Introduction
Multiple myeloma (MM) is a plasma cell disorder,
characterized by anemia, renal disease, lytic bone disease, and
immune dysfunction. MM is one of the most common types of
hematological malignancy in China (1). Although currently approved treatments
for newly diagnosed MM, including high-dose chemotherapy followed
by autologous transplantation, and novel drugs, including
proteasome inhibitors and immunomodulatory agents (IMiDs), have led
to increased survival rates, the majority of patients will
eventually relapse and become refractory to treatment (2). Therefore, patients with relapsed or
refractory MM have an unmet requirement for safe and efficacious
novel therapies.
Arsenic trioxide (ATO) has been suggested as an
option for the treatment of relapsing or refractory MM. In
vitro, ATO has been found to induced myeloma cell apoptosis,
and monotherapy with ATO results in partial response rates between
0 and 17%, and minimal responses between 7 and 33%, resulting in
mean overall response rates of 30% for treatment of myeloma
(3–5).
In order to improve the treatment response rates in
patients with MM, ATO has been combined with other novel drugs,
including proteasome inhibitors, IMiDs and dexamethsone, for the
treatment of MM. The overall response rates in these combined
regimens vary widely between 12 and 100% (6–8). It
is necessary to identify novel combinations of ATO with other
drugs, to offer novel mechanisms for treating MM.
The observation that histone deacetylases (HDAC) may
be involved in various types of hematologic malignancy has led to
the development of HDAC inhibitors as potential antitumor agents
(9). VPA, as one type of HDACI,
has the unique advantage of oral dosage, and can achieve its
effective concentration with low toxicity, providing a useful tool
for investigating the mechanism of the HDAC inhibitor. The present
study aimed to investigate the synergistic effects of VPA and ATO
and its underlying mechanism.
Materials and methods
Cells and reagents
The RPMI8226 myeloma cell line was obtained from the
Shanghai Cell Bank of the Chinese Academy of Sciences(Shanghai,
China). The cells were cultured in RPMI-1640 medium, supplemented
with 10% heat-inactivated fetal bovine serum (FBS; HyClone,
Beijing, China), 100 U/ml penicillin, 100 mg/ml streptomycin and 2
mmol/l glutamine (North China Pharmaceutical Co., Ltd.,
Shijiazhuang, China) at 37°C in humidified air containing 5%
CO2. The culture medium was replaced every 3 days. ATO
(Harbin Yida Pharmaceutical Co, Ltd., Harbin, China) was stored at
room temperature, VPA powder (Dalian Meilun Biological Technology
Co, Ltd., Dalian, China) was dissolved in 0.9% NaCl to produce a 60
mM stock solution. The VPA solution was diluted in 0.9% NaCl just
prior to use. All experiments were performed using cells in the
logarithmic phase.
The rabbit anti-human monoclonal Bcl-2, Bax, Caspase
8, Caspase 9, LSD1 and JMJD2B antibodies, and rabbit anti-human
polyclonal acetylated-H3 and H3K9me2 antibodies were purchased from
Cell Signaling Technology Inc. (Danvers, MA, USA), and mouse
anti-human monoclonal β-actin antobidy was purchased from Beijing
Sinopept Biotechnology, Co., Ltd. (Beijing, China). The chromatin
immunoprecipitation (CHIP) assay kit was purchased from EMD
Millipore (Billerica, MA, USA).
Cell viability assays
To evaluate the growth inhibitory effect of VPA and
ATO on the myeloma cells, a Cell Counting kit (CCK)-8 colorimetric
assay was used, according to the manufacturer’s instructions.
Briefly, the RPMI8226 cells were inoculated into each well of
96-well culture plates, at a density of 1×106cells/l
with 100 μl for each well, in the presence of VPA (1 or 5
mmol/l) or ATO (4 μmmol/l) or the two drugs in combination,
at the same concentrations, for 24, 48 and 72 h at 37°C in a 5%
CO2 incubator. CCK-8 solution (10 μl) was added
to each well of the plate during the last 2 h of incubation. This
was followed by measurement of absorbance at a wavelength of 490 nm
using an absorbance microplate reader (ELx808, Bio-Tek, Winooski,
VT, USA). Interactions between the two drugs were determined using
the gold (Zheng Jun) formula (10). Q = E (a+b)/(Ea+Eb-Ea x Eb), where
Ea was the cell inhibition rate of RPMI8226 cells treated with ATO,
Eb was the cell inhibition rate of RPMI8226 cells treated with VPA,
and E (a+b) was the cell inhibition rate of RPMI8226 cells treated
with ATO combined with VPA. When Q<0.85, the combination of the
two drugs had an antagonistic effect; when Q was between 0.85 and
1.15, the combination of the two drugs had a simple additive
effect; and when Q>1.15, the combination of the two drugs had a
synergistic effect.
Observation of cell morphology
The RPMI8226 cells were cultured in 25 ml culture
flasks at a concentration of 5×104/l (5 ml in each
flask). The subgroups and treatment methods used were the same as
those used for the CCK-8 assay. The cells were harvested after 2
days and were observed using inverted microscopy (CX31; Olympus
Corporation, Tokyo, Japan).
Staining with annexin V-fluorescein
isothiocya-nate/propidium iodide (PI) and detection of
apoptosis
Apoptosis was determined by staining the cells with
annexin V-FITC/PI (Jiamay Biotechnology Co., Ltd., Beijing, China),
according to the manufacturer’s instructions. The stained cells
were then analyzed by flow cytometry (FACSCanto; BD Biosciences,
Franklin Lakes, NJ, USA). The rates of apoptosis were quantified
using FlowJo software (version 7.6; Tree star, Inc., Ashland, OR,
USA).
Semi-quantitative PCR analysis
The total RNA were extracted with RNA fast200
(Fastagen Biotechnology Co., Ltd., Shanghai, China). The RNA
content and purity were measured using a DU-600 spectrophotometer
(Beckman Coulter, Fullerton, CA, USA). The required A260/A280 ratio
was 1.8–2.0. The RNA (1 μg) was reverse transcribed to cDNA
using oligo (dT) 18 primers and M-MlV reverse transcriptase (Thermo
Fisher Scientific, Pittsburgh, PA, USA). The qPCR analyses for the
mRNA transcripts were performed using the following primers: Bcl-2,
forward 5′-GAACTGGGGGAGGATTGTGG-3′ and reverse
5′-CCGGTTCAGGTACTCAGTCA-3; Bax, forward
5′-AAGCTGAGCGAGTGTCTCCGGCG-3′ and reverse
5′-GCCACAAAGATGGTCACTGTCTGCC-3′; Caspase 8, forward
5′-CTGGGAAGGATCGACGATATA-3′ and reverse 5′-CATGTCCTGCATTTTGATGG-3′;
Caspase 9, forward 5′-AGCCAGATGCTGTCCCATAC-3′ and reverse
5′-CAGGAGACAAAACCTGGGAA-3′; LSD1, forward 5′-GCCAGGCATTGGAAGTTGT-3′
and reverse 5′-TGACCGCCCTATGCAAGG-3′; and HDAC1, forward
5′-GCTCCATCCGTCCAGATAACA-3′ and reverse 5′-TGCC ACAGAACCACCAGTAGA
(Dingguo Changsheng Co., Ltd., Beijing, China). The qPCR was
performed using the following cycles: 35 cycles at 94°C 45 sec,
50°C for 45 sec, and 72°C for 60 sec. The qPCR products of Bcl-2,
Bax, Caspase 8, Caspase 9, LSD1 and HDAC1 (124, 284, 117, 201, 234
and 128 bp, respectively) were verified by 1.2% agarose gel
electrophoresis stained by ethidium bromide (GE Healthcare Life
Sciences, Piscataway, NJ, USA). The mRNA expression of β-actin was
used as control.
Western blotting
The total protein was isolated from the cell pellet
using radioimmunoprecipitation buffer (Beyotime Institute of
Biotechnology, Shanghai, China), and quantified using a
Bicinchoninic Acid kit (Beyotime Biotechnology, Shanghai, China). A
total of 35 μg total protein from the cell lysate was then
separated on 12% SDS-polyacrylamide gels and transferred onto
cellulose acetate membranes (Beyotime Biotechnology) at a constant
current of 320 mA for 1 h. The membranes were blocked with 5%
bovine serum albumin (HyClone) in Tris-buffered saline with 0.5
ml/l Tween-20 (TBS-T) and probed overnight at 4°C with 1:1,000
dilutions of Bcl-2, Bax, Caspase 8, Caspase 9, LSD1, acetylated H3
and H3K9me2 primary antibodies. The membranes were then washed five
times with TBS-T and incubated with a 1:3,000 dilution of
anti-rabbit horesreadish peroxidase-conjugated secondary antibody
for 2 h at room temperature. The membranes were washed again five
times with TBS-T, and the proteins were visualized using enhanced
chemuluminescence (EMD Millipore). β-actin was used as an internal
control.
Chromatin immunoprecipitation
The level of histone methylation of the Bcl-2 gene
promoter and the acetylation level of the Bax gene promoter were
determined using a CHIP assay (EMD Millipore), according to the
manufacturer’s instructions. The RPMI8226 cells were inoculated
into each well of 6-well culture plates, at a concentration of
1×106cells/l, and treated with VPA or ATO alone or with
the two in combination, for 48 h at 37°C in a 5% CO2
incubator. The histones were cross-linked to DNA by adding
formaldehyde (Xian Chemical Reagent Factory, Xian, China) directly
to the culture medium to a final concentration of 1%, followed by
incubation for 10 min at 37°C, and the addition of 0.125 M glycine
(Amresco, LLC, Solon, OH, USA) to terminate the cross-linking. The
cells were washed twice using cold PBS, containing protease
inhibitors (Roche Diagnostics, Basel, Switzerland), and centrifuged
for 4 min at 2,000 × g at 4°C, followed by resuspending the cells
in SDS lysis buffer (1×106cells/200 μl; Amresco,
LLC). Sonicate lysate (200 μl; EMD Millipore) was used to
shear the DNA to lengths between 200 and 1,000 bp. The sonicated
cell supernatant was diluted 10-fold in CHIP dilution buffer (EMD
Millipore), and primary antibody, including anti RNA polymerase
antibody as positive control, normal rabbit IgG antibodies as
negative control and antibodies against acety-lated H3 and H3K9me2
for detecting the histone acetlyation and methylation of genes,
were added to the pre-cleared 2 ml supernatant and incubated
overnight at 4°C with constant rotation. Subsequently the protein A
agarose/antibody/chromatin complex was washed using CHIP Washing
Dilution (EMD Millipore) for 3–5 min with rotation. The The
cross-links were reversed to recover the DNA for qPCR detection.
The following primers were used for the Bcl-2 gene promoter:
Forward 5′-CCAGTTGCTGCAGTTTGGAAT-3′ and reverse
5′-TTGGACCATGTCTGGTGTCC-3′. The primer used for qPCR of the Bax
gene promoter were: Forward 5′-ACGCTCCAGAATAACTGCC-3′ and reverse
5′-GGTTTGCGCTGCGAGATAAG-3′. The qPCR reaction mixture contained 10
ng cDNA, 2x Taq PCR Green Mix (Dingguo Changsheng Co., Ltd.,
Beijing, China), 1 μl forward and reverse primers, and
H2O to make upto a total volume of 25 μl. The PCR
cycling conditions were set as follows: one cycle at 95°C for 5
min, 30 cycles at 94°C for 45 sec, 58°C for 45 sec and 72°C for 60
sec, and then one cycle at 72°C for 6 min.
Statistical analysis
All data are expressed as the mean ± standard
deviation. Statistical analyses of data were performed using
Student’s t-test on SPSS version 17.0 (SPSS Inc., Chicago, IL,
USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
Effects of VPA and/or ATO on the
proliferation of RPMI8226 cells
In the preliminary experiment, VPA and ATO inhibited
the proliferation of the RPMI8226 cells in a time- and
dose-dependent manner. The combination of the two drugs had a
synergistic effect with a Q-value >1.15. The growth rate of the
RPMI8226 cells in the combined drug groups was significantly
inhibited compared to those observed in the single drug groups
(P<0.05; Fig. 1).
Morphological observation
On examining the morphology of the RPMI8226 cells,
the cells exhibited a decrease in number, with disordered
arrangement, an increased number of cell fragments and increased
cell apoptosis. These features were observed at a high
magnification under an inverted fluorescent microscope (Fig. 2).
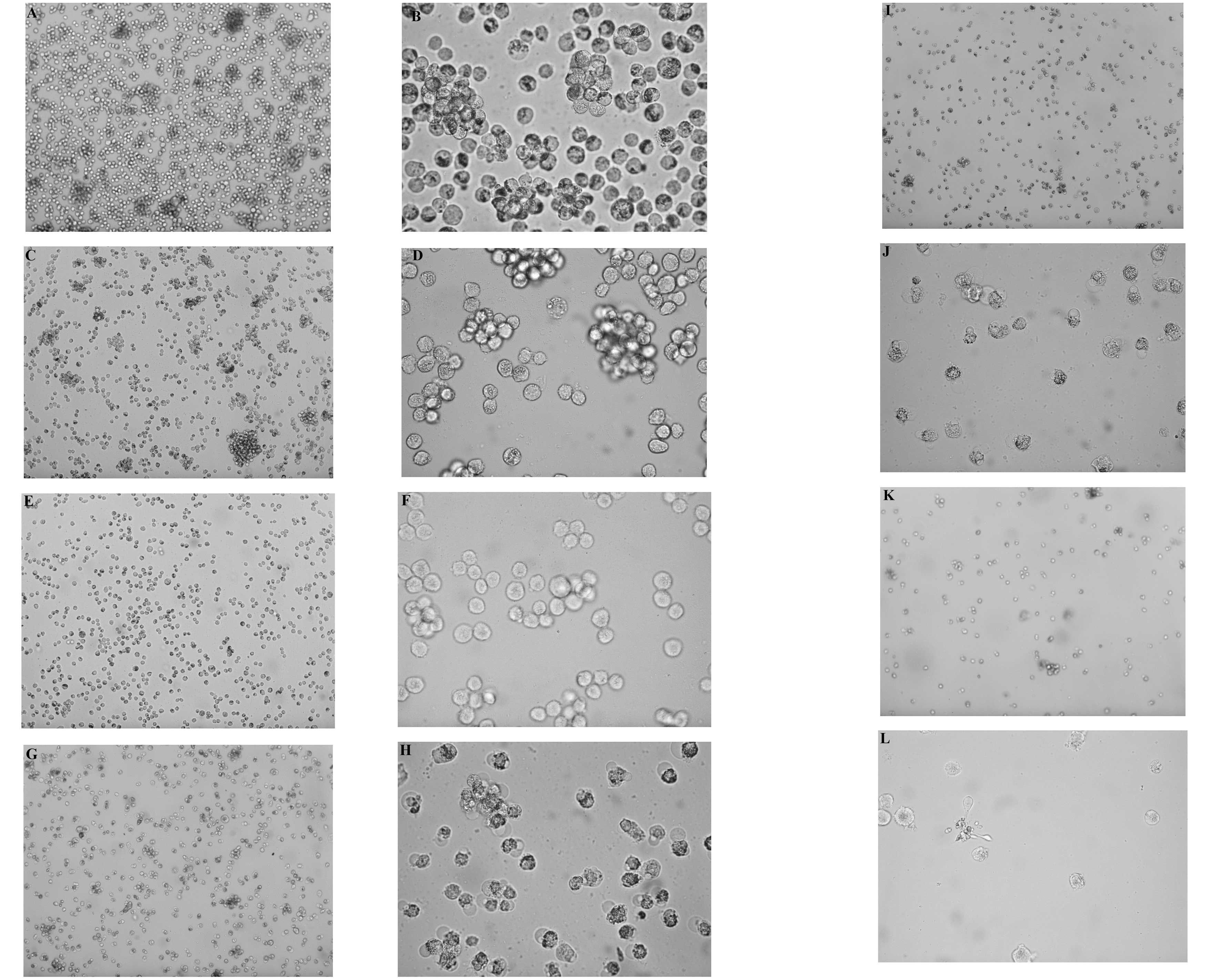 | Figure 2RPMI8226 cells cultured for 48 h,
viewed under an inverted fluorescent microscope. (A) Control group
(magnification, ×100), (B) control group (magnification, ×400), (C)
VPA 1 mmol/l (magnification, ×100), (D) VPA 1 mmol/l
(magnification, ×400), (E) VPA 5 mmol/l (magnification, ×100), (F)
VPA 5 mmol/l (magnification, ×400), (G) ATO 4 μmol/l
(magnification, ×100), (H) ATO 4 μmol/l (magnification,
×400). VPA, valproic acid; ATO, arsenic trioxide. (I) VPA 1
mmol/l+ATO 4 μmol/l (magnification, ×100), (J) VPA 1
mmol/l+ATO 4 μmol/l (magnification, ×400), (K) VPA 5
mmol/l+ATO 4 μmol/l (magnification, ×100), (L) VPA 5
mmol/l+ATO 4 μmol/l (magnification, ×400). VPA, valproic
acid; ATO, arsenic trioxide. |
VPA and ATO treatment induces myeloma
cell apoptosis
The present study also determined the percentage of
apoptosis of the RPMI8226 cells following exposure to VPA, ATO, or
the two combined, by flow cytometry using an annexin V FITC/PI
assay (Fig. 5). The apoptotic
rates of the RPMI8226 cells in the combined drug groups were
significantly increased compared with those of the single drug
groups (P<0.05; Fig. 3).
 | Figure 5Protein expression levels of Bcl-2,
Bax, Caspase 8, Caspase 9, acetylated H3, LSD1 and H3K9me2 in the
RPMI8226 cells, analyzed by western blot analysis. 1, control
group; 2, VPA 1 mmol/l; 3, VPA 5 mmol/l; 4, ATO 4 μmol/l; 5,
VPA 1 mmol/l+ATO 4 μmol/l; 6, VPA 5 mmol/l+ATO 4
μmol/l. VPA, valproic acid; ATO, arsenic trioxide. |
Increased mRNA expression levels of Bax,
Caspase 8 and Caspase 9 and decreased mRNA expression levels of
Bcl-2 and HDAC1
The mRNA expression levels of Bcl-2 and HDAC1 were
significantly decreased, and the mRNA expression levels of Bax,
Caspase 8 and Caspase 9 were significantly increased following
exposure of the cells to VPA + ATO for 48 h, compared with exposure
to either of the drugs alone (P<0.05; Fig. 4).
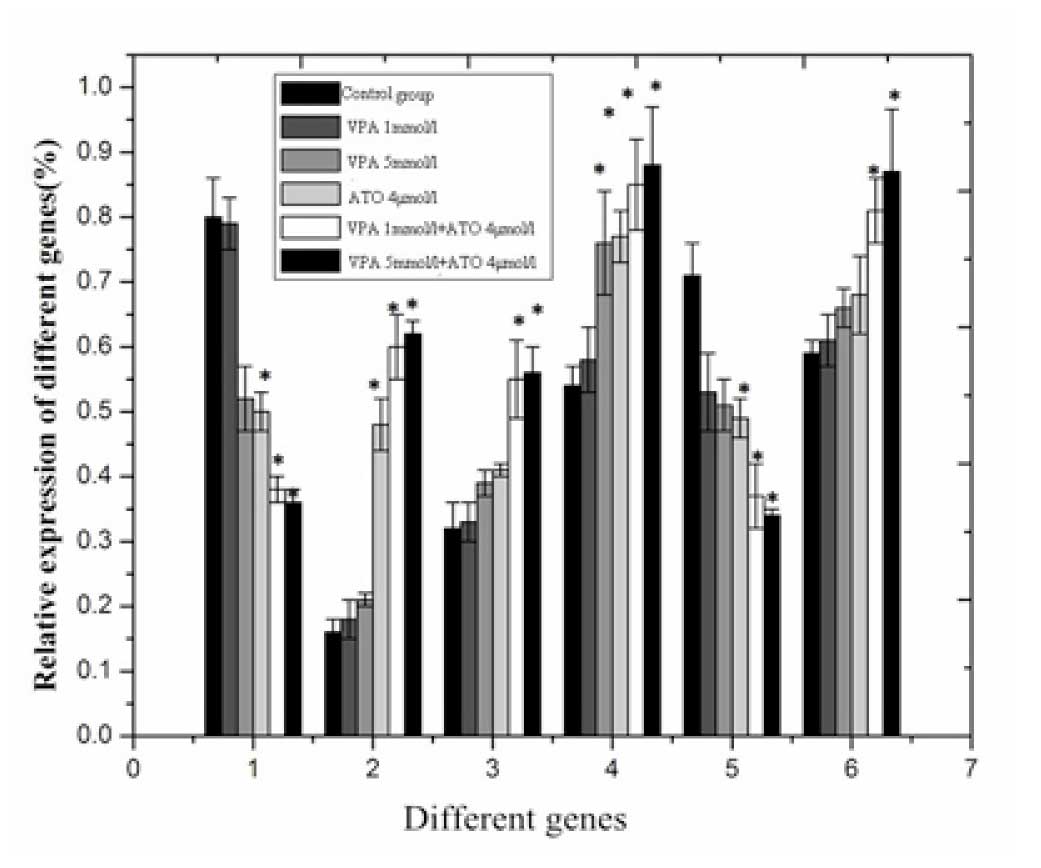 | Figure 4mRNA expression levels of Bax, Bcl-2,
Caspase 8, Caspase 9, HDAC1 and LSD1 in the RPMI8226 cells: 1,
ABcl-2/β-actin; 2, ABax/β-actin; 3, ACaspase
8/β-actin; 4, ACaspase 9/β-actin; 5, AHDAC1/β-actin; 6,
ALSD1/β-actin. *P<0.05, vs. control. Data are
expressed as the mean ± standard deviation VPA, valproic acid; ATO,
arsenic trioxide. |
Protein expression levels of Bcl-2, Bax,
Caspase 8, Caspase 9, acetylated H3, LSD1 and H3K9me2 in RPMI 8226
cells with VPA, ATO, or combined treatment
The protein expression levels of Bcl-2 and H3K9me2
were significantly decreased, and the protein expression levels of
Bax, Caspase 8, Caspase 9, acetylated H3 and LSD1 were
significantly increased following exposure to VPA + ATO for 48 h,
compared with either of the drugs alone (P<0.05; Fig. 5).
Histone acetylation and methylation state
of the Bcl-2 and Bax gene promoters
The level of histone methylation of the Bcl-2 gene
promoter and the level of acetylation of the Bax gene promoter were
increased in the combination groups compared with the single drug
groups (Fig. 6, Table I).
 | Figure 6(A) Acetylation modification of the
Bax gene promoter and (B) methylation modification of the Bcl-2
gene promoter in the RPMI8226 cells using a CHIP assay: 1, control
group; 2, VPA 1 mmol/l; 3, VPA 5 mmol/l; 4, ATO 4 μmol/l; 5,
VPA 1 mmol/l+ATO 4 μmol/l; 6, VPA 5 mmol/l+ATO 4
μmol/l. -, negative control (normal rabbit immunoglobulin
G); +, positive control (anti-RNA polymase antibody). VPA, valproic
acid; ATO, arsenic trioxide. |
 | Table IAcetylation level of the Bax gene
promoter and methylation level of the Bcl-2gene promoter. |
Table I
Acetylation level of the Bax gene
promoter and methylation level of the Bcl-2gene promoter.
| Group | ABax/A
positive control |
ABcl-2/A positive
control |
|---|
| Control group | 0.31±0.01 | 0.23±0.02 |
| VPA 1 mmol/l | 0.33±0.03 | 0.25±0.01 |
| VPA 5 mmol/l | 0.30±0.02 | 0.30±0.03 |
| ATO 4
μmol/l | 0.31±0.05 | 0.37±0.02 |
| VPA 1 mmol/l+ATO 4
μmol/l |
0.68±0.02a,b |
0.52±0.05a,b |
| VPA 5 mmol/l+ATO 4
μmol/l |
0.84±0.05a,b |
0.68±0.04a,b |
Discussion
MM is a B-cell malignancy, characterized by the
accumulation of monoclonal plasma cells and the production of
monoclonal immunoglobulin. Traditional chemotherapy and
hematopoietic stem cell transplantation can extend the overall
survival rates of patients with MM, however, almost all patients
with MM eventually develop chemoresistance (2). The development of novel therapeutic
options, including proteasome inhibitors and IMiDs, has improved
treatment outcomes, however, patients often develop relapsed and
refractory MM, thus requiring alternative treatment approaches.
Histone acetyltransferases and histone deacetylases (HDACs) control
the acetylation status of proteins and affect a broad array of
physiologic processes involved in cell growth and survival,
including cell cycle, apoptosis and protein folding (9). The observation that HDACs may be
involved in various hematological malignancies, including MM, has
led to the development of HDAC inhibitors (HDACIs) as potential
antitumor agents. Several types of HDACI have been used to treat
patients with MM in clinical trials, including vorinostat (SAHA)
and panobinostat, however, the response is poor (11–13).
The modest, yet encouraging, single-agent activity observed with
HDAC inhibitors in heavily pretreated patients with MM has led to
their evaluation in combination with other novel therapeutic agents
(14–18). The clinical activity of HDAC
inhibitors in combination with proteasome inhibitors, IMiDs and
conventional cytotoxic agents has been demonstrated in heavily
pretreated MM patients, supporting the continued evaluation of
these regimens in this patient population (19). VPA is a well-tolerated
anticonvulsant, which exerts antitumor activity as a histone
deacetylase inhibitor. In vitro exposure of
interleukin-6-dependent or -independent MM cells to VPA inhibits
cell proliferation in a time- and dose-dependent manner and induces
apoptosis (20). In a cohort of
severe combined immunodeficienct mice bearing human MM xenografts,
VPA was observed to induce tumor growth inhibition and improve
survival rates in treated animals compared with controls (20).
ATO has long been used as a therapeutic agent. It
was first used to treat acute promyelocytic leukemia and has been
suggested as an option for the treatment of relapsing or refractory
multiple myeloma (21).
Monotherapy with ATO results in partial response rates between 0
and 17% and minimal response rates of 7–33%, resulting in a mean
overall response rate of 30% (22,23).
Previously, the combination of ATO with ascorbic acid,
dexamethasone, bortezomib or thalidomide have been used to treat
patients with refractory or relapsed MM. The overall response rates
of using ATO in addition to dexamethasone, melphalan or other
cytostatic agents varies considerably, between 12–100%. Complete
remission has been achieved in the minority of cases (0–25%). The
duration of response in previous studies has also varied, ranging
between 0 and 24 months (24–28).
Therefore, it is necessary to identify novel therapeutic
combinations.
It has been reported that VPA combined with ATO
induces the apoptosis of HL-60 and K562 cells (29). In the present study, VPA and ATO
inhibited the proliferation of the RPMI8226 cells. The combination
of the two drugs had a synergistic effect (Q-value >1.15). The
apoptotic rate of the RPMI8226 cells in the combined drug group (5
mmol/l VPA +4 μmol/l ATO) was 75.11±1.16%. The apoptotic
rate of the RPMI8226 cells in the 5 mmol/l VPA and 4 μmol/l
ATO single drug group was 11.65±0.20% and 47.74±0.18%,
respectively. In addition, the mRNA and protein expression levels
of Caspase 8 and Caspase 9 increased in the combination groups
compared with the single drug groups. The expression of Bcl-2 was
decreased and the expression of Bax was increased following
combined treatment with VPA and ATO. Therefore, it was hypothesized
that VPA combined with ATO induced the apoptosis of MM cells
through the intrinsic and extrinsic apoptotic pathway.
VPA, as a histone deacetylase inhibitor can increase
histone acetylation levels. Previous studies have demonstrated that
ATO can combine with protein-rich cysteine or contain thiol to
exert antitumor effects (30).
Histone deacetylase, which contains cysteine readily combines with
ATO. It has been observed that ATO inhibits the expression of
histone acetyl-transferase 6 in the NCI-H929 myeloma cell line and
primary myeloma cells (30). In
the present study, the mRNA expression level of HDAC1 decreased,
and the mRNA expression level of LSD1 increased, therefore, the
protein expression levels of acetylated H3 and H3K9me2 increased
significantly following exposure to VPA combined with ATO for 48 h,
compared with the single drug groups (P<0.05). VPA and ATO may,
therefore, coordinately regulate histone acetylation and
methylation.
Epigenetic changes, including histone modification
is correlated with cells apoptosis (31). In the present study, the histone
acetylation and methylation state of the Bcl-2 and Bax gene
promoters were detected by chromatin immunoprecipitation. The
methylation level of the Bcl-2 gene promotor and acetylation level
of the Bax gene promoter were both increased. Histone acetylation
activated the transcription of certain genes and methylation
inhibited the transcription of several genes, therefore, the
expression levels of the Bcl-2 gene family may be regulated by the
methylation and acetylation levels of the Bcl-2 and Bax gene
promoters, and involved in the apoptosis of RPMI 8226 cells. In
order to provide an evidence-based basis for the clinical
application of VPA combined with ATO for the treatment of MM,
further investigation is required to determine whether other
mechanisms of apoptosis in RPMI8226 cells are induced by VPA and
ATO.
Acknowledgments
The authors would like to thank Dr Jing Li, Dr
Meiyin Qi and Dr Yue Teng for their technical assis tance.
References
|
1
|
He X, Yang K, Chen P, et al: Arsenic
trioxide-based therapy in relapsed/refractory multiple myeloma
patients: A meta-analysis and systematic review. Onco Targets Ther.
7:1593–1599. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Spitzer TR, Sachs DH and Cosimi B:
Multiple myeloma. N Engl J Med. 364:23642011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hussein MA, Saleh M, Ravandi F, et al:
Phase 2 study of arsenic trioxide in patients with relapsed or
refractory multiple myeloma. Br J Haematol. 125:470–476. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sanaat Z, Rezazadeh M, Gharamaleki JV, et
al: Arsenic trioxide in patients with refractory multiple myeloma:
A prospective, phase II, single-arm study. Acta Med Iran.
49:504–508. 2011.PubMed/NCBI
|
|
5
|
Rousselot P, Larghero J, Arnulf B, et al:
A clinical and pharmacological study of arsenic trioxide in
advanced multiple myeloma patients. Leukemia. 9:1518–1521. 2004.
View Article : Google Scholar
|
|
6
|
Wu K, van Droogenbroeck J, Beksac M, et
al: Treatment with asenic trioxide, ascorbic acid and dexamethasone
in advanced myeloma patients: Preliminary findings of a
multicenter, phase II study. Blood. 106:367b2005.
|
|
7
|
Berenson JR, Boccia R, Siegel D, et al:
Efficacy and safety of melphalan, arsenic trioxide and ascorbic
acid combination therapy in patients with relapsed or refractory
multiple myeloma: A prospective, multicentre, phase II, single-arm
study. Br J Haematol. 135:174–183. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bertenson J, Marous J, Ferretti D, et al:
A phase I/II trial evaluation the combination of arsenic trioxide,
bortezomib and ascorbic acid for patients with relapsed or
refractory multiple myeloma. Blood. 106:25652005.
|
|
9
|
Matthews GM, Newbold A and Johnstone RW:
Intrinsic and extrinsic apoptotic pathway signaling as determinants
of histone deacetylase inhibitor antitumor activity. Adv Cancer
Res. 116:165–197. 2012.PubMed/NCBI
|
|
10
|
Ye BG, Lin FA, Shen JZ, et al: Synergistic
effects of VPA and As2O3 on Molt-4 cells in
vitro and its possible mechanisms. Zhongguo shi yan xue ye xue za
zhi. 16:1288–1292. 2008.PubMed/NCBI
|
|
11
|
Dimopoulos M, Siegel DS, Lonial S, et al:
Vorinostat or placebo in combination with bortezomib in patients
with multiple myeloma (VANTAGE 088): a multicentre, randomised,
double-blind study. Lancet Oncol. 14:1129–1140. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang M, Martin T, Bensinger W, et al:
Phase 2 dose-expansion study (PX-171–006) of carfilzomib,
lenalidomide and low-dose dexamethasone in relapsed or progressive
multiple myeloma. Blood. 122:3122–3128. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kaufman JL, Fabre C, Lonial S, et al:
Histone Deacetylase Inhibitors in Multiple Myeloma: Rationale and
Evidence for Their Use in Combination Therapy. Clin Lymphoma
Myeloma Leuk. 13:370–376. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Richardson P, Mitsiades C, Colson K, et
al: Phase I trial of oral vorinostat (suberoylanilide hydroxamic
acid, SAHA) in patients with advanced multiple myeloma. Leuk
Lymphoma. 49:502–507. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Galli M, Salmoiraghi S, Golay J, et al: A
phase II multiple dose clinical trial of histone deacetylase
inhibitor ITF2357 in patients with relapsed or progressive multiple
myeloma. Ann Hematol. 89:185–190. 2010. View Article : Google Scholar
|
|
16
|
Niesvizky R, Ely S, Mark T, et al: Phase 2
trial of the histone deacetylase inhibitor romidepsin for the
treatment of refractory multiple myeloma. Cancer. 117:336–342.
2011. View Article : Google Scholar
|
|
17
|
DeAngelo DJ, Spencer A, Bhalla KN, et al:
Phase Ia/II, two-arm, open-label, dose-escalation study of oral
panobinostat administered via two dosing schedules in patients with
advanced hematologic malignancies. Leukemia. 27:1628–1636. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gimsing P, Hansen M, Knudsen LM, et al: A
phase I clinical trial of the histone deacetylase inhibitor
belinostat in patients with advanced hematological neoplasia. Eur J
Haematol. 81:170–176. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Richardson PG, Mitsiades CS, Laubach JP,
et al: Preclinical data and early clinical experience supporting
the use of histone deacetylase inhibitors in multiple myeloma. Leuk
Res. 37:829–837. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Neri P, Tagliaferri P, Di Martino MT, et
al: In vivo anti-myeloma activity and modulation of gene expression
profile induced by valproic acid, a histone deacetylase inhibitor.
Br J Haematol. 143:520–531. 2008.PubMed/NCBI
|
|
21
|
Lengfelder E, Hofmann WK and Nowak D:
Treatment of acute promyelocytic leukemia with arsenic trioxide:
Clinical results and open questions. Expert Rev Anticancer Ther.
13:1035–1043. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Berenson JR and Yeh HS: Arsenic compounds
in the treatment of multiple Myeloma: A new role for a historical
remedy. Clin Lymphoma Myeloma. 7:192–198. 2006. View Article : Google Scholar
|
|
23
|
Sanaat Z, Rezazadeh M, Gharamaleki JV, et
al: Arsenic trioxide in patients with refractory multiple myeloma:
A prospective, phase II, single-arm study. Acta Med Iran.
49:504–508. 2011.PubMed/NCBI
|
|
24
|
Held LA, Rizzieri D, Long GD, et al: A
Phase I study of arsenic trioxide (Trisenox), ascorbic acid and
bortezomib (Velcade) combination therapy in patients with
relapsed/refractory multiple myeloma. Cancer Invest. 31:172–176.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sanaat Z, Rezazadeh M, Gharamaleki JV, et
al: Arsenic trioxide in patients with refractory multiple myeloma:
a prospective, phase II, single-arm study. Acta Med Iran.
49:504–508. 2011.PubMed/NCBI
|
|
26
|
Sharma M, Khan H, Thall PF, et al: A
randomized phase 2 trial of a preparative regimen of bortezomib,
high-dose melphalan, arsenic trioxide and ascorbic acid. Cancer.
118:2507–2515. 2012. View Article : Google Scholar
|
|
27
|
Takahashi S: Combination therapy with
arsenic trioxide for hematological malignancies. Anticancer Agents
Med Chem. 10:504–510. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Röllig C and Illmer T: The efficacy of
arsenic trioxide for the treatment of relapsed and refractory
multiple myeloma: a systematic review. Cancer Treat Rev.
35:425–430. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Peng CY, Jiang J, Zheng HT, et al:
Growth-inhibiting effects of arsenic trioxide plus epigenetic
therapeutic agents on leukemia cell lines. Leuk Lymphoma.
51:297–303. 2010. View Article : Google Scholar
|
|
30
|
Qu X, Du J, Zhang C, et al: Arsenic
trioxide exerts antimyeloma effects by inhibiting activity in the
cytoplasmic substrates of histone deacetylase 6. PLoSOne.
7:e322152012. View Article : Google Scholar
|
|
31
|
Selokar NL, St John L, Revay T, et al:
Effect of histone Deacetylase inhibitor Valproic acid treatment on
donor cell growth characteristics, cell cycle arrest, apoptosis and
handmade cloned Bovine Embryo production efficiency. Cell
Reprogram. 15:531–542. 2013. View Article : Google Scholar : PubMed/NCBI
|




















