited by an acidic pH (10). FM3 and B16-G4F melanoma cell lines
are amelanotic and lack tyrosinase activity. Treatment of these
cells with BafA1 leads to rapid melanin synthesis, which is
independent of de novo synthesis of tyrosinase (11), indicating that melanosome pH
controls tyrosinase activity and melanin synthesis.
The c.1122G>C mutation (L374F) in MATP is
associated with skin color variation in humans. The ancestral form,
L374, is nearly fixed in Africa and Japan (12,13).
The L374F mutation is thought to have occurred only once, and the
F374 allele is associated with light-colored skin and predominates
in the European population (14,15).
A previous study using cultured melanocytes revealed that
homozygous F374 melanocytes have less melanin and lower tyrosinase
activity compared with L374 cells (16). This indicates that MATP influences
tyrosinase biosynthesis or stability, in addition to possibly
regulating tyrosinase activity by controlling melanosome pH. A
previous study in the mouse underwhite (uw) mutant suggested that
MATP may also have a role in melanosome development (17).
It is difficult to investigate the effects of
mutations on MATP function directly, as the transported substrates
of this protein have not been identified to date. Therefore it may
be helpful to utilize related transporters as a model with which to
study MATP activity. The closest homologs to MATP with known
transport function are sucrose transporters from plants. The
substrate specificity of sucrose transporters has been analyzed
(18); they transport a wide range
of glucosides including sucrose. Amino acid positions that
contribute to substrate specificity have also been investigated
(19). In the current study, two
important polymorphisms in SLC45A2 that result in single amino acid
changes in MATP were studied. The effects of these changes on MATP
function are not known. The corresponding mutations were created in
a sucrose transporter and the effects on transport activity were
analyzed.
Materials and methods
Constructs
Site-directed mutagenesis of OsSUT1 in Gateway entry
vector pCR8/GW (Invitrogen Life Technologies, Carlsbad, CA, USA)
was performed using the QuikChange II site-directed mutagenesis kit
(Agilent Technologies, Santa Clara, CA, USA). The following primers
were used: Forward: 5′-catgcgtgggtctatacagtAataggtgcacctc-3′ and
reverse: 5′-gaggtgcacctatTactgtatagacccacgcatg-3′ for OsSUT1_D111N
(to change Asp111 to Asn) and forward:
5′-cagggcaggtgcatttggcTtCctactgaattcgattgtg-3′ and reverse:
5′-cacaatcgaattcagtagGaAgccaaatgcacctgccctg-3′ for OsSUT1_L363F (to
change Leu363 to Phe). Upper case letters denote the changes
introduced into the primer sequences. All introduced DNA sequence
changes were verified by sequencing the entire insert. Sequencing
services were provided by ACGT, Inc. (Wheeling, IL, USA). For
oocyte expression, the constructs in the entry vector were
recombined with pOO2/GW (20).
cRNA for oocyte injection was prepared from templates linearized
with MluI, using the SP6 mMessage mMachine kit (Invitrogen Life
Technologies). Each oocyte was injected with 50 ng of RNA. For
yeast expression, entry clones were recombined with pDR196/GW
(21).
Preparation of oocytes and two-electrode
voltage-clamping (TEVC)
Xenopus laevis oocytes were prepared and TEVC
was performed as described previously (4). Oocytes were bathed in modified sodium
Ringer solution (115 mM NaCl, 2 mM KCl, 1.8 mM CaCl2, 1
mM MgCl2 and 5 mM MES-Tris, at pH 5.6). Recording
pipettes were filled with 1 M KCl and showed tip resistances of 1.5
to 3 megaohms. Currents were measured using a Dagan TEV 200A
amplifier (Dagan Corp., Minneapolis, MN, USA). Currents were
filtered online at 200 Hz and digitized at 2,000 Hz using pClamp
5.5.1 (Axon Instruments, Inc., Union City, CA, USA). The holding
potential was −40 mV and voltage pulses from −142 to +62 mV were
applied for 200 ms. Mean steady-state currents are presented.
Sucrose-dependent currents were obtained by subtracting an average
of background currents prior to and following sucrose application.
Sucrose in modified sodium Ringer solution was added at the
concentrations indicated in the figure legends.
Yeast 14C-sucrose uptake
assay
The uptake of radio-labeled 14C-sucrose
into yeast cells was determined as described previously (22). Briefly, yeast strain, SEY6210 (MATα
leu2-3,112 ura3-52 his3-Δ200 lys2-801 trpl-Δ901 suc2-Δ9) (23), transformed with OsSUT1,
OsSUT1_D111N or OsSUT1_L363F in pDR196/GW (empty vector pDR196 was
used as control) was grown to mid-log phase in SD-URA [1.7 g/l
Difco™ yeast nitrogen base (Becton Dickinson, Franklin Lakes, NJ,
USA), 5 g/l ammonium sulfate, 20 g/l glucose and addition of the
required supplements]. Cells were harvested by centrifugation for 5
min at 2,000 × g, washed with 25 mM Na-phosphate buffer (pH 4) and
resuspended at an OD600 of 20. For uptake assays the
cells were incubated at 30°C in the presence of 10 mM glucose and
14C-labeled sucrose (final concentration 1 mM) for 5
min, then collected onto glass fiber filters (type A/C; Pall
Corporation, Ann Arbor, MI, USA) by vacuum filtration and washed
with 20 ml ice-cold 10 mM sucrose. The filters containing the
collected cells were counted in a scintillation counter and the
amount of sucrose taken up was calculated.
Statistical analysis
Means and standard deviations were calculated using
Microsoft Excel 2011 (Microsoft Corp., Redmond, WA, USA). GraphPad
Prism software version 5.0a (GraphPad Software, La Jolla, CA, USA)
was used for non-linear regression analyses of transporter kinetics
and to perform one-way analysis of variance followed by Tukey’s
multiple comparison test. P<0.05 was considered to indicate a
statistically significant difference.
Results
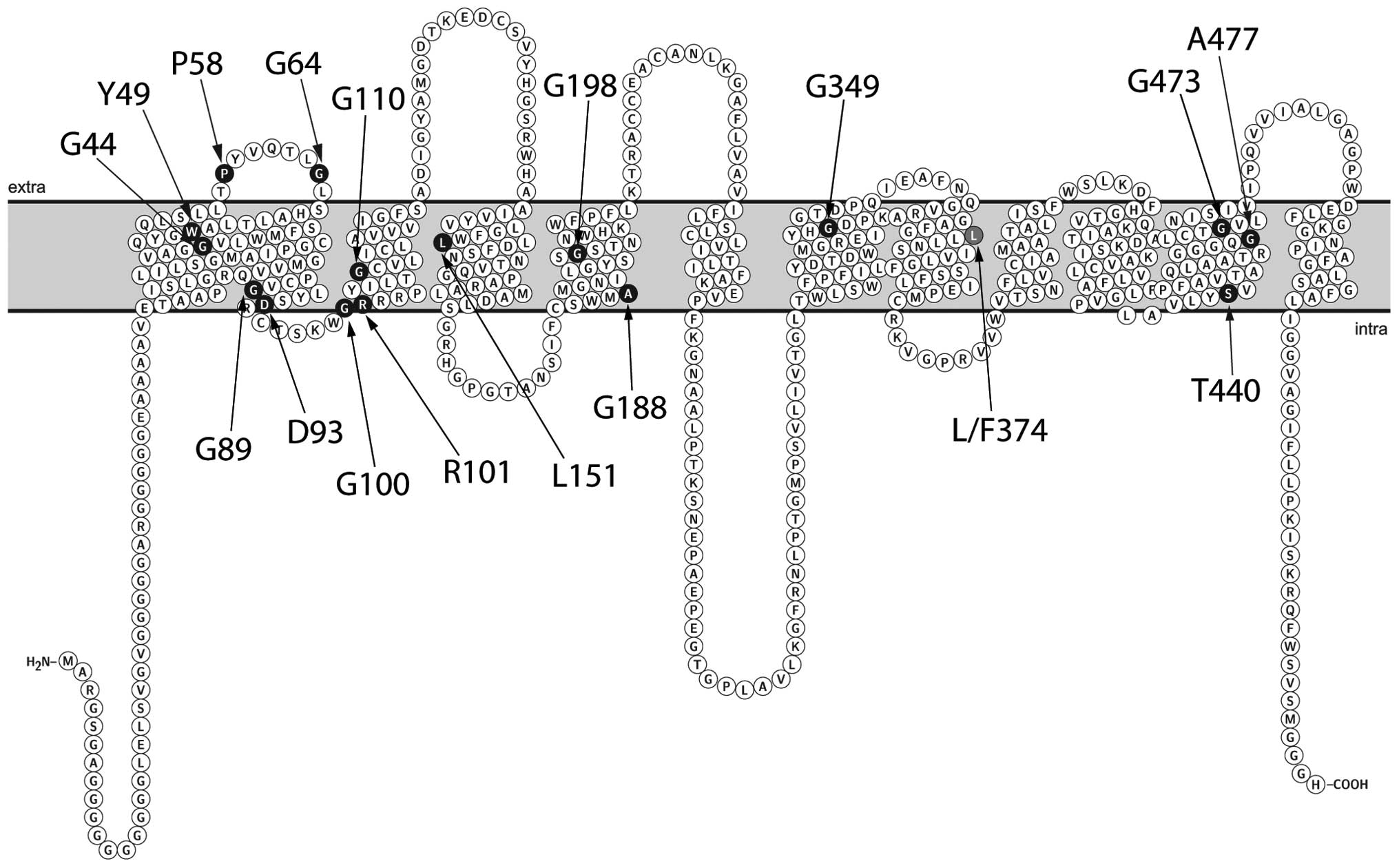
Conserved positions in MATP and sucrose
transporters
More than 50 alleles of SLC45A2 have been identified
in patients with OCA4. Many of them are predicted to result in
amino acid changes in the MATP protein. MATP is distantly related
to plant sucrose transporters, with ~25% similarity at the amino
acid level between plant sucrose transporters and MATP. Sequence
identity between MATP and sucrose transporters is predominantly
within the membrane-spanning domains. A number of the mutations
that are known to lead to OCA4 in humans result in amino acid
changes at positions conserved with plant sucrose transporters, and
those are also located primarily within membrane-spanning domains
(Fig. 1). Table I summarizes a list of known OCA4
alleles and the corresponding amino acids in the rice sucrose
transporter OsSUT1 (24). Of the
identified amino acids, thirteen are perfectly conserved between
humans and plants, and five are similar amino acids (Table I).
 | Table IAA positions in MATP causing OCA4 or
pigmentation phenotypes, which are conserved in plant sucrose
transporters. |
Table I
AA positions in MATP causing OCA4 or
pigmentation phenotypes, which are conserved in plant sucrose
transporters.
| A, OCA4 alleles
with identical residues in OsSUT1 |
|---|
|
|---|
| OCA4 allele | OsSUT1 residue | Reference(s) |
|---|
| G44R | G62 | 38 |
| P58A/S | P76 | 36,37 |
| G64S | G82 | 44 |
| G89R | G107 | 45 |
| D93N | D111 | 25 |
| G100S | G118 | 38 |
| R101C | R119 | 38 |
| G110R | G127 | 35 |
| L151P | L175 | 35 |
| G198D | G222 | 38 |
| G349R | G340 | 35 |
| G473D | G467 | 45 |
| A511E | A506 | 25 |
|
| B, OCA4 alleles
with similar residues in OsSUT1 |
|
| OCA4 allele | OsSUT1 residue | Reference |
|
| Y49C | W67 | 45 |
| G188D | A212 | 46 |
| G188V | A212 | 37 |
| T440A | S449 | 45 |
| A477T | G471 | 36 |
|
C, Non-OCA
polymorphism
|
| OCA4 allele | OsSUT1 residue | Reference |
|
| L/F374 | L363 | 6 |
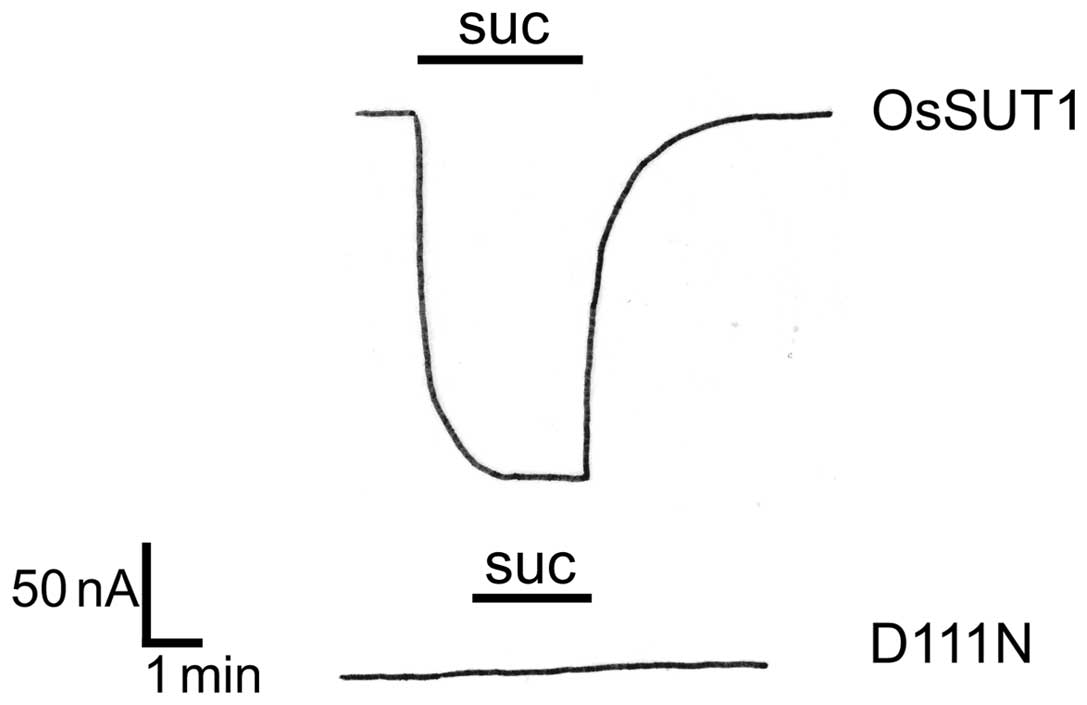
Changing Asp111 to Asn in OsSUT1
inactivates sucrose transport activity
The D93N allele of SLC45A2 has been identified in
Turkish (25) and Korean (26) patients with OCA4, and due to their
strong phenotype, it is likely that this change abolishes function
of MATP. D93 is conserved in animal MATP homologs and in plant
sucrose transporters. This position corresponds to D111 in the
OsSUT1 sucrose transporter. Site-directed mutagenesis of Asp to Asn
(D111N) was performed, and the mutated protein was subsequently
expressed in Xenopus oocytes. While the wild-type OsSUT1
induced large currents in the presence of applied sucrose, as a
result of the transport of the co-transported H+
(Fig. 2) (20), the OsSUT1_D111N mutant did not
induce any currents under the same conditions (Fig. 2). This is consistent with an
inactivation of the transporter due to the introduced amino acid
change.
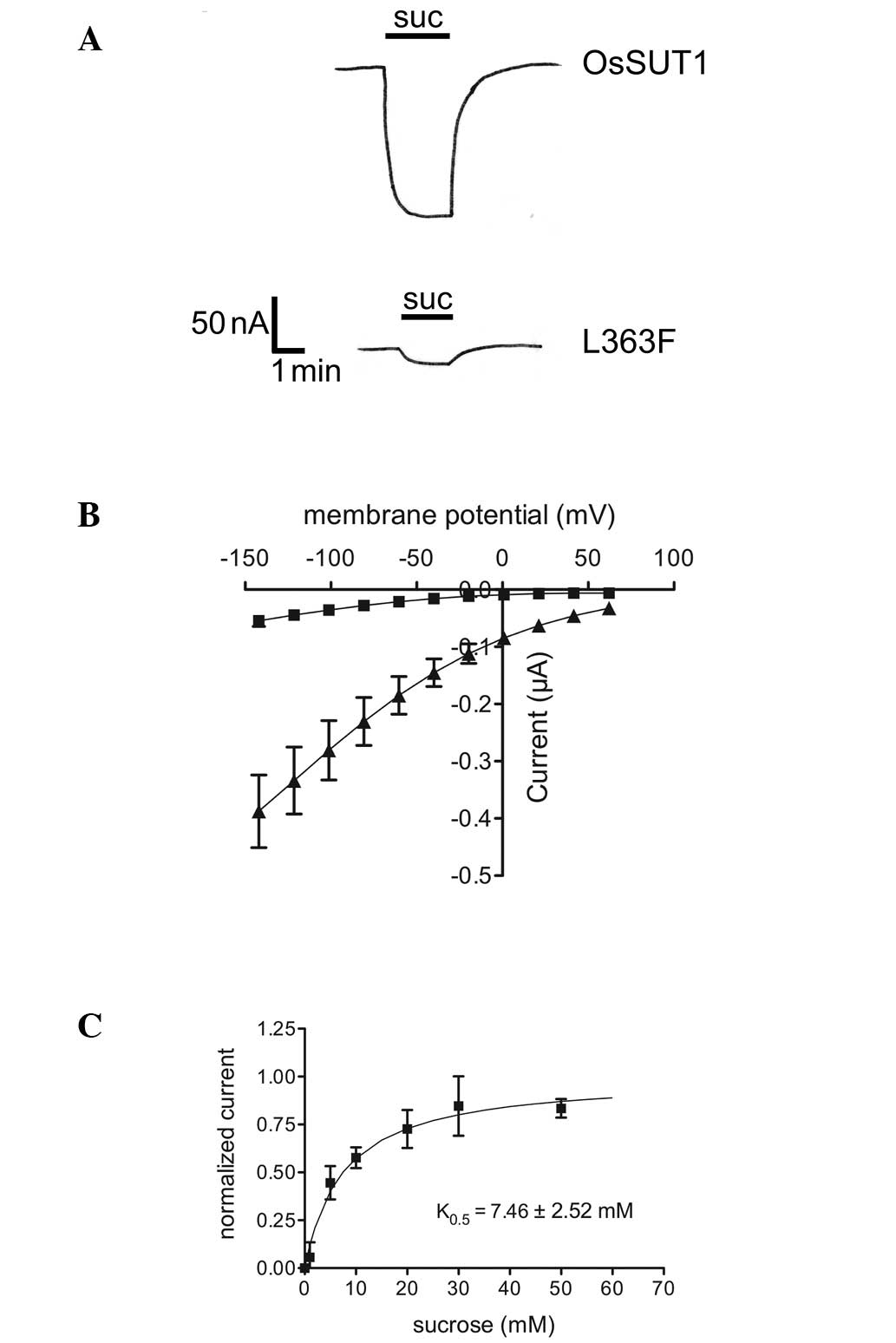
Changing Leu363 to Phe in OsSUT1 reduces
sucrose trans- port activity but does not affect the apparent
substrate affinity
One polymorphism that is not implicated in OCA4,
although it is known to be correlated with differences in
pigmentation in human populations, is L/F374. Since
loss-of-function mutations in SLC45A2 result in OCA4, a lower
activity of MATP is thought to result in reduced pigmentation. In
accordance with this, mutations in SLC45A2 that lower transcription
result in a reduction in pigmentation (27). The F374 allele is generally found
in individuals with lighter skin and hair (12,14,15,28).
In plants, the corresponding position contains a Leu (L363), and is
conserved in all plant sucrose transporters.
The effect of L374F on activity was examined by
replacing L363 with Phe in the rice sucrose transporter, OsSUT1.
Transport activity was analyzed in oocytes by TEVC. Compared with
the wild-type transporter, the OsSUT1_L363F mutant produced only
~10% of the current in the presence of 10 mM sucrose at a membrane
potential of −40 mV (Fig. 3A). The
transport activity of OsSUT1_L363F was lower than that of the wild
type transporter at all membrane potentials tested (Fig. 3B). However, the apparent affinity
of the mutated transporter was not different from that of the wild
type OsSUT1. OsSUT1_L363F had a K0.5 of 7.46 mM sucrose
at pH 5.6 and a membrane potential of −100 mV (Fig. 3C). The K0.5 of wild-type
OsSUT1 was previously reported to be 7.5 mM at pH 5.6 (20).
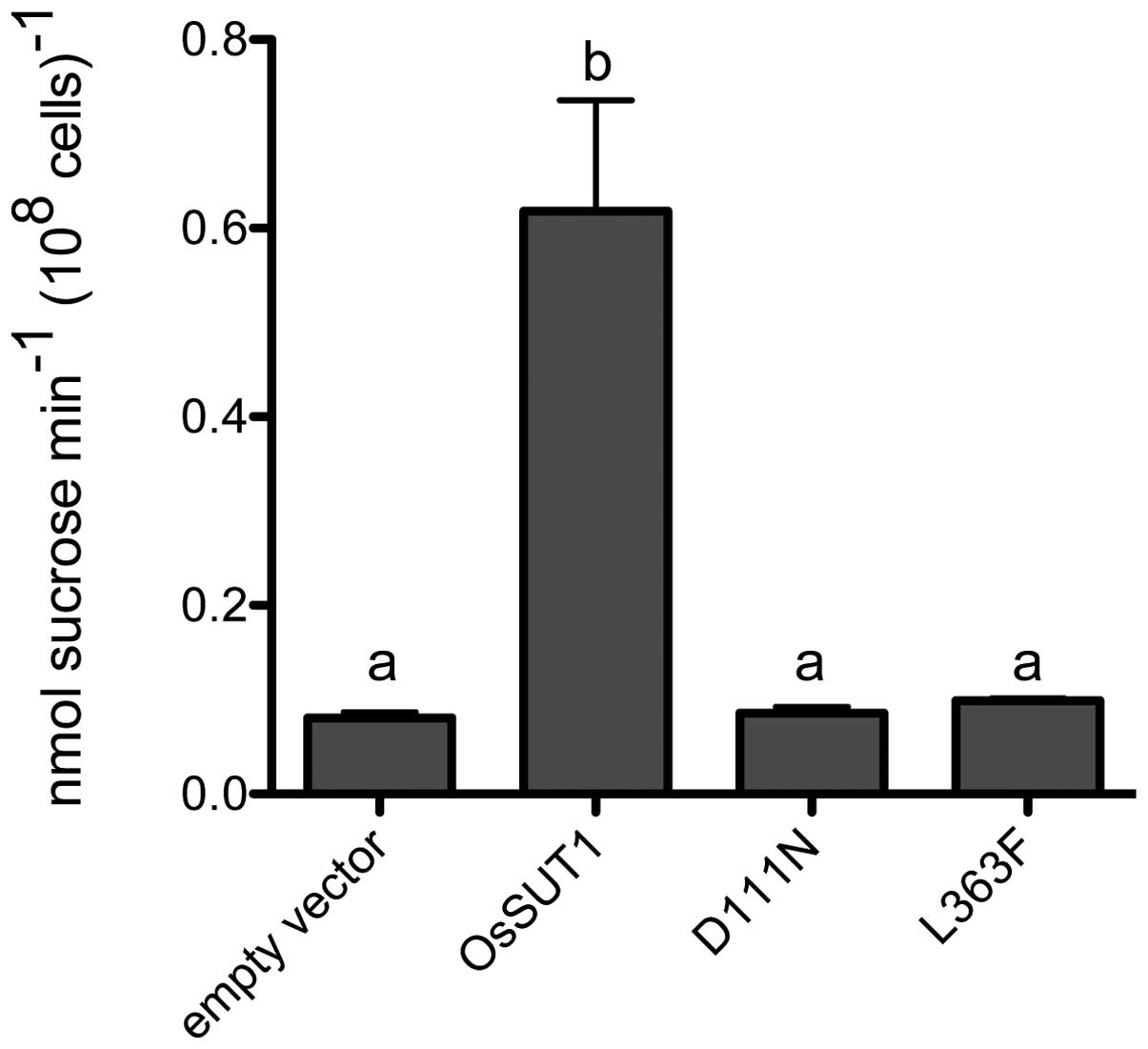
In order to confirm the results using an independent
heterologous system, OsSUT1 and the two mutants, OsSUT1_D111N and
OsSUT1_L363F, were expressed in yeast, and sucrose uptake was
determined using 14C-sucrose as a substrate. Consistent
with the results from oocytes, the D111N mutant showed no
significant sucrose uptake above the background level (empty
vector). Sucrose uptake by yeast expressing the L363F mutant was
also not statistically different from the vector control (Fig. 4).
Discussion
Worldwide, ~1 in 17,000 people are affected by
oculocutaneous albinism (OCA) (29), a heritable autosomal recessive
condition that is defined by a lack of pigmentation in hair, skin
and eyes. It is projected that 1 in 70 people carry an allele for
albinism (30). Besides the
visible effects on pigmentation, patients also experience a variety
of problems with vision as a consequence of the lack of pigment in
the retina. OCA subtypes are differentiated based on underlying
mutations. OCA1 is caused by mutations in the tyrosinase gene,
encoding an enzyme critical for melanin synthesis. In OCA2, the
OCA2 gene is affected, which encodes the P-protein, a transporter
with homology to bacterial
Na+/H+-antiporters. OCA3 is characterized by
mutations in the tyrosinase-related protein 1 (TRP1) gene, while
OCA4 is caused by mutations in SLC45A2, which encodes a membrane
protein that is localized to melanosomes and has homology to plant
sucrose transporters. Melanin synthesis occurs in melanosomes,
which are specialized organelles of melanocytes. Key steps of
melanin synthesis are catalyzed by the enzyme tyrosinase, a
copper-dependent glycoprotein with a single transmembrane-spanning
domain. Polymorphisms in SLC45A2 produce similar effects in other
animals. In horse, D153N in the SLC45A2 homolog MATP is associated
with a cream coat color (31). In
mouse, an SLC45A2 homolog is encoded by the uw gene (6,17),
while mutations in the corresponding gene b in medaka fish reduce
melanin content (7) and in birds,
plumage color is also controlled by alleles of the gene encoding
MATP (32). In zebrafish,
bafilomycin, an inhibitor of V-ATPase, has been found to rescue
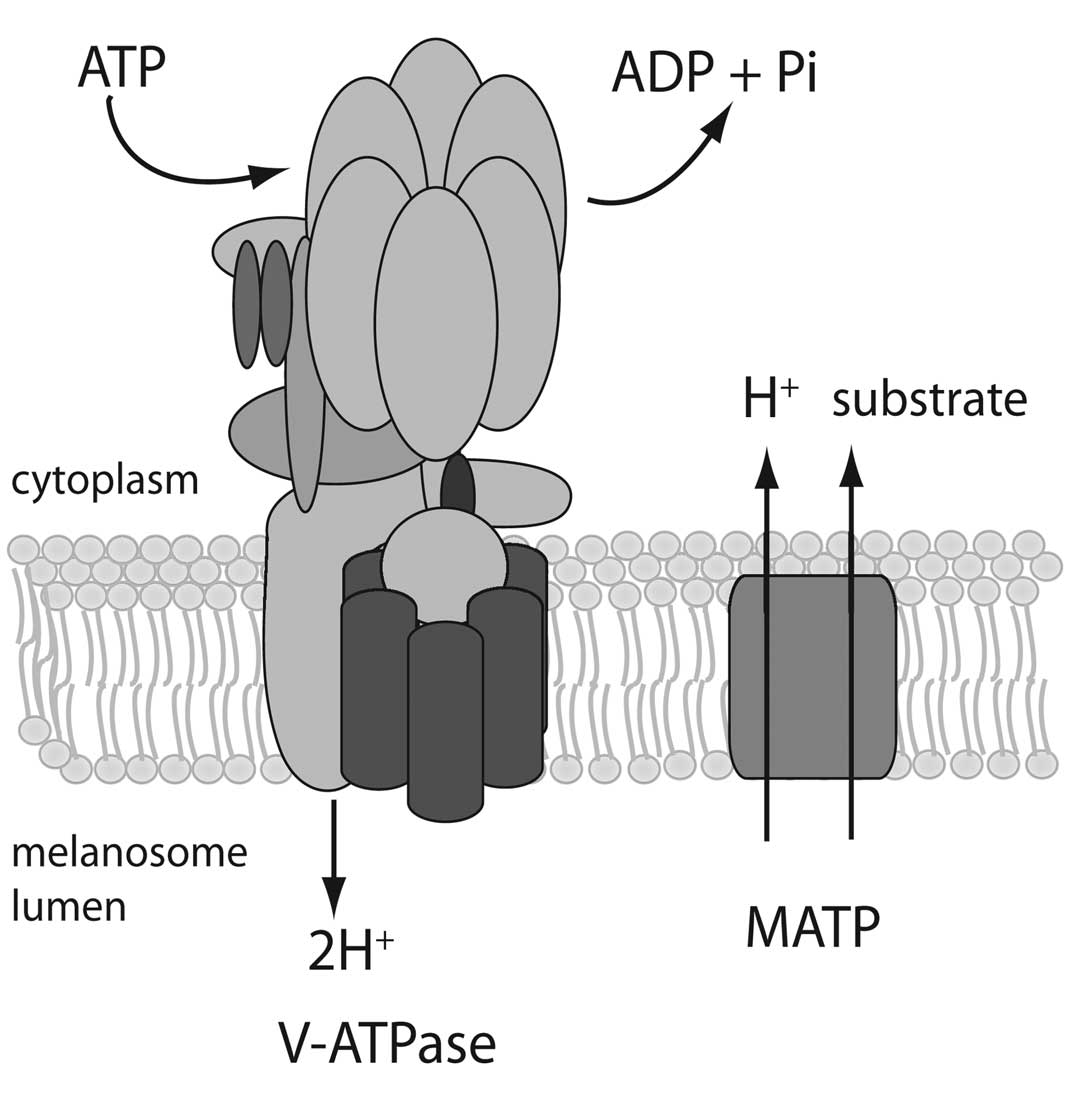
melanin synthesis in an SLC45A2 mutant (1). This indicates that MATP activity may
increase the lumenal pH of melanosomes and thereby stimulate
melanin synthesis. A model for V-ATPase and MATP in the melanosome
membrane is presented in Fig. 5.
In this model, V-ATPase acidifies the melanosome lumen, inhibiting
tyrosinase activity, and MATP functions to stimulate tyrosinase by
increasing lumenal pH. However, transport activity has not been
assayed for an MATP homolog from any animal, and the substrates and
transport mechanisms remain unknown. SLC45A2 has been expressed in
Xenopus oocytes and in yeast, without detection of sucrose
transport activity (Ward and Reinders, unpublished result). The
closest homologs to SLC45A2 with known transport function are
sucrose transporters (SUTs) from plants (18). SUTs are H+-coupled
uptake transporters (symporters) that transport sucrose and
H+ into the cytoplasm. They are localized to the plasma
membrane, with the exception of type III sucrose transporters,
which are found in the vacuole membrane (4,33).
Certain mutations in SLC45A2, isolated from patients
with OCA4, produce a truncated MATP protein that is likely to be
non-functional (6,25,34–39).
Polymorphisms that produce single amino acid substitutions in MATP,
and that lead to OCA4 or result in pigmentation phenotypes, are
more diffi-cult to interpret. For these alleles, it may be useful
to have a biochemical assay for the MATP protein in order to
determine whether transport activity is altered and whether Vmax or
substrate affinity, or other activities are affected. In the
absence of a direct assay of MATP, this group used transport assays
of the related plant sucrose transporters as a model to test the
effect of SLC45A2 mutations. A number of the point mutations that
cause OCA4 result in changes in amino acids that are conserved
between animal and plant homologs (Table I). In the present study, the
effects of two SLC45A2 mutations, D93N that causes OCA4, and L374F
that is related to light skin color in Europeans were tested.
When the MATP D93N mutation was reproduced in
OsSUT_D111N, sucrose transport activity was not detectable
(Fig. 2). A 3D model of OsSUT1
locates D111 at the cytoplasmic edge of TMS2 (40). D111 is conserved in all plant
sucrose transporters, and the lack of activity of the D111N mutant
indicates that it may possess an important function. This result
suggests that the D93N MATP mutant, which causes OCA4, lacks
transport activity. The L374F mutation in SLC45A2 is hypothesized
to have occurred recently and to have undergone positive selection
during the last 5,000 years (41,42).
Selection for lighter pigmentation may have been driven by a
requirement for greater exposure to sunlight, necessary for vitamin
D biosynthesis, at locations distant from the equator.
The results indicate that although sequence identity
is only 25% between MATP and sucrose transporters, the conserved
residues may be important for transport function in each of these
transporters. Therefore, information on the structure-function
relation of sucrose transporters may be useful in predicting the
effects of mutations on the function of MATP. The sucrose
transporter 3D structure has been modeled (40), based on the crystal structure of
lactose permease and other members of the major facilitator
superfamily obtained from E. coli. This produced a
refinement in the transmembrane model for sucrose transporters as
presented in Fig. 1. As in lactose
permease, conserved charged amino acids within membrane spans in
the sucrose transporter, OsSUT1, were found to be important for
transport function (40). Further
studies may be conducted in order to determine whether other
mutations corresponding to OCA alleles affect the function of
sucrose transporters. Conversely, the effects of numerous sucrose
transporter mutations on transport activity have been investigated
in detail (19,40,43)
and these may be used to predict the effect of corresponding
mutations in SLC45A2.
Acknowledgments
This study was supported by The Division of Chemical
Sciences, Geosciences, and Biosciences, Office of Basic Energy
Sciences of the U.S. Department of Energy (grant no.
DE-FG02-10ER15886).
References
|
1
|
Dooley CM, Schwarz H, Mueller KP, et al:
Slc45a2 and V-ATPase are regulators of melanosomal pH homeostasis
in zebrafish, providing a mechanism for human pigment evolution and
disease. Pigment Cell Melanoma Res. 26:205–217. 2013. View Article : Google Scholar
|
|
2
|
Reddy VS, Shlykov MA, Castillo R, Sun EI
and Saier MH Jr: The major facilitator superfamily (MFS) revisited.
FEBS J. 279:2022–2035. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chandran D, Reinders A and Ward JM:
Substrate specificity of the Arabidopsis thaliana sucrose
transporter AtSUC2. J Biol Chem. 278:44320–44325. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Reinders A, Sivitz AB, Starker CG, Gantt
JS and Ward JM: Functional analysis of LjSUT4, a vacuolar sucrose
transporter from Lotus japonicus. Plant Mol Biol. 68:289–299. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sivitz AB, Reinders A and Ward JM:
Analysis of the transport activity of barley sucrose transporter
HvSUT1. Plant Cell Physiol. 46:1666–1673. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Newton JM, Cohen-Barak O, Hagiwara N, et
al: Mutations in the human orthologue of the mouse underwhite gene
(uw) underlie a new form of oculocutaneous albinism, OCA4. Am J Hum
Genet. 69:981–988. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fukamachi S, Shimada A and Shima A:
Mutations in the gene encoding B, a novel transporter protein,
reduce melanin content in medaka. Nat Genet. 28:381–385. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Du J and Fisher DE: Identification of
Aim-1 as the underwhite mouse mutant and its transcriptional
regulation by MITF. J Biol Chem. 277:402–406. 2002. View Article : Google Scholar
|
|
9
|
Schulz A, Beyhl D, Marten I, et al:
Proton-driven sucrose symport and antiport are provided by the
vacuolar transporters SUC4 and TMT1/2. Plant J. 68:129–136. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ancans J, Tobin DJ, Hoogduijn MJ, Smit NP,
Wakamatsu K and Thody AJ: Melanosomal pH controls rate of
melanogenesis, eumelanin/phaeomelanin ratio and melanosome
maturation in melanocytes and melanoma cells. Exp Cell Res.
268:26–35. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ancans J and Thody AJ: Activation of
melanogenesis by vacuolar type H(+)-ATPase inhibitors in
amelanotic, tyrosinase positive human and mouse melanoma cells.
FEBS lett. 478:57–60. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nakayama K, Fukamachi S, Kimura H, Koda Y,
Soemantri A and Ishida T: Distinctive distribution of AIM1
polymorphism among major human populations with different skin
color. J Hum Genet. 47:92–94. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lucotte G and Yuasa I: Near fixation of
374l allele frequencies of the skin pigmentation gene SLC45A2 in
Africa. Biochem Genet. 51:655–665. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yuasa I, Umetsu K, Harihara S, et al:
Distribution of the F374 allele of the SLC45A2 (MATP) gene and
founder-haplotype analysis. Ann Hum Genet. 70:802–811. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yuasa I, Umetsu K, Watanabe G, Nakamura H,
Endoh M and Irizawa Y: MATP polymorphisms in Germans and Japanese:
the L374F mutation as a population marker for Caucasoids. Int J
Legal Med. 118:364–366. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cook AL, Chen W, Thurber AE, et al:
Analysis of cultured human melanocytes based on polymorphisms
within the SLC45A2/MATP, SLC24A5/NCKX5, and OCA2/P loci. J Invest
Dermatol. 129:392–405. 2009. View Article : Google Scholar
|
|
17
|
Costin GE, Valencia JC, Vieira WD,
Lamoreux ML and Hearing VJ: Tyrosinase processing and intracellular
trafficking is disrupted in mouse primary melanocytes carrying the
underwhite (uw) mutation. A model for oculocutaneous albinism (OCA)
type 4. J Cell Sci. 116:3203–3212. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Reinders A, Sivitz AB and Ward JM:
Evolution of plant sucrose uptake transporters. Front Plant Sci.
3:222012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Reinders A, Sun Y, Karvonen KL and Ward
JM: Identification of amino acids important for substrate
specificity in sucrose transporters using gene shuffling. J Biol
Chem. 287:30296–30304. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sun Y, Reinders A, LaFleur KR, Mori T and
Ward JM: Transport activity of rice sucrose transporters OsSUT1 and
OsSUT5. Plant Cell Physiol. 51:114–122. 2010. View Article : Google Scholar :
|
|
21
|
Loqué D, Lalonde S, Looger LL, von Wirén N
and Frommer WB: A cytosolic trans-activation domain essential for
ammonium uptake. Nature. 446:195–198. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Reinders A and Ward JM: Functional
characterization of the alpha-glucoside transporter Sut1p from
Schizosaccharomyces pombe, the first fungal homologue of plant
sucrose transporters. Mol Microbiol. 39:445–454. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Robinson JS, Klionsky DJ, Banta LM and Emr
SD: Protein sorting in Saccharomyces cerevisiae: isolation of
mutants defective in the delivery and processing of multiple
vacuolar hydrolases. Mol Cell Biol. 8:4936–4948. 1988.PubMed/NCBI
|
|
24
|
Aoki N, Hirose T, Scofield GN, Whitfeld PR
and Furbank RT: The sucrose transporter gene family in rice. Plant
Cell Physiol. 44:223–232. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Rooryck C, Morice-Picard F, Elçioglu NH,
Lacombe D, Taieb A and Arveiler B: Molecular diagnosis of
oculocutaneous albinism: new mutations in the OCA1-4 genes and
practical aspects. Pigment Cell Melanoma Res. 21:583–587. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ko JM, Yang JA, Jeong SY and Kim HJ:
Mutation spectrum of the TYR and SLC45A2 genes in patients with
oculocutaneous albinism. Mol Med Rep. 5:943–948. 2012.PubMed/NCBI
|
|
27
|
Graf J, Voisey J, Hughes I and van Daal A:
Promoter polymorphisms in the MATP (SLC45A2) gene are associated
with normal human skin color variation. Hum Mutat. 28:710–717.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Graf J, Hodgson R and van Daal A: Single
nucleotide polymorphisms in the MATP gene are associated with
normal human pigmentation variation. Hum Mutat. 25:278–284. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Witkop CJ: Depigmentations of the general
and oral tissues and their genetic foundations. Ala J Med Sci.
16:330–343. 1979.PubMed/NCBI
|
|
30
|
Grønskov K, Ek J and Brondum-Nielsen K:
Oculocutaneous albinism. Orphanet J Rare Dis. 2:432007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Mariat D, Taourit S and Guérin G: A
mutation in the MATP gene causes the cream coat colour in the
horse. Genet Sel Evol. 35:119–133. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gunnarsson U, Hellström AR,
Tixier-Boichard M, et al: Mutations in SLC45A2 cause plumage color
variation in chicken and Japanese quail. Genetics. 175:867–877.
2007. View Article : Google Scholar :
|
|
33
|
Endler A, Meyer S, Schelbert S, et al:
Identification of a vacuolar sucrose transporter in barley and
Arabidopsis mesophyll cells by a tonoplast proteomic approach.
Plant Physiol. 141:196–207. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Grønskov K, Ek J, Sand A, et al: Birth
prevalence and mutation spectrum in danish patients with autosomal
recessive albinism. Invest Ophthalmol Vis Sci. 50:1058–1064. 2009.
View Article : Google Scholar
|
|
35
|
Wei A, Wang Y, Long Y, et al: A
comprehensive analysis reveals mutational spectra and common
alleles in Chinese patients with oculocutaneous albinism. J Invest
Dermatol. 130:716–724. 2010. View Article : Google Scholar
|
|
36
|
Rundshagen U, Zühlke C, Opitz S, Schwinger
E and Käsmann-Kellner B: Mutations in the MATP gene in five German
patients affected by oculocutaneous albinism type 4. Hum Mutat.
23:106–110. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Inagaki K, Suzuki T, Shimizu H, et al:
Oculocutaneous albinism type 4 is one of the most common types of
albinism in Japan. Am J Hum Genet. 74:466–471. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hutton SM and Spritz RA: Comprehensive
analysis of oculocutaneous albinism among non-Hispanic caucasians
shows that OCA1 is the most prevalent OCA type. J Invest Dermatol.
128:2442–2450. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lezirovitz K, Nicastro FS, Pardono E, et
al: Is autosomal recessive deafness associated with oculocutaneous
albinism a “coincidence syndrome”? J Hum Genet. 51:716–720. 2006.
View Article : Google Scholar
|
|
40
|
Sun Y, Lin Z, Reinders A and Ward JM:
Functionally important amino acids in rice sucrose transporter
OsSUT1. Biochemistry. 51:3284–3291. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wilde S, Timpson A, Kirsanow K, et al:
Direct evidence for positive selection of skin, hair, and eye
pigmentation in Europeans during the last 5,000 y. Proc Natl Acad
Sci USA. 111:4832–4837. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Beleza S, Santos AM, McEvoy B, et al: The
timing of pigmentation lightening in Europeans. Mol Biol Evol.
30:24–35. 2013. View Article : Google Scholar
|
|
43
|
Sun Y and Ward JM: Arg188 in rice sucrose
transporter OsSUT1 is crucial for substrate transport. BMC Biochem.
13:262012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Sengupta M, Chaki M, Arti N and Ray K:
SLC45A2 variations in Indian oculocutaneous albinism patients. Mol
Vis. 13:1406–1411. 2007.PubMed/NCBI
|
|
45
|
Inagaki K, Suzuki T, Ito S, et al:
Oculocutaneous albinism type 4: six novel mutations in the
membrane-associated transporter protein gene and their phenotypes.
Pigment Cell Res. 19:451–453. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Wei AH, Yang XM, Lian S and Li W: Genetic
analyses of Chinese patients with digenic oculocutaneous albinism.
Chin Med J (Engl). 126:226–230. 2013.
|
|
47
|
Omasits U, Ahrens CH, Müller S and
Wollscheid B: Protter: interactive protein feature visualization
and integration with experimental proteomic data. Bioinformatics.
30:884–886. 2014. View Article : Google Scholar
|