Introduction
Gastric cancer is the fourth most common type of
cancer and the second leading cause of cancer-associated mortality
globally (1). Although the
incidence and rates of mortality have decreased over the past 20
years, patients with gastric cancer are generally diagnosed at an
advanced stage and the prognosis is often poor (2). Furthermore, the molecular mechanisms
involved in the development and progression of gastric cancer
remain to be elucidated (3,4).
The deregulation of oncogenes or tumor suppressors,
including microRNAs (miRNAs/miRs), has been observed to be involved
in the development and progression of gastric cancer (5,6). As
a type of small noncoding RNA, miRNAs are able to modulate multiple
cellular processes by affecting the post-transcriptional processes
regulating gene expression, which are able to promote or inhibit
the development and progression of human malignancies (7). It has been widely-accepted that the
upregulation or downregulation of miRNAs is able to affect the
expression of various proteins and therefore is involved in
tumorigenesis through mediating proliferation and invasion
(8).
Aberrant expression of certain miRNAs, including
miR-133a, has been found to be correlated with the development,
progression and prognosis of gastric cancer. In addition, miR-133a
generally acts as a tumor suppressor in various types of cancer,
including gastric cancer (9),
non-small cell lung cancer (10),
ovarian cancer (11), osteosarcoma
(12) and esophageal squamous cell
carcinoma (12). More recently,
Qiu et al (9) reported that
miR-133a is able to inhibit proliferation, migration, invasion and
cell cycle progression via targeting transcription factor
specificity protein 1 in gastric cancer cells. As miRNAs are able
to target various mRNAs, other oncogenes may also be involved in
miR-133a-mediated cell proliferation and invasion in gastric cancer
cells (5).
The present study aimed to examine the roles of
miR-133a and FSCN1 in the regulation of the malignant phenotypes of
gastric cancer cells. In addition, the association between miR-133a
and FSCN was investigated.
Materials and methods
Reagents
Dulbecco’s modified Eagle’s medium (DMEM), fetal
bovine serum (FBS), TRIzol reagent, Lipofectamine 2000, SYBR green
assay kit, miRNA reverse transcription kit, and all the miRNA
mimics and the inhibitor were purchased from Invitrogen Life
Technologies (Carlsbad, CA, USA). The bicinchoninic acid (BCA)
protein assay kit and an enhanced chemiluminescence (ECL) kit were
purchased from Pierce Biotechnology, Inc. (Rockford, IL, USA). MTT
was purchased from Biosharp (Hefei, China). Hairpin-it™ miRNAs qPCR
quantitation kit and U6 small nuclear RNA were purchased from
Gene-Pharma (Shanghai, China). PsiCHECK™2 vector was purchased from
Promega Corporation (Madison, WI, USA). A quick-change
site-directed mutagenesis kit was purchased from Stratagene (La
Jolla, CA, USA). Mouse anti-FSCN1 monoclonal antibody (cat. no.
ab49815) and mouse anti-GAPDH (cat. no. ab8245) monoclonal antibody
were purchased from Abcam (Cambridge, UK). An Annexin V-fluorescein
isothiocyanate (FITC) apoptosis detection kit was purchased from BD
Pharmingen (San Diego, CA, USA) A Transwell chamber was obtained
from Corning Inc. (Corning, NY, USA).
Tissue specimen collection
The present study was approved by the Ethical
Committee of Central South University (Changsha, China). Informed
consent was obtained from the patients. A total of 18 gastric
carcinoma tissues and normal gastric tissues were obtained from the
Department of General Surgery, Xiangya Hospital of Central South
University (Changsha, China) between November 2012 and June 2013
The gastric carcinoma tissue samples were obtained during surgical
resection and confirmed by pathological examination. Normal gastric
tissue was obtained during surgery as a result of gastrorrhagia and
confirmed by pathological examination. The tissue samples were
immediately frozen in liquid nitrogen following surgical
removal.
Cell culture
HGC-27, GC7901 and AGS human gastric carcinoma
cells, and GES-1 normal gastric mucosa epithelial cells were
obtained from the Cell Bank of Central South University, and
cultured in DMEM with 10% FBS at 37°C in a humidified incubator
containing 5% CO2.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) assay
According to the manufacturer’s instructions, total
RNA was extracted using TRIzol reagent. Subsequently, an miRNA
reverse transcription kit was used to convert RNA into cDNA.
Subsequently, the expression levels of miRNAs were evaluated using
the Hairpin-it™ miRNAs qPCR quantitation kit, according to the
manufacturer’s instructions. The relative expression of the miRNAs
were analyzed using the 2−ΔΔCt method. The U6 small
nuclear RNA was used for normalization. The expression of FSCN1
mRNA was detected using the SYBR green qPCR assay kit. Expression
of GAPDH was used as an endogenous control. The specific primer
sequences were as follows: Forward: 5′-CCAGGGTATGGACCTGTCTG-3′ and
reverse: 5′-GTGTGGGTACGGAAGGCAC-3′ for FSCN1; and forward:
5′-GGAGCGAGATCCCTCCAAAAT-3′ and reverse:
5′-GGCTGTTGTCATACTTCTCATGG-3′ for GAPDH.
Western blotting
The tissues or cells were lysed in
radioim-munoprecipitation assay buffer. The protein was quantified
using a BCA protein assay kit. The proteins (60 μg) were
separated by 10% SDS-PAGE, and transferred onto a polyvinylidene
difluoride (PVDF) membrane (Pierce, Rockford, IL, USA), which was
then incubated with Tris-buffered saline with Tween 20 containing
5% milk at room temperature for 3 h. The PVDF membrane was then
incubated with rabbit anti-FSCN1 and GAPDH primary antibodies,
respectively, at room temperature for 3 h. Following three washes
with phosphate-buffered saline with Tween 20, the membrane was
incubated with the rabbit anti-mouse secondary antibodies (cat. no.
ab175743; Abcam) at room temperature for 40 min. Chemiluminescence
detection was performed using an ECL kit. The relative protein
expression was analyzed using Image-Pro plus software 6.0 (Media
Cybernetics, Rockville, MD, USA), presented as the density ratio
versus GAPDH.
Transfection
In accordance with the manufacturer’s instructions,
transfection was performed using Lipofectamine 2000. For FSCN1
functional analysis, the cells were transfected with FSCN1-specific
small interfering RNA or a pcDNA3.1-FSCN1 plasmid. For miR-133a
functional analysis, AGS cells were transfected with the scrambled
miRNA as a nega tive control, miR-133a mimics, or miR-133a
inhibitor.
Dual luciferase reporter assay
A quick-change site-directed mutagenesis kit was
used to generate a mutant type 3′-untranslated region (UTR) of
FSCN1, according to the manufacturer’s instructions. The wild or
mutant type 3′-UTR of FSCN1 was inserted into the psiCHECK™2
vector, respectively. After AGS cells were cultured to ~70%
confluence, they were transfected with psiCHECK™2-FSCN1-3′-UTR or
psiCHECK™2-mutant FSCN1 -3′-UTR vector, with or without 100 nM
miR-133a mimics, respectively. After transfection for 48 h, the
luciferase activities were determined using an LD400 luminometer
(Beckman Coulter, Fullerton, CA, USA). Renilla luciferase
activity was normalized to firefly luciferase activity.
Cell proliferation analysis
MTT was used to perform cell proliferation analysis,
according to the manufacturer’s instructions. Briefly, for each
group, 1×104 cells per well were plated in a 96-well
plate, and incubated for 0, 12, 24 or 48 h at 37°C and 5%
CO2. To assess cell proliferation, 10 μl MTT (5
mg/ml) was added to each well and then incubated for 4 h at 37°C
and 5% CO2. The supernatant was removed, and 100
μl dimethyl sulfoxide was added to dissolve the precipitate.
The absorbance was detected at 492 nm with a Microplate Reader
(Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Apoptosis analysis
The Annexin V-FITC apoptosis detection kit was used
to perform cell apoptosis analysis, according to the manufacturer’s
instructions. Briefly, cells were resus-pended in binding buffer,
then 2.5 μl Annexin V and 5 μl propidium iodide were
added. Following incubation for 15 min in the dark, 400 μ1
binding buffer was added. Cell apoptosis was determined via flow
cytometry (FACSCalibur C6, Becton-Dickinson, Franklin Lakes, NJ,
USA).
Invasion assay
A cell suspension containing 5×105
cells/ml was prepared in serum-free media. Subsequently, 300
μl cell suspension was added into the upper chamber, and 500
μl RPMI 1640 containing 10% FBS was added into the lower
chamber. Following incubation for 24 h, non-invading cells as well
as the matrix gel on the interior of the inserts was removed using
a cotton-tipped swab. Invasive cells on the lower surface of the
membrane were stained with 0.1% crystal violet for 20 min, then
rinsed with water and air dried. A total of five fields were
randomly selected and the cell number was counted under a
microscope (CX22; Olympus, Tokyo, Japan).
Statistical analysis
The results are expressed as the mean ± standard
deviation of three independent experiments. Statistical analysis of
the differences was performed using a one-way analysis of variance
with SPSS 17.0 software (SPSS, Inc., Chicago, IL, USA). P<0.05
was considered to indicate a statistically significant
difference.
Results
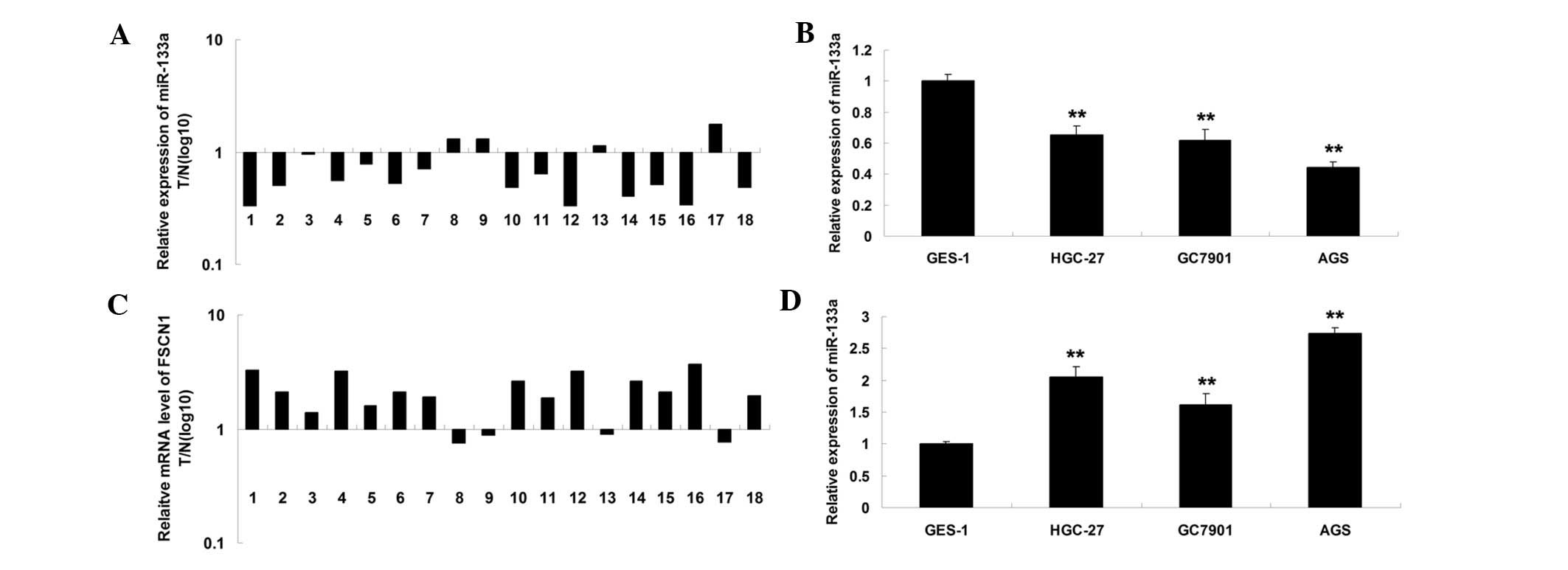
miR-133a is downregulated and FSCN1 is
upregulated in gastric carcinoma tissues and cells
The expression level of miR-133a was initially
examined in gastric carcinoma tissues and their matched normal
adjacent tissues. As shown in Fig.
1A, the expression level of miR-133a in gastric carcinoma
tissues was significantly reduced. In addition, it was also
downregulated in gastric carcinoma cell lines, when compared with
normal gastric epithelial cells (Fig.
1B). Subsequently, the expression level of FSCN1 was determined
using RT-qPCR. The present findings revealed that the mRNA level of
FSCN1 was upregulated in gastric carcinoma tissues, compared with
their matched normal adjacent tissues (Fig. 1C). In addition, the mRNA level of
FSCN1 was also increased in gastric carcinoma cell lines, when
compared with normal gastric epithelial cells (Fig. 1D). Accordingly, the present data
suggested that miR-133a is downregulated while FSCN1 is upregulated
in gastric carcinoma.
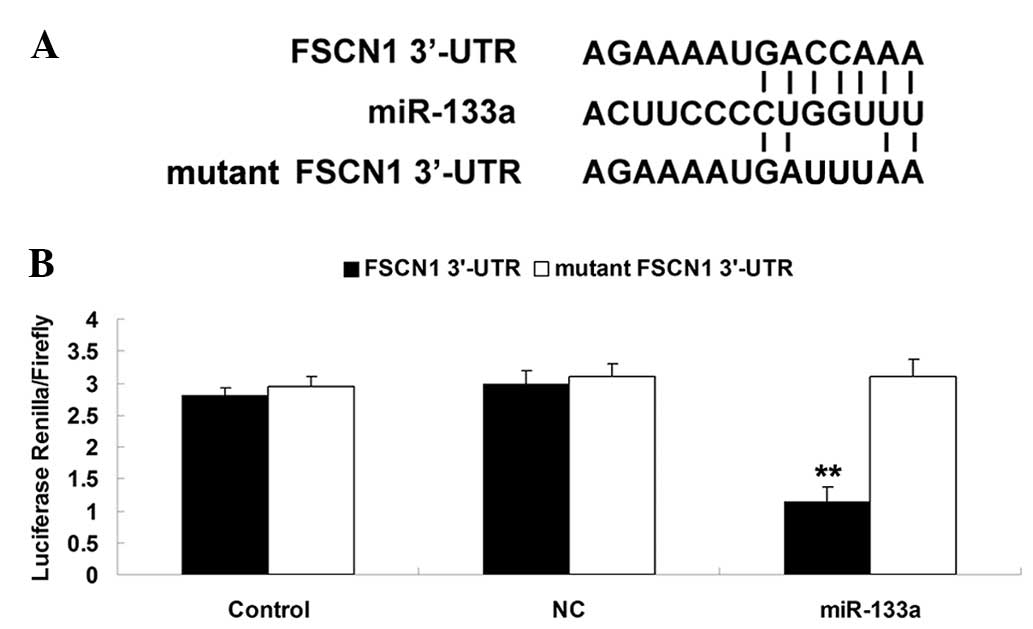
FSCN1 is a target of miR-133a in AGS
gastric carcinoma cells
As shown in Fig.
2A, the putative seed sequences for miR-133a at the 3′UTR of
FSCN1 was indicated based on bioinformatics analysis. To further
confirm that FSCN1 is a target of miR-133a, the wild and mutant
forms of the FSCN1 3′-UTR were generated (Fig. 2A). Subsequently, a luciferase
reporter assay was performed using AGS gastric carcinoma cells. As
shown in Fig. 2B, the luciferase
activity was markedly decreased in AGS gastric carcinoma cells
co-transfected with miR-133a mimics and the wild type 3′UTR of
FSCN1, but unaltered in AGS cells co-transfected with miR-133a
mimics and mutant FSCN1 3′UTR. The present data indicated that
miR-133a FSCN1 is a target of miR-133a in AGS gastric carcinoma
cells.
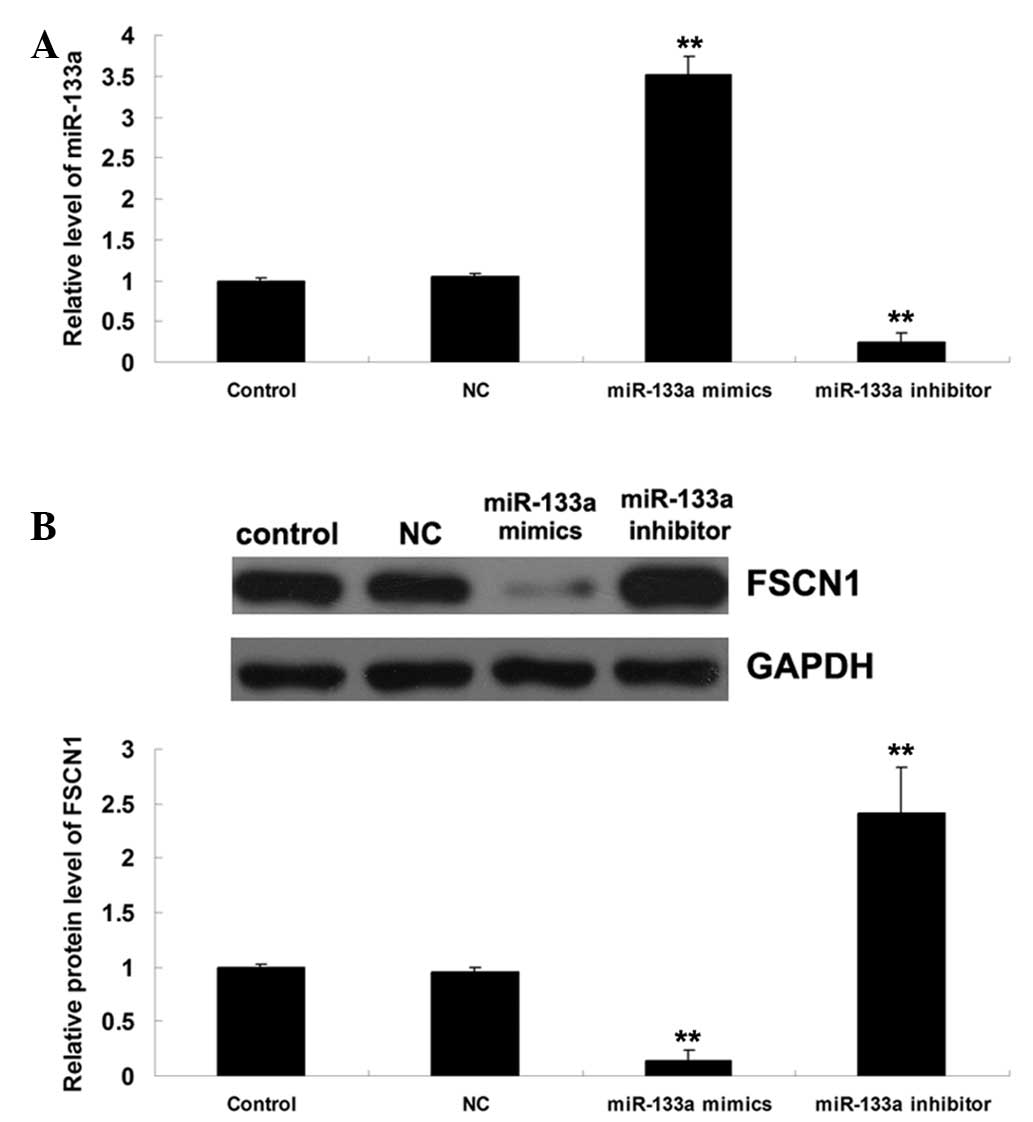
miR-133a negatively regulates the protein
expression of FSCN1 in AGS gastric carcinoma cells
AGS cells were transfected with miR-133a mimics and
inhibitor, and the protein expression of FSCN1 was determined in
AGS cells. As shown in Fig. 3A,
the transfection was efficient. It was then demonstrated that the
protein level of FSCN1 was reduced following upregulation of
miR-133a, but increased following inhibition of miR-133a (Fig. 3B). Therefore, it was suggested that
miR-133a negatively regulates the protein expression of FSCN1 in
AGS gastric carcinoma cells.
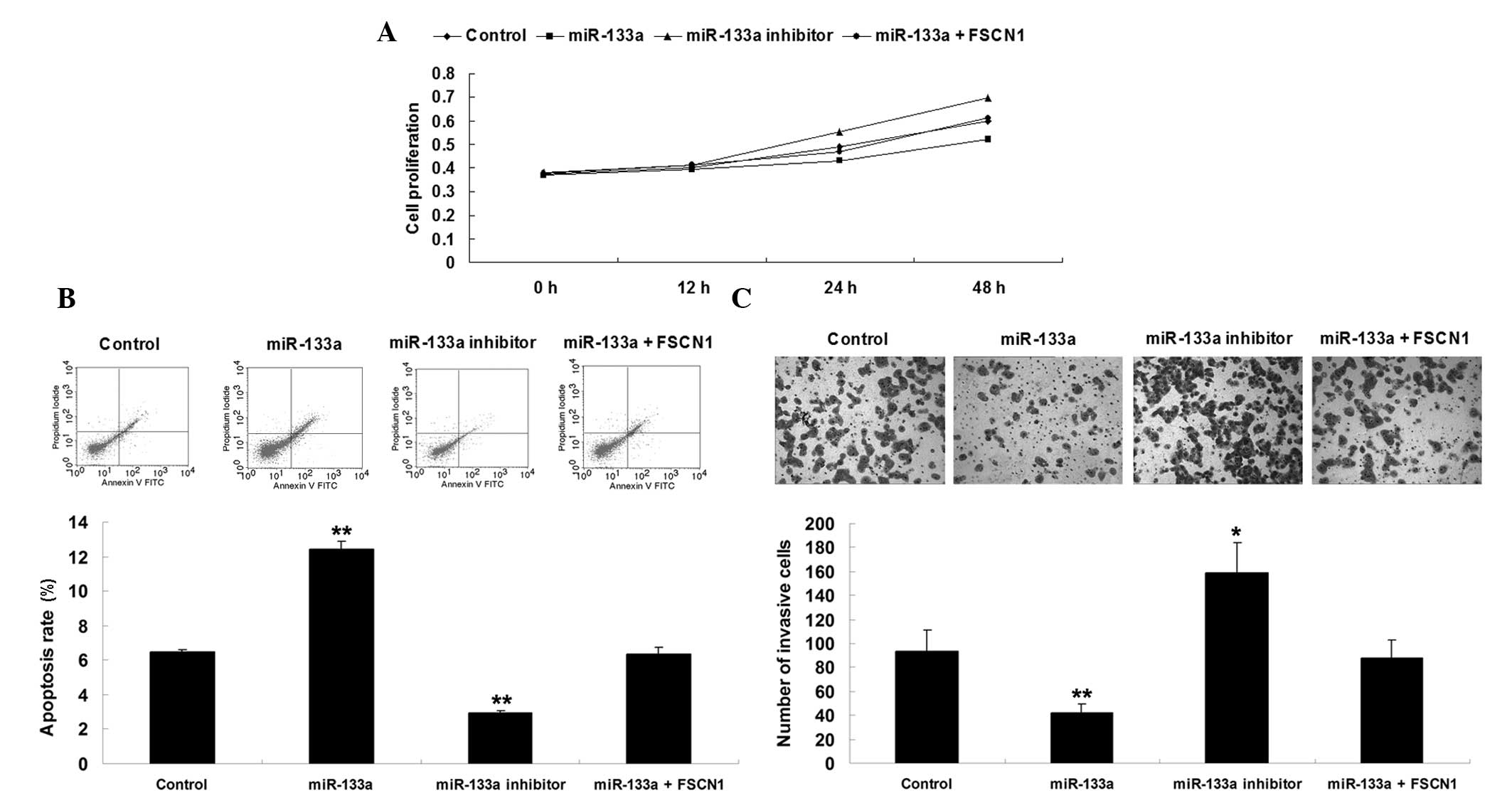
Effects of miR-133a and FSCN1 on AGS cell
proliferation, apoptosis and invasion
At present, whether miR-133a is important in the
regulation of cell proliferation, apoptosis, and invasion in
gastric carcinoma cells via targeting FSCN1 remains to be
elucidated. To investigate this mechanism, four groups were
assigned. In the control group, AGS cells were cultured without
transfection. In the miR-133a group, AGS cells were transfected
with miR-133a mimics. In the miR-133a inhibitor group, AGS cells
were transfected with miR-133a inhibitor. In the miR-133a + FSCN1
group, AGS cells were co-transfected with miR-133a mimics and FSCN1
plasmid. Subsequently, analysis of cell proliferation, apoptosis
and invasion were performed. As shown in Fig. 4, overexpression of miR-133a
inhibited proliferation and invasion, while promoted apoptosis in
AGS cells, which was reversed by upregulation of FSCN1. By
contrast, downregulation of miR-133a enhances proliferation and
invasion, while suppressing apoptosis in gastric cancer cells.
Accordingly, it was theorized that the role of miR-133a in the
regulation of cell proliferation, apop-tosis and invasion was,
partly at least, through the inhibition of FSCN1.
Discussion
miRNAs are able to recognize target mRNAs based on
complete or incomplete sequence complementarity and prevent their
protein expression by binding to the 3′-UTR of the target mRNA
(5). In the present study, it was
identified that FSCN1 is a target of miR-133a in AGS gastric cancer
cells, and it was observed that FSCN1 was upregulated while
miR-133a was downregulated in gastric cancer tissues and cell
lines, when compared with normal gastric tissues and gastric
epithelial cells.
In addition, it was hypothesized that the effect of
miR-133a on proliferation, apoptosis and invasion in AGS gastric
cancer cells may be, partly at least, through the direct inhibition
of FSCN1. It has been well-established that deregulation of miRNAs
is associated with the development and progression of cancer,
including gastric cancer (6). In
the present study, it was demonstrated that miR-133a was
significantly downregu-lated in gastric carcinoma tissues and cell
lines. In addition, Qiu et al (9) also revealed that the expression level
of miR-133a was reduced in gastric carcinoma tissues and cell
lines, consistent with the present findings. Furthermore, they
identified that overexpression of miR-133a induced G1
cell cycle arrest and inhibited cell proliferation, migration and
invasion in gastric carcinoma cells (9). In the present study, it was also
demonstrated that miR-133a upregulation inhibited cell
proliferation and invasion in AGS gastric cancer cells. However,
the molecular regulatory mechanism by which miR-133a regulates
gastric cancer remains to be elucidated.
In the present study, it was identified that FSCN1
was involved in the effect of miR-133a on AGS cell proliferation,
apoptosis and invasion. FSCN1 is a member of the FSCN family of
actin-binding proteins. FSCN proteins are responsible for
organization of F-actin into parallel bundles, and are involved in
the formation of actin-based cellular protrusions. It has been
well-established that FSCN1 is important in the regulation of cell
adhesion motility and cellular interactions (14,15).
In addition, overexpression of FSCN1 has been observed to be
associated with the metastasis of multiple types of cancer by
increasing cell motility (16,17).
In addition, the deregulation of FSCN1 has been reported in gastric
cancer. Tsai et al (18)
revealed that among 60 samples of poorly-differentiated gastric
adenocarcinomas, over half exhibited moderate or marked FSCN1
expression. Notably, a higher expression of FSCN1 correlated
directly with more-advanced cancer stages and inversely with
survival rates, suggesting that aberrant upregulation of FSCN1 may
be involved in the progression of gastric adenocarcinomas. Fu et
al (19) investigated the
molecular mechanism by which FSCN1 may be involved in the
regulation of gastric cancer. The authors revealed that FSCN1 is
involved in TGF-β1-induced gastric cancer cell invasion and
metastasis. In addition, another study demonstrated that inhibition
of FSCN1 expression suppressed the proliferation and metastasis of
gastric cancer cells (20). In the
present study, it was also observed that the expression of FSCN1
was upregulated in gastric cancer tissues. Based on these studies
and the present data, it was hypothesized that FSCN1 may become a
promising therapeutic target for gastric cancer.
Furthermore, the expression level of FSCN1 has been
reported to be mediated by several microRNAs, including miR-145,
miR-451 and miR-133b (21–23). In addition, the association between
miR-133a and FSCN1 has been reported in several other types of
cancer, including esophageal carcinoma, breast cancer and bladder
cancer (13,24–26).
For example, Akanuma et al (13) observed that the mRNA level of FSCN1
was upregulated in esophageal squamous cell carcinoma tissues and
inversely correlated with the expression level of miR-133a. The
authors further demonstrated that miR-133a inhibited cell
proliferation and invasion, partly as least, via inhibition of
FSCN1 in esophageal squamous cell carcinoma cells. Chiyomaru et
al (24) revealed that
miR-133a had a suppressive role through targeting FSCN1 in bladder
cancer. In breast cancer, the expression of miR-133a was reduced in
cancerous breast tissue compared with that of normal tissue and
benign tumor tissue, and miR-133a was found to suppress tumor cell
invasion and migration via targeting FSCN1 (26).
In conclusion, the present study indicated that the
anti-oncogenic activity of miR-133a involved inhibition of the
target gene FSCN1. The present study suggested that miR-133a may be
a potential therapeutic target for gastric cancer.
Acknowledgments
The present study was supported by the Fundamental
Research Funds for the Central Universities of Central South
University (grant no. 2177-72150050583).
References
|
1
|
Piazuelo MB and Correa P: Gastric cancer:
Overview. Colomb Med (Cali). 44:192–201. 2013.
|
|
2
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kuo CY, Chao Y and Li CP: Update on
treatment of gastric cancer. J Chin Med Assoc. 77:345–353. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Piazuelo MB and Correa P: Gastric cancer:
Overview. Colomb Med (Cali). 44:192–201. 2013.
|
|
5
|
Ambros V: The functions of animal
microRNAs. Nature. 431:350–355. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li PF, Chen SC, Xia T, et al: Non-coding
RNAs and gastric cancer. World J Gastroenterol. 20:5411–5419. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bartel DP: MicroRNAs: genomics,
biogenesis, mechanism and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hung CH, Chiu YC, Chen CH and Hu TH:
MicroRNAs in hepatocellular carcinoma: carcinogenesis, progression
and therapeutic target. BioMed Res Int. 2014:4864072014. View Article : Google Scholar
|
|
9
|
Qiu T, Zhou X, Wang J, et al: MiR-145,
miR-133a and miR-133b inhibit proliferation, migration, invasion
and cell cycle progression via targeting transcription factor Sp1
in gastric cancer. FEBS Lett. 588:1168–1177. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang LK, Hsiao TH, Hong TM, et al:
MicroRNA-133a suppresses multiple oncogenic membrane receptors and
cell invasion in non-small cell lung carcinoma. PLoS One.
9:e967652014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Guo J, Xia B, Meng F and Lou G: miR-133a
suppresses ovarian cancer cell proliferation by directly targeting
insulin-like growth factor 1 receptor. Tumour Biol. 35:1557–1564.
2014. View Article : Google Scholar
|
|
12
|
Fujiwara T, Katsuda T, Hagiwara K, et al:
Clinical relevance and therapeutic significance of microRNA-133a
expression profiles and functions in malignant
osteosarcoma-initiating cells. Stem Cells. 32:959–973. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Akanuma N, Hoshino I, Akutsu Y, et al:
MicroRNA-133a regulates the mRNAs of two invadopodia-related
proteins, FSCN1 and MMP14, in esophageal cancer. Br J Cancer.
110:189–198. 2014. View Article : Google Scholar :
|
|
14
|
Jayo A and Parsons M: Fascin: a key
regulator of cytoskeletal dynamics. Int J Biochem Cell Biol.
42:1614–1617. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yang S, Huang FK, Huang J, et al:
Molecular mechanism of fascin function in filopodial formation. J
Biol Chem. 288:274–284. 2013. View Article : Google Scholar :
|
|
16
|
Bi JB, Zhu Y, Chen XL, et al: The role of
fascin in migration and invasion of urothelial carcinoma of the
bladder. Urol Int. 91:227–235. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tan VY, Lewis SJ, Adams JC and Martin RM:
Association of fascin-1 with mortality, disease progression and
metastasis in carcinomas: a systematic review and meta-analysis.
BMC Med. 11:522013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tsai WC, Jin JS, Chang WK, et al:
Association of cortactin and fascin-1 expression in gastric
adenocarcinoma: correlation with clinicopathological parameters. J
Histochem Cytochem. 55:955–962. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fu H, Hu Z, Wen J, Wang K and Liu Y:
TGF-beta promotes invasion and metastasis of gastric cancer cells
by increasing fascin1 expression via ERK and JNK signal pathways.
Acta Biochim Biophys Sin (Shanghai). 41:648–656. 2009. View Article : Google Scholar
|
|
20
|
Fu H, Wen JF, Hu ZL, Luo GQ and Ren HZ:
Knockdown of fascin1 expression suppresses the proliferation and
metastasis of gastric cancer cells. Pathology. 41:655–660. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen MB, Wei MX, Han JY, et al:
MicroRNA-451 regulates AMPK/mTORC1 signaling and fascin1 expression
in HT-29 colorectal cancer. Cell Signal. 26:102–109. 2014.
View Article : Google Scholar
|
|
22
|
Adammek M, Greve B, Kässens N, et al:
MicroRNA miR-145 inhibits proliferation, invasiveness and stem cell
phenotype of an in vitro endometriosis model by targeting multiple
cytoskeletal elements and pluripotency factors. Fertil Steril.
99:1346–1355. e52013. View Article : Google Scholar
|
|
23
|
Yamamoto H, Kohashi K, Fujita A and Oda Y:
Fascin-1 over-expression and miR-133b downregulation in the
progression of gastrointestinal stromal tumor. Mod Pathol.
26:563–571. 2013. View Article : Google Scholar
|
|
24
|
Chiyomaru T, Enokida H, Tatarano S, et al:
miR-145 and miR-133a function as tumour suppressors and directly
regulate FSCN1 expression in bladder cancer. Br J Cancer.
102:883–891. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kano M, Seki N, Kikkawa N, et al: miR-145,
miR-133a and miR-133b: Tumor-suppressive miRNAs target FSCN1 in
esophageal squamous cell carcinoma. Int J Cancer. 127:2804–2814.
2010. View Article : Google Scholar
|
|
26
|
Wu ZS, Wang CQ, Xiang R, et al: Loss of
miR-133a expression associated with poor survival of breast cancer
and restoration of miR-133a expression inhibited breast cancer cell
growth and invasion. BMC Cancer. 12:512012. View Article : Google Scholar : PubMed/NCBI
|