Introduction
The utility of bacteria as agents for the treatment
of cancer was described over a century ago and continues to be
investigated for their therapeutic value as delivery agents for
anti-cancer drugs and vectors for gene therapy (1–3).
Additionally, contemporary strategies have also studied the use of
bacterial products, including proteins, enzymes, immunotoxins and
secondary metabolites for their anti-tumor properties (1–3). An
advantage of these alternatives is the lack of systemic infection
associated with the use of live, attenuated and engineered
bacterial strains for cancer therapy (1–3).
In the present study, the secondary metabolite
violacein, a pigment produced by the bacteria Chromobacterium
violaceum, was investigated as an anti-tumor agent, which has
been shown to have medicinal applications as an antibiotic and
anti-trypanosoma agent (4,5). The use of bacterial metabolites such
as violacein as anti-cancer agents is supported by pre-clinical
studies with other microbial products, including
farnesyltransferase inhibitors (6–14),
prodigines (15–17) and epothilones (18–22),
which have displayed positive therapeutic efficacy in several types
of cancer and were subsequently examined in phase I and II clinical
trials. More specifically, violacein has been shown to have
anti-cancer properties in leukemia (23,24)
and colon cancer cells (25–27),
as well as in Ehrlich ascites tumors by Bromberg et al
(28) using an in vivo
mouse model. In contrast to these studies, the present study used
violacein extracted from a Chromobacterium violaceum strain
native to a copper basin in Tennessee, which was demonstrated here
to have anti-proliferative effects on cell lines derived from solid
tumors, as well as displayed an inhibitory effect on cancer cell
migration, extending the anti-tumorigenic properties of this
bacterial-produced metabolite.
Materials and methods
Cell conditions and reagents
U87 (glioblastoma), A549 (lung) and MCF7 (breast)
cancer cells were purchased from the American Type Culture
Collection (Manassas, VA, USA). All cell lines were maintained in
Dulbecco’s Modified Eagle’s Medium (DMEM; Invitrogen Life
Technologies, Carlsbad, CA, USA) containing 10% fetal bovine serum
(Invitrogen Life Technologies), 2 mM L-glutamine (Invitrogen Life
Technologies), 100 nM MEM non-essential amino acids (Invitrogen
Life Technologies) and penicillin-streptomycin (Invitrogen Life
Technologies) at 37°C and 5% CO2.
Isolation and characterization of
violacein
Chromobacterium violaceum was collected from
environmental soil and water samples at the Tennessee Copper Mining
Company site (Ducktown, TN, USA), also termed the Copper Basin.
Chromobacterium violaceum were inoculated in Luria-Bertani
broth (LB) media, streaked and grown on agar plates for 48 h at
30°C, until colonies formed. The colonies were subsequently
selected and grown as cultures in LB broth media for 24–48 h at
30°C. The cultures were used to extract and purify violacein, as
described previously (29).
Cultures were centrifuged at 14,629 × g in 100% ethanol for 15 min
at 4°C and the supernatant was collected, and violacein was
separated and extracted using chloroform. The violacein was allowed
to dry for 24 h and dissolved in 50% ethanol (Fisher Scientific,
Fair Lawn, NJ, USA). Violacein was subsequently purified with
reverse phase column chromatography and characterized by liquid
chromatography-mass spectrometry and ultraviolet-visible
spectroscopy.
Crystal violet cell proliferation
assay
Cells were plated in 24-well plates, treated with
250 nM, 500 nM and 1 μM violacein and allowed to incubate
for 48 h ([vehicle controls were treated with dimethyl sulfoxide
(DMSO; Amresco, LLC, Solon, OH, USA)] for dose-response
experiments. For time-course experiments, cells were treated with 1
μM violacein and allowed to incubate for 1, 3 and 5 days.
Subsequently, tissue culture medium was removed, the cell monolayer
was fixed with 100% methanol for 5 min and stained with 0.5%
crystal violet in 25% methanol for 10 min. Cells were then washed
three times for 5 min each with distilled water to remove excess
dye and allowed to dry overnight at room temperature. The
incorporated dye was then solubilized in 0.1 M sodium citrate
(Sigma-Aldrich; St. Louis, MO, USA) in 50% ethanol. Next, 100
μl treated and control samples were transferred to 96-well
plates and optical densities read at 540 nm using an X-mark
microplate absorbance spectrophotometer (Bio-Rad, Hercules, CA,
USA).
Cell motility
Motility assays were conducted according to the
manufacturer’s instructions (Cell Biolabs Inc., San Diego, CA,
USA). A cell suspension containing 0.5–1.0×106 cells/ml
was prepared in serum-free media with vehicle (DMSO) or 1 μM
violacein, while 500 μl of media containing 10% fetal bovine
serum was added to the lower chamber of the migration plate. 300
μl of the cell suspension containing vehicle or 1 μM
violacein was then added to the inside of each insert and allowed
to incubate for 24 hours at 37°C and 5% CO2.
Subsequently, non-migratory cells were removed from the plate
inserts (per manufacturer’s instructions), migratory cells were
counterstained, solubilized, and optical density densities read at
595 nm using a X-mark microplate absorbance spectrophotometer
(Bio-Rad Laboratories).
Western blot analysis
Cells were plated and treated with 1 μM
violacein for 24 h or vehicle (DMSO), rinsed with
phosphate-buffered saline (Bio-Rad Laboratories, Hercules, CA,
USA), and lysed with CelLytic M Cell lysis reagent (Sigma-Aldrich,
St Louis, MO, USA). Protein concentrations were subsequently
determined using the Bradford reagent (cat. no. B6916;
Sigma-Aldrich). Proteins were separated by 8% SDS-PAGE (Bio-Rad
Laboratories) and then transferred to nitrocellulose membranes. For
immunoblotting, nitrocellulose membranes were incubated with
phosphorylated (p) Akt (rabbit monoclonal; cat. no. 4606), cleaved
poly(ADP ribose) polymerase (PARP; rabbit polyclonal; cat. no.
9544), p44/42 (rabbit monoclonal; cat. no. 4370), and pS6-ribosomal
protein (rabbit monoclonal; cat. no. 4858) and β-actin (rabbit
polyclonal; cat. no. 4967), purchased from Cell Signaling
Technology (Danvers, MA, USA) and all diluted 1:25, recognizing
target proteins overnight at 4°C. The membranes were then incubated
with a horseradish peroxidase-conjugated goat anti-rabbit secondary
antibody (1:100; cat. no. 7074; Cell Signaling Technology) for 3 h
at room temperature, analyzed using an enhanced chemiluminescence
detection system with SuperSignal West Pico Chemilluminescent
Substrate (cat. no. 34080; Thermo Fisher Scientific, Waltham, MA,
USA) and visualized by autoradiography using a BioSpectrum UVP
Imaging System (BioSpectrum, Upland, CA, USA). Actin was used as
loading control.
Statistical analysis
Values are expressed as the mean ± standard error.
Significance was determined using Student’s t-test. P<0.05 was
considered to indicate a statistically significant difference
between values.
Results
Violacein antagonizes brain, lung and
breast cancer cell proliferation
Lung and breast cancer are two of the most common
types of cancer found in men and women, and have high incidence of
metastasis to the brain (30). The
present study therefore evaluated the effects of violacein on three
solid tumor cell lines, U87 (brain), A549 (lung) and MCF7 (breast).
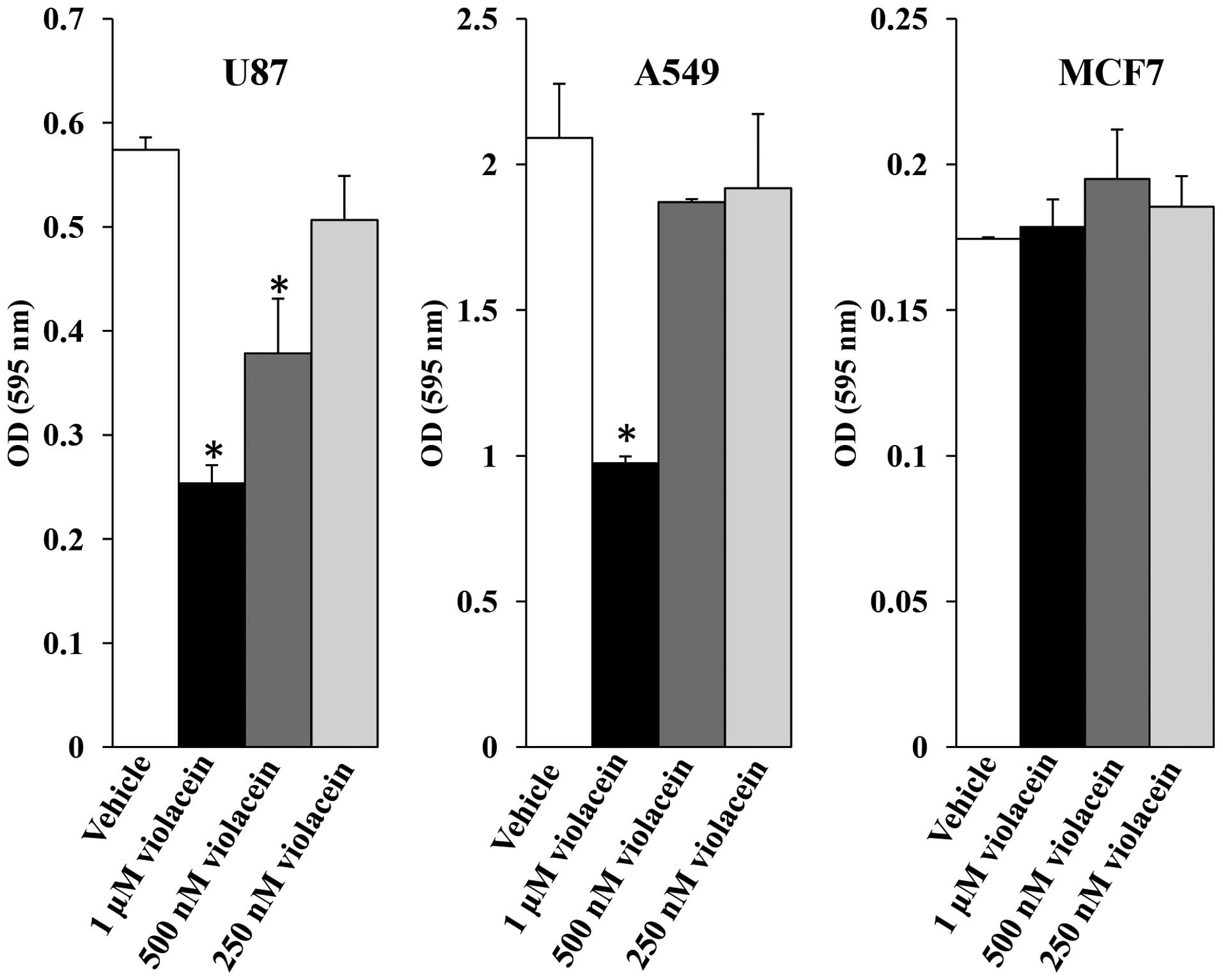
Dose-response experiments were performed at the outset to assess
the effects of several violacein concentrations (250 nM, 500 nM and
1 μM) on cell viability. Cell viability dose-response assays
revealed a 56-, 34- and 12%-decrease in U87 cells treated with 1
μM, 500 nM and 250 nM violacein as compared to the viability
of vehicle treated control cells, respectively, while A549 cells
displayed a 54, 11 and 8%-decrease in viability when treated with
identical concentrations compared to that of vehicle-treated
control cells (Fig. 1). By
contrast, MCF7 cell viability did not decrease in response to
violacein exposure using this assay.
The anti-proliferative effects of violacein have
been attributed to its ability to elicit a cytotoxic response in
several human cancers (23,27,31–33),
as well as induce differentiation, a cytostatic response observed
in leukemia cells by Melo et al (26). To gain better insight into the
effect of violacein on cell proliferation in brain, lung and breast
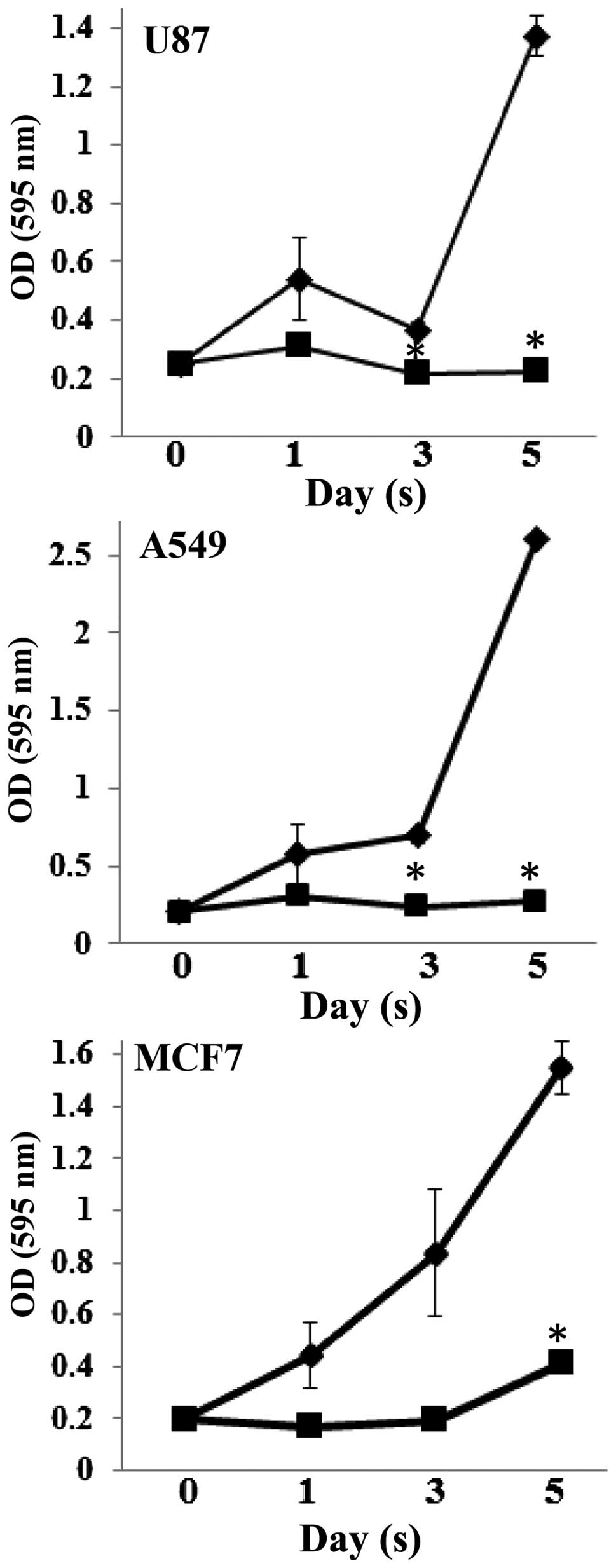
cancer cells, time course experiments were preformed in the present
study. Examination of U87, A549 and MCF7 cells treated with 1
μM violacein revealed a reduction in cell proliferation of
all three cell lines over a five-day period, with a statistically
significant difference (P<0.05) in cell viability observed on
day five between vehicle-treated control cells and
violacein-treated cells (Fig. 2).
Time course analysis also revealed that U87 and A549 cells were
more sensitive to violacein treatment as compared to MCF7 cells,
which displayed a two-fold increase in cell viability five days
post-violacein treatment as compared to cell viability on day 0
(Fig. 2). These data were
consistent with dose response experiments that also showed that U87
and A549 cells were more sensitive to violacein exposure than MCF7
cells. The findings of the present study parallel a study by
Menezes et al (34), which
showed that crude extracts of violacein were differentially toxic
to several human tumor cell lines, including the
multi-drug-resistant ovarian tumor cell line NCI/ADR-RES.
Violacein promotes PARP and p44/42
mitogen-activated protein kinase (MAPK) signaling
The mechanistic effects of violacein on cellular
responses have proven to be varied and tumor type-specific. Of
note, violacein has been shown to upregulate p53, p27 and p21,
which are negative regulators of cell cycle progression (24), and to induce reactive oxygen
species-mediated apoptotic cell death in colon cancer cells
(23). Additionally, Ferreira
et al (27) demonstrated
that violacein promoted leukemia cell death via tumor necrosis
factor signaling. To address the mechanisms of the
anti-proliferative response of violacein on solid tumor cells
described above, several intracellular signaling proteins were
analyzed for changes in their expression when exposed to various
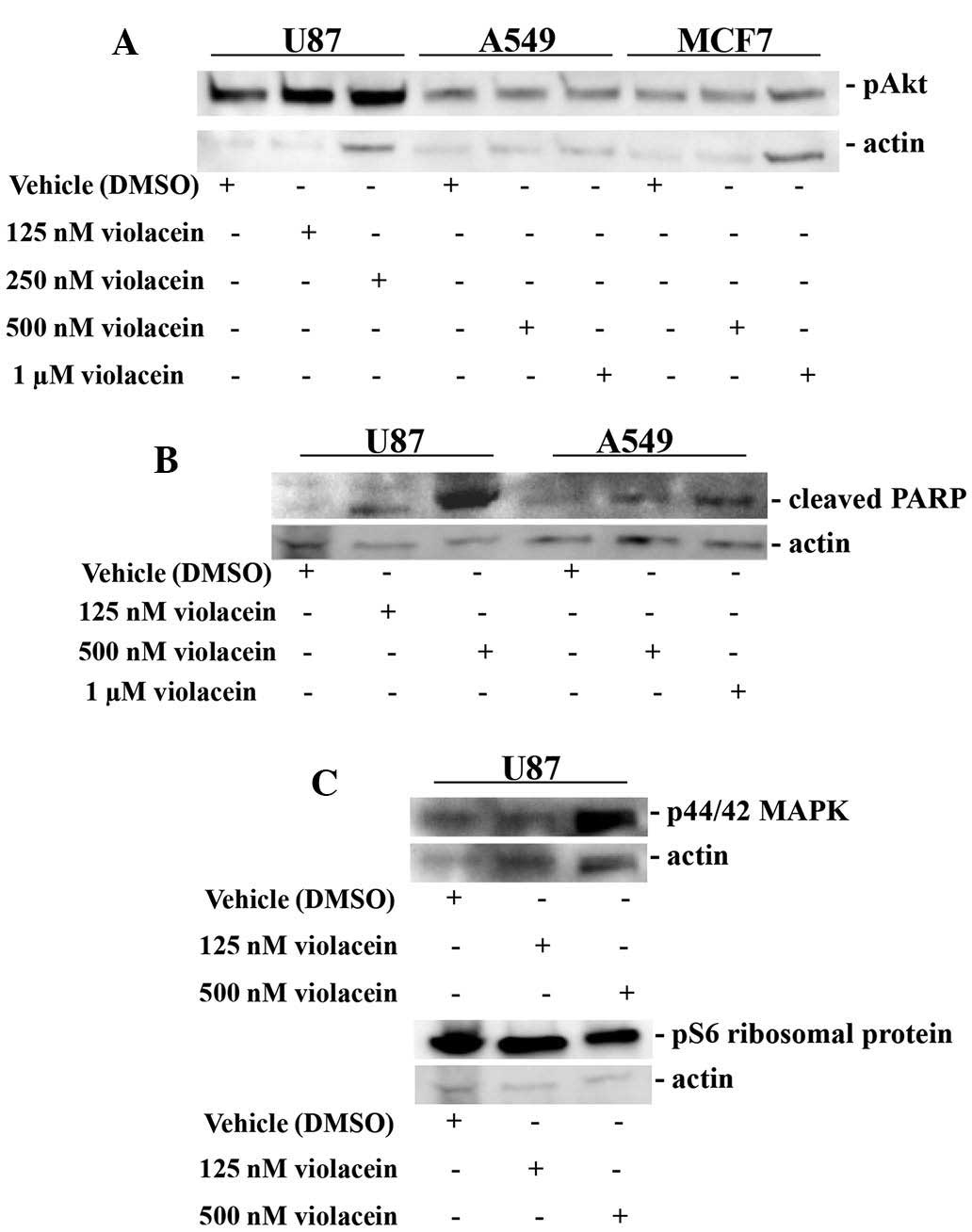
concentrations of this agent. The survival and pro-apoptotic
proteins, Akt and PARP, were examined in U87, A549 and MCF7 cells
exposed to violacein. A substantial induction of cleaved PARP was
observed in U87 brain tumor cells treated with 500 nM violacein, as
well as in A549 lung cancer cells treated with 1 μM
violacein (Fig. 3). By contrast,
no changes in the activity of Akt expression were observed in cells
exposed to violacein (Fig. 3).
Additional protein expression analysis revealed that 500 nM
violacein upregulated p-44/42 MAPK in U87 cells. This result was
similar to that observed in leukemia cells, which displayed
increased p38 MAPK expression in response to violacein (27). Furthermore, violacein did not
affect the expression levels of pS6 ribosomal protein (Fig. 3), providing additional evidence
along with the lack of expressional changes of pAkt protein
described above, that this secondary metabolite does not
mechanistically influence the translational control signaling
network.
Impairment of tumor cell migration in
response to violacein
The effect of violacein on cellular biological
processes that underlie metastatic invasion has not been
sufficiently investigated to date, to the best of our knowledge. In
the present study, the ability of violacein to inhibit cellular
processes that contribute to the metastatic invasion of cancers,
which leads to therapeutic resistance and recurrence of this
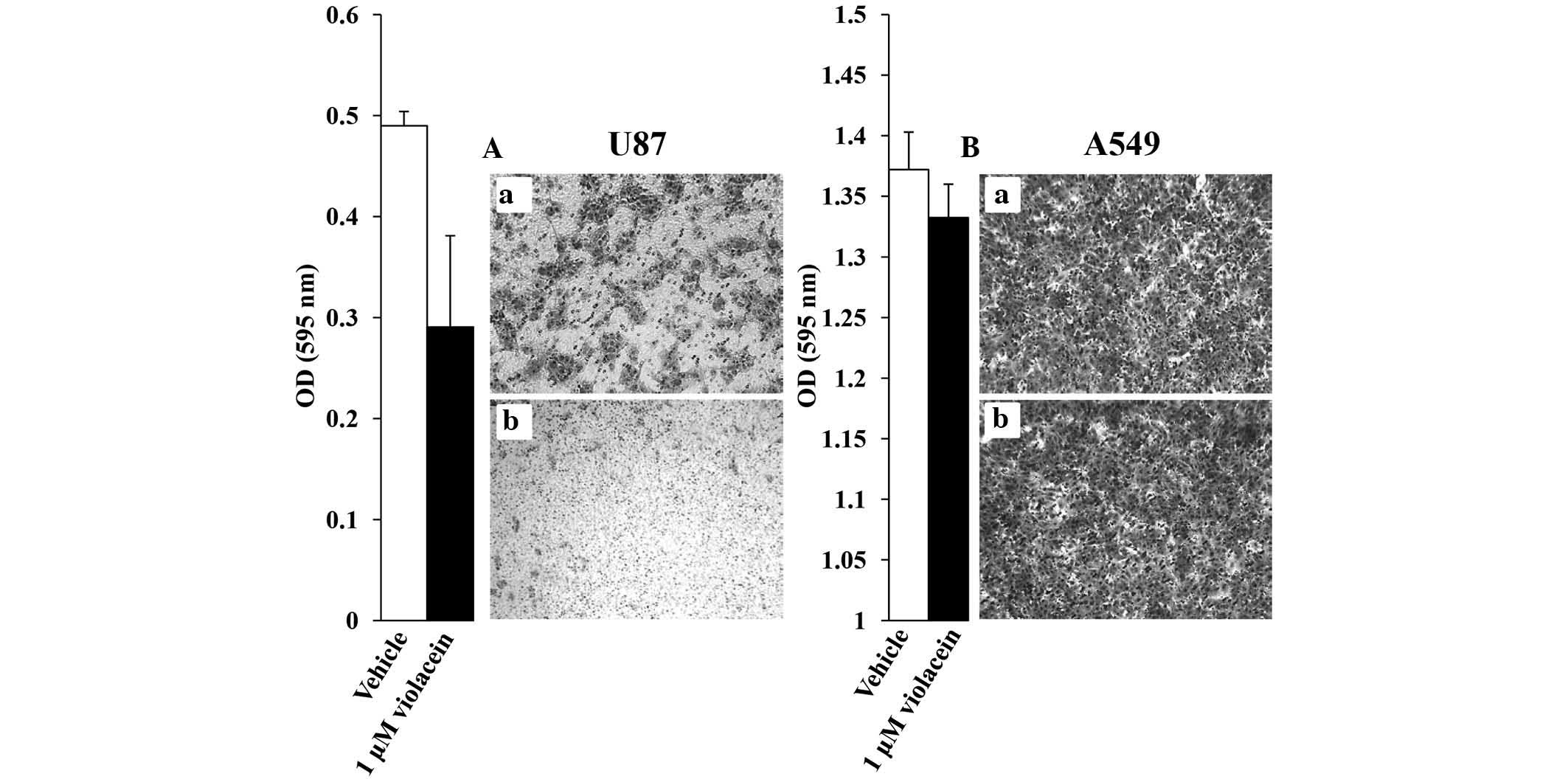
disease, were therefore evaluated. Boyden chamber assays revealed
that 1 μM violacein decreased the migration of U87 cells by
40% as compared to that of vehicle-treated control cells, while a
diminutive decrease was observed in A549 violacein-treated cells
(Fig. 4). The anti-migratory
response, particularly of U87 cells, may be attributed in part to
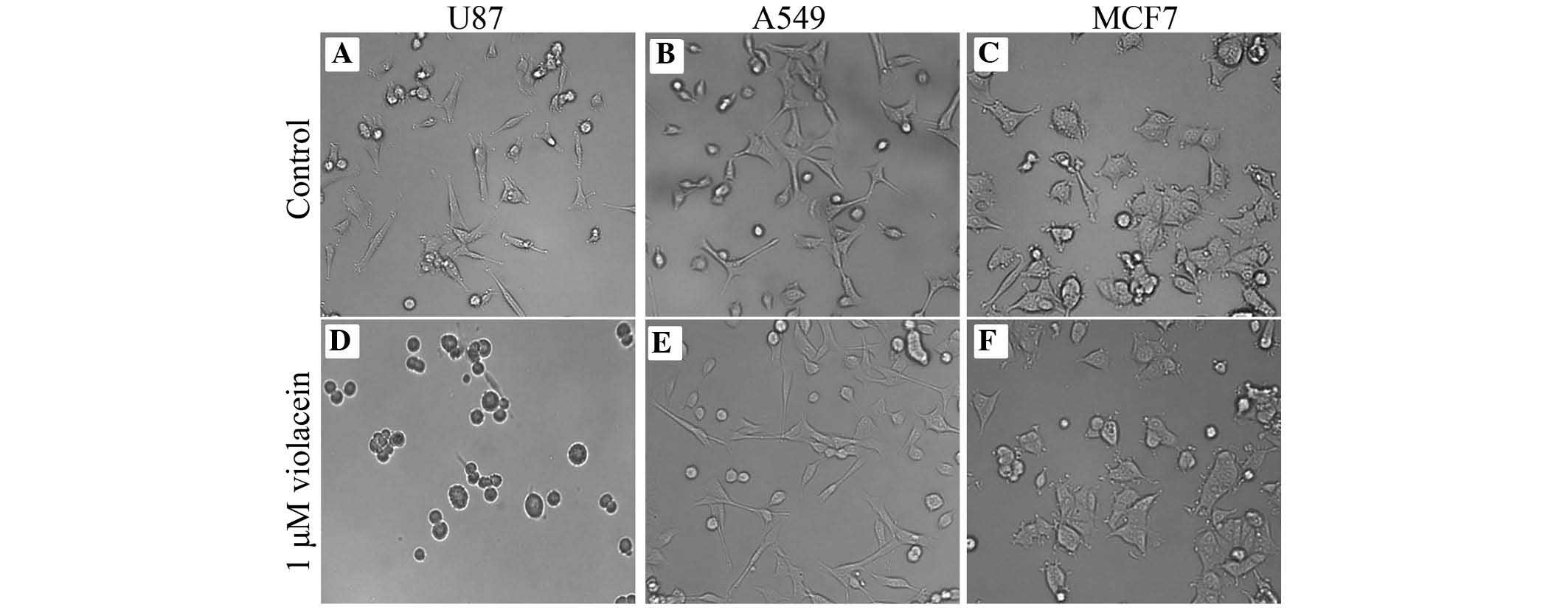
violacein-induced morphological changes (Fig. 5). U87 cells exposed to 500 nM
violacein appeared to detach from the cell substratum and exhibited
a round cellular phenotype three hours post-exposure, which was not
observed in A549 and MCF7 cells after treatment with violacein
(Fig. 5). However, cell adhesion
assays performed revealed that violacein did not affect the
adhesive properties of U87 brain tumor cells (data not shown). It
should also be mentioned that U87 cells re-attached and displayed
normal cell morphology when examined 24 h after violacein exposure,
indicating the violacein-induced morphological changes were not a
consequence of cells undergoing cell death. In conclusion, these
results suggested that violacein-induced morphological changes had
an impact on the migratory ability of U87 brain tumor cells.
Discussion
The efficacy of therapeutic agents used to treat
human cancers is dependent upon their ability to antagonize
molecular signaling events that contribute to the survival of tumor
cells and induce regulatory mechanisms that promote their death.
The present study investigated a novel secondary metabolite,
violacein, produced by a chromobacterium, for its utility as an
agent that can promote tumor cell death in solid tumor-derived cell
lines. It was shown that violacein considerably reduced the
proliferative capacity of lung and brain cancer cells, and to a
much lesser extent that of breast cancer cells, providing an
indication that cancers are differentially sensitive to this agent.
This difference in sensitivity and responsiveness was also
manifested mechanistically as demonstrated in the ability of
violacein to promote apoptotic cell death by upregulating cleaved
PARP, a downstream target of the effector pro-apoptotic molecule
caspase 3, in brain and lung cancer cells. Additionally, p44/42, a
known apoptosis-promoting regulator and caspase 3 activator, was
increased in brain tumor cells treated with violacein. These
results established that violacein-induced apoptosis observed in
the present study likely occurs via the intrinsic pathway,
particularly in brain tumor cells, which is consistent with studies
on leukemia, fibrosarcoma and colon cancer cells that have
demonstrated the apoptosis-promoting properties of violacein by
intrinsic as well as extrinsic pathways (23,27,35).
It should also be noted that violacein has been described to elicit
other types of cell death, as shown in resistant leukemia cells
that underwent cell death as a consequence of endoplasmic reticulum
stress and breakdown of the golgi apparatus, underscoring the tumor
cell killing capacity of violacein via different cell death
mechanisms (25).
Although several studies, including the present
study, have provided evidence of violacein’s ability to promote
tumor cell death, its role as an agent that can prevent the
metastatic invasion of cancer cells has received little attention.
The present study showed that violacein inhibited brain tumor cell
migration, likely as a consequence of disrupting sub-cellular
domain structures of the actin filamentous network, including the
lamellipodia and filopodia, that led to a round cellular phenotype
that compromised the motility of these cells. To the best of our
knowledge, the present study was the first to show that violacein
inhibits cancer cell migration, extending the anti-malignant
properties of this agent. Additionally, the anti-migratory effects
of violacein on cancer cells demonstrated in the present study are
further supported by a recent study by Platt et al (36), which showed that violacein
inhibited the secretion of the pro-cell migratory inflammatory
chemokine CXCL12 in breast cancer cells. These results suggested
that violacein may have therapeutic applications that prevent brain
tumor cells from invading normal brain tissue, as well as inhibit
brain metastases from breast cancer, the most common source of
metastatic brain tumors in women (30).
In conclusion, violacein has potential as a
therapeutic agent to treat cancer due to its versatility to cause
cell death in several types of cancer and prohibit metastatic
invasion. The potential of violacein to be used as a cancer drug is
further supported by the recent U.S. Federal Drug Administration’s
approval of romidepsin, a histone deactylase inhibitor produced by
Chromobacterium violaceum, for the treatment of T-cell
lymphoma (37), as well as a
renewed interest in cancer therapies utilizing bacteria and
bacterial products (1–3) to selectively target cancer cells.
Acknowledgments
The present study was supported in part by a
National Institutes of Health-National Cancer Institute U54 grant
(5U54CA163066).
References
|
1
|
Forbes NS: Engineering the perfect
(bacterial) cancer therapy. Nat Rev Cancer. 10:785–794. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Patyar S, Joshi R, Byrav DS, Prakash A,
Medhi B and Das BK: Bacteria in cancer therapy: A novel
experimental strategy. J Biomed Sci. 17:212010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bernardes N, Seruca R, Chakrabarty AM and
Fialho AM: Microbial-based therapy of cancer: Current progress and
future prospects. Bioeng Bugs. 1:178–190. 2010. View Article : Google Scholar
|
|
4
|
Andrighetti-Fröhner CR, Antonio RV,
Creczynski-Pasa TB, Barardi CR and Simões CM: Cytotoxicity and
potential antiviral evaluation of violacein produced by
Chromobacterium violaceum. Mem Inst Oswaldo Cruz. 98:843–848. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Vaishnav P and Demain AL: Unexpected
applications of secondary metabolites. Biotechnol Adv. 29:223–229.
2011. View Article : Google Scholar
|
|
6
|
Ito T, Kawata S, Tamura S, Igura T, Nagase
T, Miyagawa JI, Yamazaki E, Ishiguro H and Matasuzawa Y:
Suppression of human pancreatic cancer growth in BALB/c nude mice
by manumycin, a farnesyl:protein transferase inhibitor. Jpn J
Cancer Res. 87:113–116. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kainuma O, Asano T, Hasegawa M, Kenmochi
T, Nakagohri T, Tokoro Y and Isono K: Inhibition of growth and
invasive activity of human pancreatic cancer cells by a
farnesyltransferase inhibitor, manumycin. Pancreas. 15:379–383.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yeung SC, Xu G, Pan J, Christgen M and
Bamiagis A: Manumycin enhances the cytotoxic effect of paclitaxel
on anaplastic thyroid carcinoma cells. Cancer Res. 60:650–656.
2000.PubMed/NCBI
|
|
9
|
She M, Pan I, Sun L and Yeung SC:
Enhancement of manumycin A-induced apoptosis by methoxyamine in
myeloid leukemia cells. Leukemia. 19:595–602. 2005.PubMed/NCBI
|
|
10
|
Frassanito MA, Cusmai A, Piccoli C and
Dammacco F: Manumycin inhibits farnesyltransferase and induces
apoptosis of drug-resistant interleukin 6-producing myeloma cells.
Br J Haematol. 118:157–165. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhou JM, Zhu XF, Pan QC, Liao DF, Li ZM
and Liu ZC: Manumycin inhibits cell proliferation and the Ras
signal transduction pathway in human hepatocellular carcinoma
cells. Int J Mol Med. 11:767–771. 2003.PubMed/NCBI
|
|
12
|
Zhou JM, Zhu XF, Pan QC, Liao DF, Li ZM
and Liu ZC: Manumycin induces apoptosis in human hepatocellular
carcinoma HepG2 cells. Int J Mol Med. 12:955–959. 2003.PubMed/NCBI
|
|
13
|
Pan J, Xu G and Yeung SC: Cytochrome c
release is upstream to activation of caspase-9, caspase-8, and
caspase-3 in the enhanced apoptosis of anaplastic thyroid cancer
cells induced by manumycin and paclitaxel. J Clin Endocrinol Metab.
86:4731–4740. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ishii T, Hayashi K, Hida T, Yamamoto Y and
Nozaki Y: TAN-1813, a novel Ras-farnesyltransferase inhibitor
produced by Phoma sp. taxonomy, fermentation, isolation and
biological activities in vitro and in vivo. J Antibiot (Tokyo).
53:765–778. 2000. View Article : Google Scholar
|
|
15
|
Nguyen M, Marcellus RC, Roulston A, Watson
M, Serfass L, Murthy Madiraju SR, Goulet D, Viallet J, Bélec L,
Billot X, et al: Small molecule obatoclax (GX15-070) antagonizes
MCL-1 and overcomes MCL-1-mediated resistance to apoptosis. Proc
Natl Acad Sci USA. 104:19512–19517. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pérez-Galán P, Roué G, Villamor N, Campo E
and Colomer D: The BH3-mimetic GX15-070 synergizes with bortezomib
in mantle cell lymphoma by enhancing Noxa-mediated activation of
Bak. Blood. 109:4441–4449. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Trudel S, Li ZH, Rauw J, Tiedemann RE, Wen
XY and Stewart AK: Preclinical studies of the pan-Bcl inhibitor
obatoclax (GX015-070) in multiple myeloma. Blood. 109:5430–5438.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fumoleau P, Coudert B, Isambert N and
Ferrant E: Novel tubulin-targeting agents: Anticancer activity and
pharmacologic profile of epothilones and related analogues. Ann
Oncol. 18(Suppl 5): v9–v15. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nettles JH, Li H, Cornett B, Krahn JM,
Snyder JP and Downing KH: The binding mode of epothilone A on
alpha, beta-tubulin by electron crystallography. Science.
305:866–869. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cheng KL, Bradley T and Budman DR: Novel
microtubule-targeting agents - the epothilones. Biologics.
2:789–811. 2008.
|
|
21
|
Bollag DM, McQueney PA, Zhu J, Hensens O,
Koupal L, Liesch J, Goetz M, Lazarides E and Woods CM: Epothilones,
a new class of microtubule-stabilizing agents with a taxol-like
mechanism of action. Cancer Res. 55:2325–2333. 1995.PubMed/NCBI
|
|
22
|
Lee FY, Borzilleri R, Fairchild CR, Kim
SH, Long BH, Reventos-Suarez C, Vite GD, Rose WC and Kramer RA:
BMS-247550: A novel epothilone analog with a mode of action similar
to paclitaxel but possessing superior antitumor efficacy. Clin
Cancer Res. 7:1429–1437. 2001.PubMed/NCBI
|
|
23
|
de Carvalho DD, Costa FT, Duran N and Haun
M: Cytotoxic activity of violacein in human colon cancer cells.
Toxicol In Vitro. 20:1514–1521. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kodach LL, Bos CL, Durán N, Peppelenbosch
MP, Ferreira CV and Hardwick JC: Violacein synergistically
increases 5-fluoro-uracil cytotoxicity, induces apoptosis and
inhibits Akt-mediated signal transduction in human colorectal
cancer cells. Carcinogenesis. 27:508–516. 2006. View Article : Google Scholar
|
|
25
|
Queiroz KC, Milani R, Ruela-de-Sousa RR,
Fuhler GM, Justo GZ, Zambuzzi WF, Duran N, Diks SH, Spek CA,
Ferreira CV, et al: Violacein induces death of resistant leukaemia
cells via kinome reprogramming, endoplasmic reticulum stress and
Golgi apparatus collapse. PLoS One. 7:e453622012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Melo PS, Justo GZ, de Azevedo MB, Durán N
and Haun M: Violacein and its beta-cyclodextrin complexes induce
apoptosis and differentiation in HL60 cells. Toxicology.
186:217–225. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ferreira CV, Bos CL, Versteeg HH, Justo
GZ, Durán N and Peppelenbosch MP: Molecular mechanism of
violacein-mediated human leukemia cell death. Blood. 104:1459–1464.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bromberg N, Dreyfuss JL, Regatieri CV,
Palladino MV, Durán N, Nader HB, Haun M and Justo GZ: Growth
inhibition and pro-apoptotic activity of violacein in Ehrlich
ascites tumor. Chem Biol Interact. 186:43–52. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Rettori D and Durán N: Production,
extraction and purification of violacein: an antibiotic pigment
produced by Chromobacterium violaceum. World Journal of
Microbiology and Biotechnology. 4:685–688. 1998. View Article : Google Scholar
|
|
30
|
Stelzer KJ: Epidemiology and prognosis of
brain metastases. Surg Neurol Int. 4(Suppl 4): S192–S202.
2013.PubMed/NCBI
|
|
31
|
Melo PS, De Azevedo MM, Frungillo L,
Anazetti MC, Marcato PD and Duran N: Nanocytotoxicity: Violacein
and violacein-loaded poly (D,L-lactide-co-glycolide) nanoparticles
acting on human leukemic cells. J Biomed Nanotechnol. 5:192–201.
2009. View Article : Google Scholar
|
|
32
|
Melo PS, Maria SS, Vidal BC, Haun M and
Durán N: Violacein cytotoxicity and induction of apoptosis in V79
cells. In Vitro Cell Dev Biol Anim. 36:539–543. 2000. View Article : Google Scholar
|
|
33
|
Saraiva VS, Marshall JC, Cools-Lartigue J
and Burnier MN Jr: Cytotoxic effects of violacein in human uveal
melanoma cell lines. Melanoma Res. 14:421–424. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Menezes CB, Silva BP, Sousa IM, Ruiz AL,
Spindola HM, Cabral E, Eberlin MN, Tinti SV, Carvalho JE, Foglio
MA, et al: In vitro and in vivo antitumor activity of crude
extracts obtained from Brazilian Chromobacterium sp isolates. Braz
J Med Biol Res. 46:65–70. 2013.
|
|
35
|
Mojib N, Nasti TH, Andersen DT, Attigada
VR, Hoover RB, Yusuf N and Bej AK: The antiproliferative function
of violacein-like purple violet pigment (PVP) from an Antarctic
Janthinobacterium sp. Ant5-2 in UV-induced 2237 fibrosarcoma. Int J
Dermatol. 50:1223–1233. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Platt D, Amara S, Mehta T, Vercuyssee K,
Myles EL, Johnson T and Tiriveedhi V: Violacein inhibits matrix
metalloproteinase mediated CXCR4 expression: Potential anti-tumor
effect in cancer invasion and metastasis. Biochem Biophys Res
Commun. 455:107–112. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
VanderMolen KM, McCulloch W, Pearce CJ and
Oberlies NH: Romidepsin (Istodax, NSC 630176, FR901228, FK228,
depsipeptide): A natural product recently approved for cutaneous
T-cell lymphoma. J Antibiot (Tokyo). 64:525–531. 2011. View Article : Google Scholar
|