Introduction
Neovascularization is a major cause of common eye
diseases, such as age-related macular degeneration (AMD), diabetic
retinopathy, and retinopathy of prematurity (ROP) that result in
blindness (1). New vessels that
are generated by neovascularization usually undergo repeated
regeneration, which can easily lead to bleeding and vessel
destruction due to stress caused by increased permeability. A
number of the pathways that result in neovascularization have been
identified (2). The majority of
these pathways, such as the protein kinase B (AKT) pathway, the
nuclear factor of activated T-cells (NFAT) signaling pathway, and
the Nox2-generated reactive oxygen species (ROS) pathway, are
associated with increased vascular endothelial growth factor (VEGF)
expression (3–5). Therefore, VEGF is a major component
of the neovascularization process.
VEGF-A, also termed VEGF, is a member of the
cysteine-knot superfamily of growth factors. The characteristics of
VEGF are determined by a cysteine residue (6,7).
Increased secretion of VEGF induces neovascularization, and
anti-VEGF therapy with bevacizumab is effective in the suppression
of neovascularization (8). Certain
conditions, such as diabetes, trauma and infection (9), can induce hypoxia, which results in
the increased expression of VEGF in the eye (10). Secretion of VEGF is also generally
observed in cancer cells. In certain types of cancer, the cell
secretes VEGF locally to stimulate the surrounding endothelial
cells for neovascularization (11). Retinal pigment epithelial (RPE)
cells are a source of VEGF in the eye (12). RPE cells form a monolayer structure
at the back of the eye, and supply nutrients and remove waste from
the surrounding tissues. Although there are several pathways that
regulate VEGF expression, the present study focuses on the
interaction between CXC-chemokine receptor 4 (CXCR4) and VEGF.
CXCR4 is a well-known hypoxia-related protein
(13). Studies of cancer cells
revealed that CXCR4 expression is increased during hypoxia and
mediates VEGF upregulation (14,15).
This suggested that CXCR4 could be a useful target for inhibition
of neovascularization.
Resveratrol (Res) is a phytoalexin present in
grapes, red wine and other food products. Res has significant
effects on inflammation, apoptosis and neovascularization (16,17).
Furthermore, Res is particularly effective as an antioxidant in
hypoxic conditions (18). Res also
prevents or mitigates the effects of eye diseases, such as retinal
detachment and diabetic retinopathy (19–22).
The main target of Res is nuclear factor (NF)-κB, which is a major
factor of the SDF-1/CXCR4 axis (23).
In this study, the major regulators of
hypoxia-induced VEGF secretion in the ARPE-19 human retinal
epithelial cell line were characterized. The present study focused
on the NF-κB, CXCR4 and VEGF pathways, and the effect of Res on
neovascularization induced by hypoxia.
Materials and methods
Reagents
High-glucose Dulbecco’s modified Eagle’s medium F12
(DMEM-F12), penicillin, streptomycin and fetal bovine serum (FBS)
were purchased from Gibco (Grand Island, NY, USA). TRIzol reagent
was purchased from Invitrogen Life Technologies (Carlsbad, CA,
USA). Res was purchased from Tocris (Ellisville, MO, USA), SDF-1
was purchased from R&D systems (Minneapolis, MN, USA) and
dimethylsulfoxide (DMSO) was purchased from Amresco (Solon, OH,
USA). Antibodies specific for hypoxia inducible factor-1α (HIF-1α;
cat. no. 3716S) and phospho-NF-κB (cat. no. 3033S) were purchased
from Cell Signaling Technology Inc. (Danvers, MA, USA), anti-CXCR4
antibodies (cat. no. ab2074) were purchased from Abcam (Cambridge,
UK), and anti-α-tubulin antibodies (cat. no. T5168) were purchased
from Sigma-Aldrich (St. Louis, MO, USA). Ammonium
pyrrolidinethiocarbamate (PDTC), NF-κB inhibitor and AMD3100 (a
CXCR4 antagonist), were purchased from Sigma-Aldrich. BD Matrigel™
Basement Membrane Matrix was purchased from BD Biociences (Bedford,
MA, USA). Resveratrol was dissolved in DMSO, PDTC and AMD3100 were
dissolved in water, and SDF-1 was dissolved in phosphate-buffered
saline (PBS).
Cell culture and treatment
ARPE-19 cells (American Type Culture Collection,
Manassas, VA, USA) were cultured in DMEM F12 supplemented with 10%
FBS, 100 U/ml penicillin, and 100 μg/ml streptomycin at 37°C
in an atmosphere with 5% CO2 at 70% confluence. For some
experiments, ARPE-19 cells were cultured in DMEM-F12 with 0.1% FBS
in the presence of designated concentrations of VEGF protein and/or
Res (10–50 μM). Cells were exposed to CoCl2 for 6
h prior to western blotting. Cells were grown as monolayer.
Western blotting
ARPE-19 cells were plated on 100-mm dishes at
1×106 cells/dish. Following treatment, cells were
harvested, centrifuged at 890 × g for 1 min, and lysed in lysis
buffer [50 mM Tris-Cl (pH 8.0), 150 mM NaCl, 0.1% sodium dodecyl
sulfate (SDS) and 0.02% sodium azide, which were purchased from
Amresco; 1% NP-40 and 0.5% sodium deoxycholate, which were
purchased from Sigma-Aldrich, and proteinase inhibitor cocktail
(containing phenylmethylsulphonyl fluoride, 100 μg/ml;
aprotinin, 1 μg/ml; leupeptin, 0.5 μg/ml; Roche,
Nutley, NJ, USA)]. Total protein concentration was determined using
a bicinchoninic acid protein assay system (Thermo Fisher
Scientific, Waltham, MA, USA). An equal volume of 4X SDS sample
buffer was added to 30 μg of protein extract and the samples
were boiled for 5 min. Equivalent quantities of total protein
(15–20 μg) were separated by SDS-PAGE on 8–10%
polyacrylamide gels and then transferred to nitrocellulose
membranes (GE Healthcare, Little Chalfont, UK) at 15 V for 30 min
using a semi-dry transfer apparatus (Bio-Rad Laboratories,
Hercules, CA, USA) submerged in transfer buffer (25 mM Tris, 192 mM
glycine, 20% methanol; pH 8.3). The membrane was blocked with 5%
skimmed milk in 0.1% Tween-20/Tris-buffered saline (TBST) and then
incubated with anti-CXCR4 (1:1,000), anti-HIF-1α (1:1,000),
anti-phospho- NF-κB (1:1,000) or anti-α-tubulin (1:10,000) antibody
at 4°C overnight. Subsequently, the blot was washed in TBST and
incubated with goat anti-rabbit (cat. no. 31430) and goat
anti-mouse (cat. no. 31460) immunoglobulin G secondary antibodies
(Thermo Fisher Scientific; 1:10,000 dilution) for 45 min at room
temperature. After washing, immunoreactivity was detected by
chemiluminescence (ECL; Advansta, Menlo Park, CA, USA) using the
LAS 3000 instrument (Fujifilm, Tokyo, Japan). The band intensities
were assessed by Multi Gauge 3.0 software (Fujifilm).
Enzyme-linked immunosorbent assay
(ELISA)
ARPE-19 cells were plated on 6-well cell culture
plates at 5×104 cells/well. Cell culture media were
supplemented with 1% FBS, 100 U/ml penicillin and 100 μg/ml
streptomycin. VEGF secretion levels were measured using a
commercial human VEGF-ELISA kit from Invitrogen Life Technologies
(Camarillo, CA, USA), according to the manufacturer’s
instructions.
Human umbilical vein endothelial cell
(HUVEC) tube formation assay
ARPE-19 cells were cultured in DMEM-F12 media with
0.1% FBS, 100 U/ml penicillin and 100 μg/ml streptomycin at
37°C and 5% CO2 in the presence of the designated
concentrations of Res (50 μM). ARPE-19 cells were classified
into three groups: Control [Normoxia conditioned medium (CM)], 1%
O2 hypoxia-treatment (Hypoxia CM) and hypoxia + Res (50
μM) co-treatment (Hypoxia + Res CM). Cells were exposed to
1% O2 for 24 h in a hypoxia chamber (MCO-5M; Sanyo,
Osaka, Japan). The CM obtained from ARPE-19 cells was transferred
to HUVECs (PromoCell, Heidelberg, Germany) that were seeded on
24-well plates coated with Matrigel™ at 1×105
cells/well. CM-treated HUVECs were incubated for 48 h. Tube
formation was analyzed by light microscopy (U-LH100HG; Olympus
Corporation, Tokyo, Japan) and ImageJ 1.46r software (National
Institutes of Health, Bethesda, MD, USA).
Statistical analysis
Data are expressed as the mean ± standard error of
the mean. One-way analysis of variance was performed using
Dunnett’s post-test. (Prism 5; GraphPad Software, La Jolla, CA,
USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
HIF-1α and CXCR4 protein expression, and
VEGF secretion profiles ARPE-19 cells treated with a hypoxia
mimetic agent
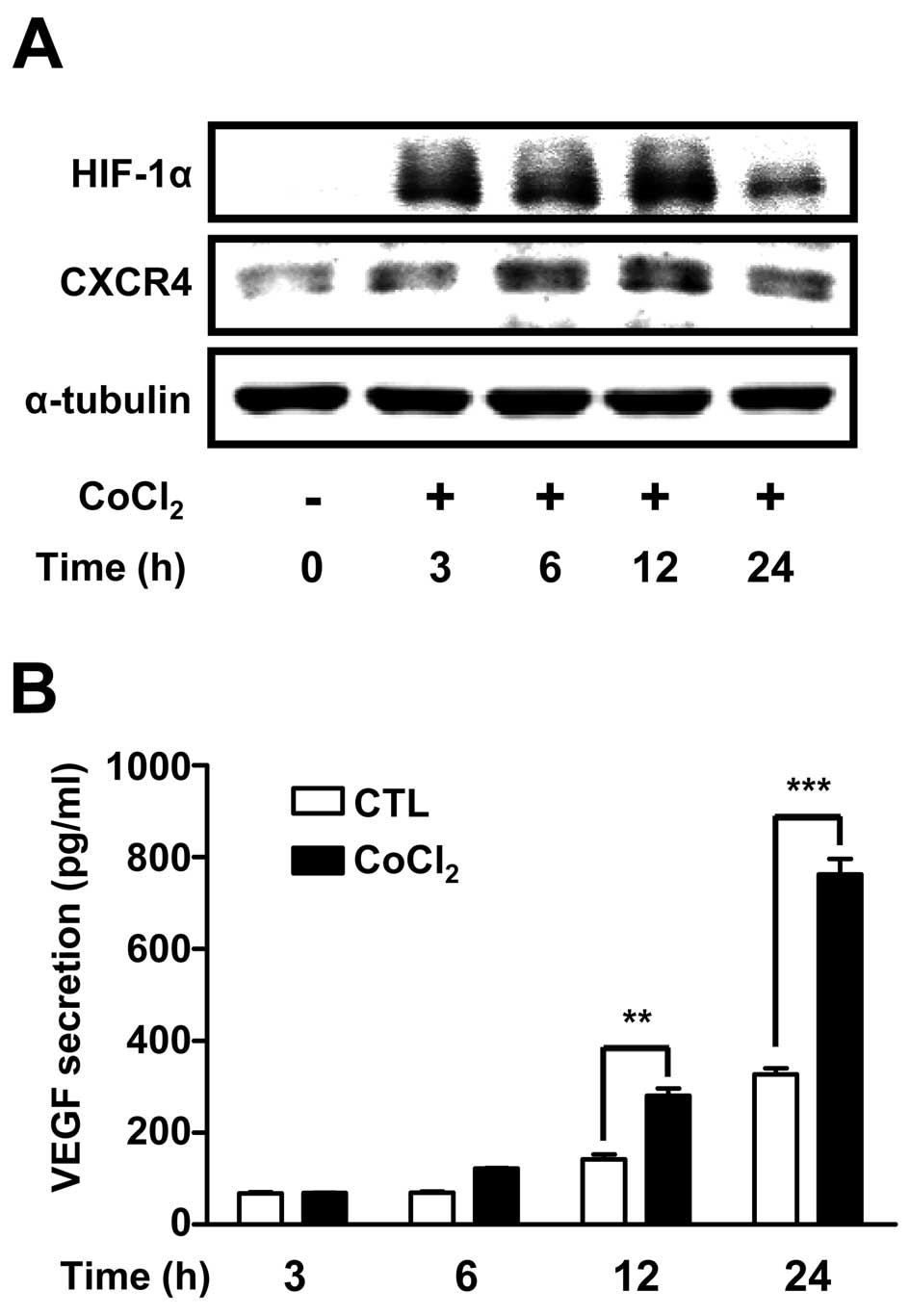
To confirm hypoxia-induced changes in protein
expression in ARPE-19 cells, cells were treated with
CoCl2 (100 μM) a hypoxia mimetic agent for 24 h.
HIF-1α and CXCR4 protein levels were determined by western
blotting. Expression of the hypoxia marker HIF-1α was increased by
~3.5-fold at 3 h and peaked by ~5-fold at 12 h. CXCR4 protein
levels also increased at 6 h (Fig.
1A). VEGF secretion, determined by ELISA, was significantly
increased at 12 h and further increased by >5-fold at 24 h
(Fig. 1B). These results showed
that CXCR4 protein was expressed in ARPE-19 cells, and that
expression of HIF-1α, CXCR4 and VEGF proteins increased after
hypoxia mimetic treatment, which were similar to the results found
under 1% O2 hypoxic conditions.
Res suppressed HIF-1α protein expression,
NF-κB phosphorylation, CXCR4 protein expression, and VEGF secretion
in ARPE-19 cells treated with a hypoxia mimetic agent
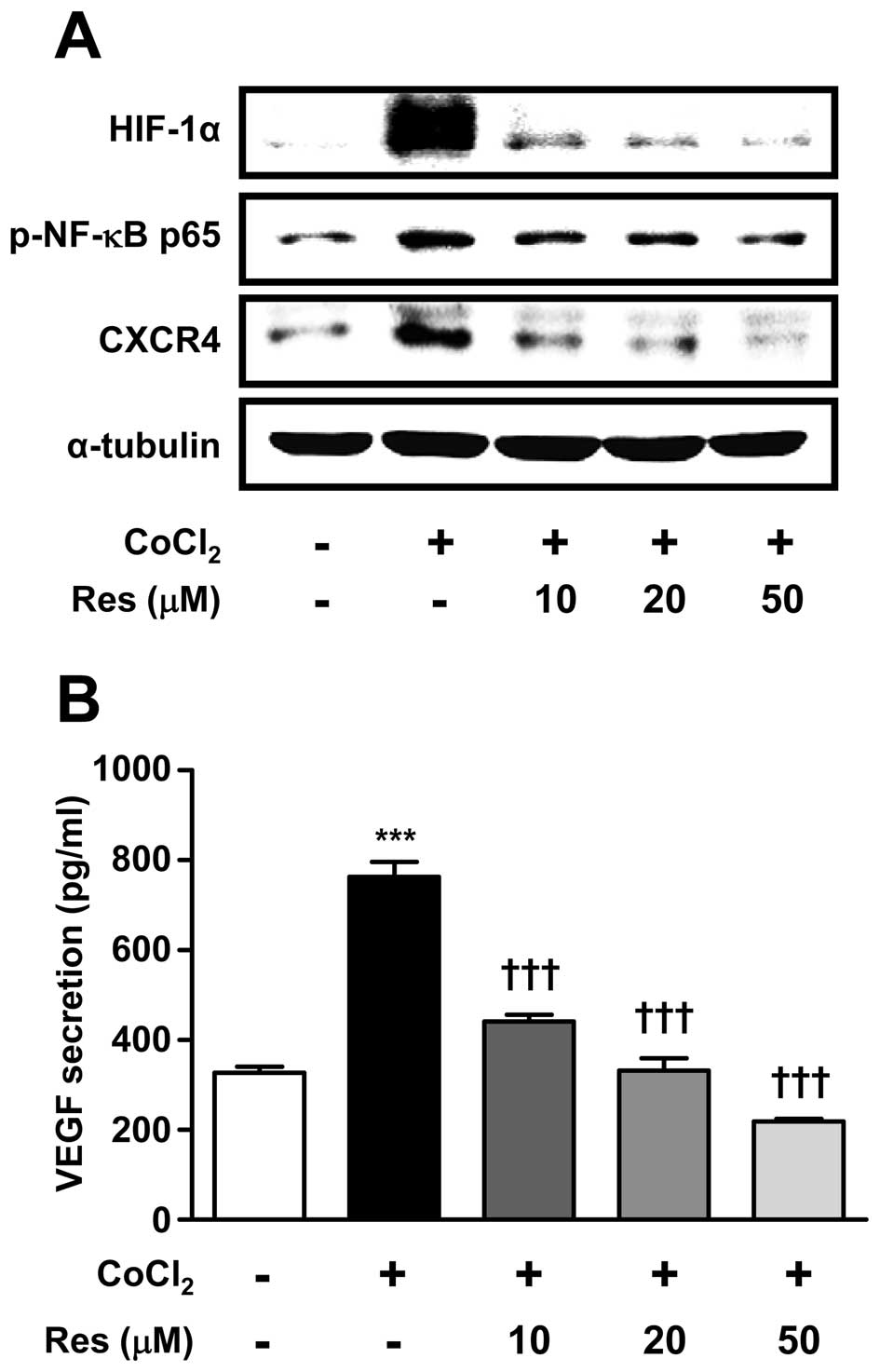
To evaluate the effects of the antioxidant Res on
the increased expression of CXCR4 protein induced by
CoCl2, cells were pre-treated with Res at three
different concentrations (10–50 μM). After 2 h,
CoCl2 (100 μM) was added for a further 6 h. The
expression of phosphorylated NF-κB protein, a major target of Res,
was also examined. Res treatment reversed the
CoCl2-induced increases in the levels of HIF-1α, p-NF-κB
and CXCR4 protein expression in a dose-dependent manner. The levels
of HIF-1α, p-NF-κB and CXCR4 protein expression were reduced by
~80, 60 and 50% by 50 μM resveratrol, respectively (Fig. 2A), and similar effects on VEGF
secretion were observed (Fig. 2B).
These results showed that 50 μM Res effectively inhibited
the phosphorylation of NF-κB, expression of HIF-1α and CXCR4, and
secretion of VEGF.
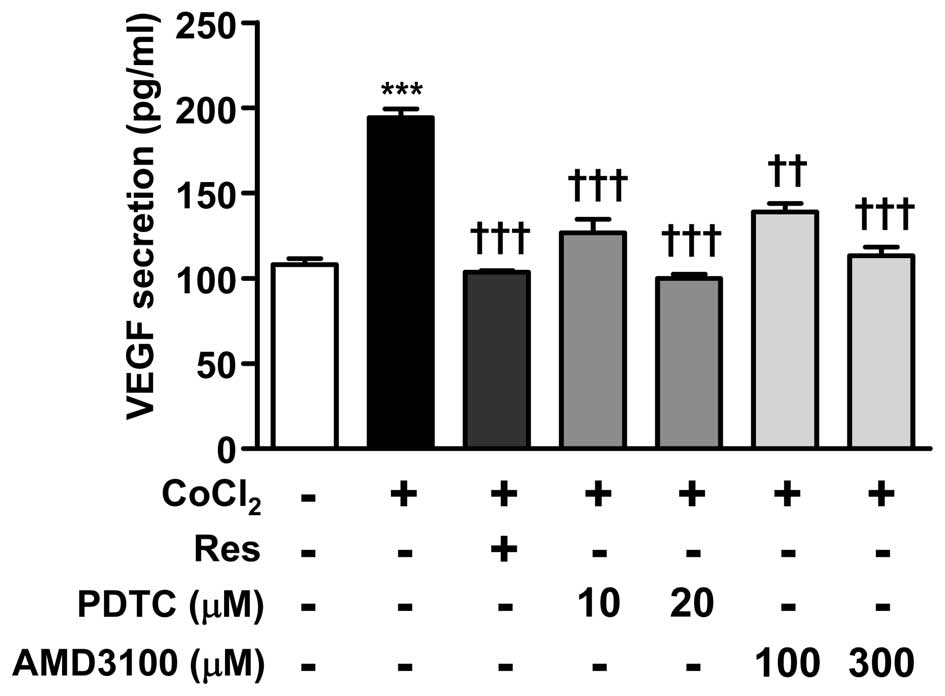
PDTC and AMD3100 suppress VEGF secretion
in hypoxia mimetic-treated ARPE-19 cells
To investigate the effects of NF-κB and CXCR4 on
VEGF secretion, cells were treated with PDTC (10 and 20 μM)
and AMD3100 (100 and 300 μM) for 12 h in combination with
CoCl2. PDTC and AMD3100 treatments dose-dependently
reduced the CoCl2-induced VEGF secretion respectively
(Fig. 3). These results showed
that Res is able to suppress VEGF secretion through inhibition of
NF-κB and CXCR4.
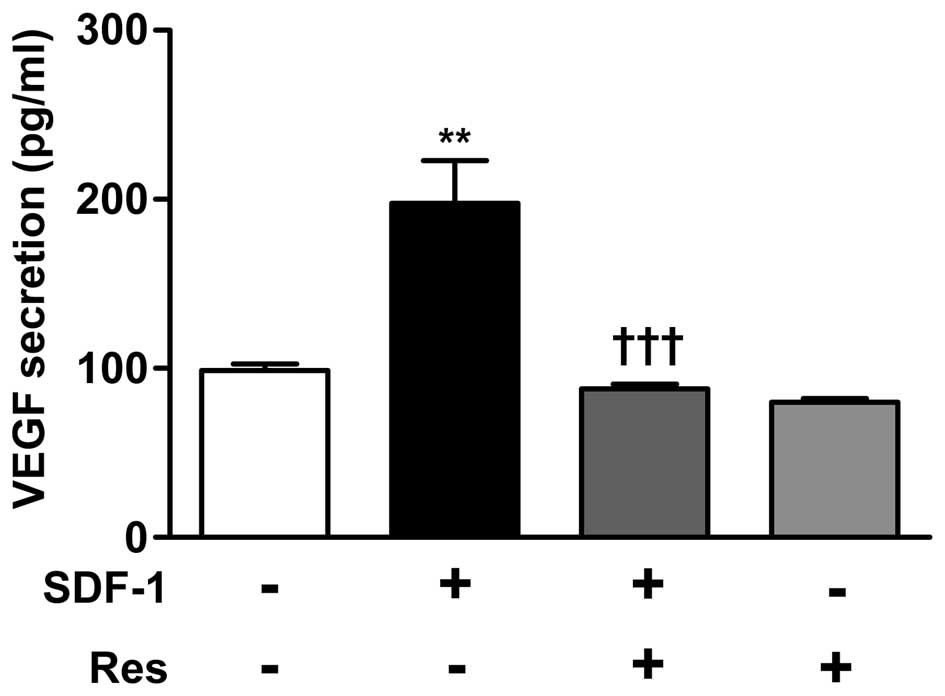
Res suppresses SDF-1-induced VEGF
secretion
As inhibition of CXCR4 suppressed VEGF secretion, it
was then analyzed whether Res can suppress VEGF secretion induced
by ligand-receptor interaction of CXCR4. Cells were treated with
SDF-1 (30 ng/ml), a ligand of CXCR4, alone or in combination with
Res (50 μM) for 12 h. SDF-1 induced-secretion of VEGF in
ARPE-19 cells was suppressed by co-treatment with Res (50
μM) (Fig. 4).
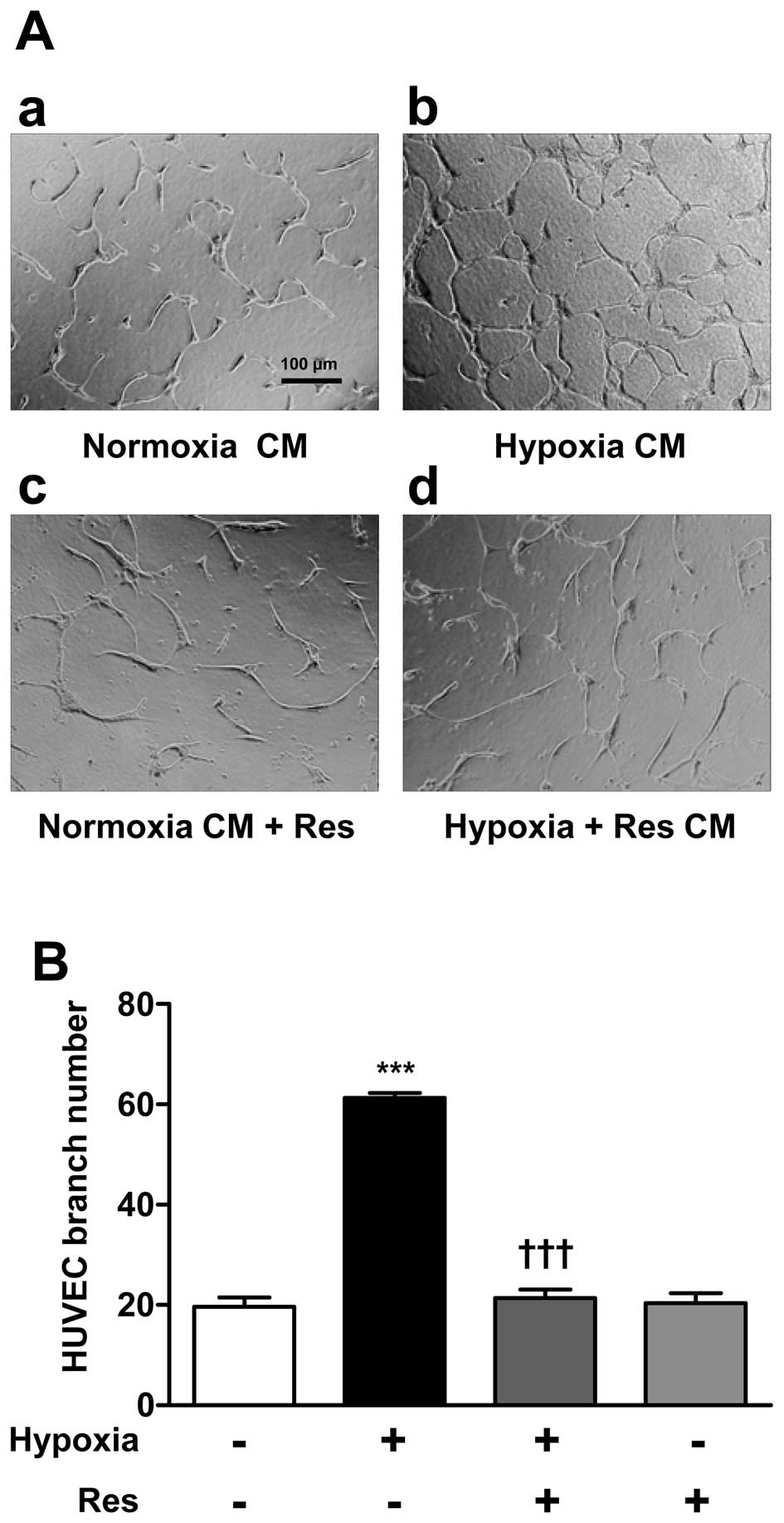
Suppression of HUVEC tube formation by CM
from Restreated ARPE-19 cells
To indirectly investigate the effect of Res on
neovascularization the HUVEC tube formation assay was used. ARPE-19
cells were classified into three groups: Control (Normoxia CM), 1%
O2 hypoxia-treatment (Hypoxia CM); and hypoxia+Res (50
μM) co-treatment (Hypoxia+Res CM). After 24 h incubation, CM
from the three experimental groups was transferred to HUVECs and
the cells were incubated for 48 h. HUVECs treated with Hypoxia CM
showed an increase in the number of branch points compared with
cells treated with Normoxia CM. HUVECs treated with Hypoxia+Res CM
showed a significant decrease in the number of branch points HUVECs
treated with Normoxia CM and Res (50 μM) served as a
negative control (Fig. 5).
Discussion
In this study, it was determined using
CoCl2-induced hypoxia mimetic conditions that there is
an association between CXCR4 and VEGF in ARPE-19 cells, and it was
assessed whether Res could effectively suppress neovascularization
by targeting interactions between CXCR4 and VEGF. Similar to other
studies, it was confirmed that CXCR4 and VEGF levels were increased
during chemically-induced hypoxia (24,25).
Furthermore, it was demonstrated that Res inhibited CXCR4
expression and VEGF secretion, and suppressed HUVEC tube
formation.
HIF-1α is widely used as a hypoxia marker. During
hypoxia, HIF-1α rapidly accumulates as the activation of enzymes
responsible for HIF-1α degradation is reduced (26). CoCl2 is a well-known
chemical inhibitor of HIF-1α degradation (27). Therefore, CoCl2-induced
HIF-1α accumulation may result in increased expression of CXCR4 and
secretion of VEGF, which is similar to the results obtained
following hypoxia.
The CoCl2-induced increase in CXCR4
expression and VEGF secretion was suppressed by Res in a
dose-dependent manner. The changes in VEGF secretion paralleled the
pattern of CXCR4 expression; therefore, it was hypothesized that
CXCR4 is important in the regulation of VEGF secretion. Recent
studies verified the interaction between CXCR4 and VEGF in several
cell types (28,29). In the present study, Res also
suppressed phosphorylation of NF-κB. Certain studies have found
that NF-κB activation is associated with CXCR4 expression (30,31).
Furthermore, NF-κB was found to be an important component of the
SDF-1/CXCR4 axis (32). NF-κB is a
typical transcription factor that is activated through
phosphorylation (23). A recent
study showed that phosphorylation of NF-κB increases during hypoxia
(33). Therefore, suppression of
NF-κB phosphorylation by Res could cause suppression of CXCR4
expression. It was hypothesized that Res inhibits phosphorylation
of NF-κB leading to suppression of CXCR4 protein expression, which
then affects VEGF secretion. The results of PDTC and AMD3100
treatment supported that. NF-κB was recently shown to be involved
in the regulation of CXCR4 transcription, suggesting that NF-κB
regulates CXCR4 at the mRNA level (34).
SDF-1 and CXCR4 are part of a ligand-receptor axis.
As SDF-1 is a ligand of CXCR4, an increase in SDF-1 induces CXCR4
activity (35). In the present
study, it was found that Res significantly affected the induction
of VEGF secretion and mRNA expression (data not shown) by SDF-1.
Thus demonstrating that Res affects the ligand-receptor interaction
of SDF-1 and CXCR4, through suppression of CXCR4 expression. The
present study suggests that Res may be a useful therapeutic agent
that suppresses VEGF secretion in RPE cells through CXCR4
inhibition, thus reducing neovascularization.
The present study also provided data supporting the
in vitro experiments using the HUVEC tube formation assay to
investigate the effects of Res on retinal neovascularization
induced by VEGF secreted from RPE cells. VEGF secretion by ARPE-19
cells was triggered by hypoxia and conditioned medium obtained from
the treated cells was applied to HUVECs. HUVEC tube formation was
significantly increased by the addition of conditioned media from
ARPE-19 cells with chemically induced hypoxia suggesting that VEGF
secretion from RPE cells substantially affected neovascularization.
Moreover, this effect could be inhibited by co-treatment of the
ARPE-19 cells with Res. Additional studies have shown that Res
effectively inhibits neovascularization in several cancer cell
types (36,37).
In conclusion, an increase in CXCR4 expression
during CoCl2 chemically induced hypoxia in ARPE-19 cells
was confirmed, accompanied by an increase in VEGF secretion. Res
suppression of these responses may be through the inhibition of
CXCR4 and NF-κB, suggesting that Res could be used as a therapeutic
agent to suppress retinal neovascularization by targeting
CXCR4.
Acknowledgments
This study was supported by a special clinical fund
of Gyeongsang National University Hospital in 2008.
References
|
1
|
Neely KA and Gardner TW: Ocular
neovascularization: clarifying complex interactions. Am J Pathol.
153:665–670. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chang JH, Garg NK, Lunde E, Han KY, Jain S
and Azar DT: Corneal neovascularization: An anti-VEGF therapy
review. Surv Opthalmol. 57:415–429. 2012. View Article : Google Scholar
|
|
3
|
Chan EC, van Wijngaarden P, Liu GS, Jiang
F, Peshavariya H and Dusting GJ: Involvement of Nox2 NADPH oxidase
in retinal neovascularization. Invest Ophthalmol Vis Sci.
54:7061–7067. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Engelmann D, Mayoli-Nüssle D, Mayrhofer C,
et al: E2F1 promotes angiogenesis through the VEGF-C/VEGFR-3 axis
in a feedback loop for cooperative induction of PDGF-B. J Mol Cell
Biol. 5:391–403. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ma JF, Von Kalle M, Plautz Q, Xu F-M,
Singh L and Wang L: Relaxin promotes in vitro tumour growth,
invasion and angio-genesis of human Saos-2 osteosarcoma cells by
AKT/VEGF pathway. Eur Rev Med Pharmacol Sci. 17:1345–1350.
2013.PubMed/NCBI
|
|
6
|
Muller YA, Christinger HW, Keyt BA and de
Vos AM: The crystal structure of vascular endothelial growth factor
(VEGF) refined to 1.93 A resolution: multiple copy flexibility and
receptor binding. Structure. 5:1325–1338. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ciulla TA, Danis RP, Criswell M and Pratt
LM: Changing therapeutic paradigms for exudative age-related
macular degeneration: antiangiogenic agents and photodynamic
therapy. Expert Opin Investig Drugs. 8:2173–2182. 1999. View Article : Google Scholar
|
|
8
|
Cheng SF, Dastjerdi MH, Ferrari G, et al:
Short-term topical bevacizumab in the treatment of stable corneal
neovascularization. Am J Ophthalmol. 154:940–948. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Campochiaro PA: Retinal and choroidal
neovascularization. J Cell Physiol. 184:301–310. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu XH, Kirschenbaum A, Yao S, et al:
Upregulation of vascular endothelial growth factor by cobalt
chloride-simulated hypoxia is mediated by persistent induction of
cyclooxygenase-2 in a metastatic human prostate cancer cell line.
Clin Exp Metastasis. 17:687–694. 1999. View Article : Google Scholar
|
|
11
|
Wang Y, Huang L, Yang Y, Xu L, Yang J and
Wu Y: Effects of autocrine vascular endothelial growth factor
(VEGF) in non-small cell lung cancer cell line A549. Mol Biol Rep.
40:3093–3099. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Saint-Geniez M, Kurihara T, Sekiyama E,
Maldonado AE and D’Amore PA: An essential role for RPE-derived
soluble VEGF in the maintenance of the choriocapillaris. Proc Natl
Acad Sci USA. 106:18751–18756. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wei L, Fraser JL, Lu ZY, Hu X and Yu SP:
Transplantation of hypoxia preconditioned bone marrow mesenchymal
stem cells enhances angiogenesis and neurogenesis after cerebral
ischemia in rats. Neurobiol Dis. 46:635–645. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Salcedo R, Wasserman K, Young HA, et al:
Vascular endothelial growth factor and basic fibroblast growth
factor induce expression of CXCR4 on human endothelial cells: In
vivo neovascularization induced by stromal-derived factor-1alpha.
Am J Pathol. 154:1125–1135. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jin F, Brockmeier U, Otterbach F and
Metzen E: New insight into the SDF-1/CXCR4 axis in a breast
carcinoma model: hypoxia-induced endothelial SDF-1 and tumor cell
CXCR4 are required for tumor cell intravasation. Mol Cancer Res.
10:1021–1031. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ozcan Cenksoy P, Oktem M, Erdem O, et al:
A potential novel treatment strategy: Inhibition of angiogenesis
and inflammation by resveratrol for regression of endometriosis in
an experimental rat model. Gynecol Endocrinol. Nov 6–2014.Epub
ahead of print. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li C, Wang L, Huang K and Zheng L:
Endoplasmic reticulum strss in retinal vascular degeneration:
Protective role of resveratrol. Invest Opthalmol Vis Sci.
53:3241–3249. 2012. View Article : Google Scholar
|
|
18
|
Jang M, Cai L, Udeani GO, et al: Cancer
chemopreventive activity of resveratrol, a natural product derived
from grapes. Science. 275:218–220. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
de la Lastra CA and Villegas I:
Resveratrol as an antioxidant and pro-oxidant agent: mechanisms and
clinical implications. Biochem Soc Trans. 35:1156–1160. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Soufi FG, Mohammad-Nejad D and Ahmadieh H:
Resveratrol improves diabetic retinopathy possibly through
oxidative stress-nuclear factor κB-apoptosis pathway. Pharmacol
Rep. 64:1505–1514. 2012. View Article : Google Scholar
|
|
21
|
Huang W, Li G, Qiu J, Gonzalez P and
Challa P: Protective effects of resveratrol in experimental retinal
detachment. PLoS One. 8:e757352013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Vin AP, Hu H, Zhai Y, et al:
Neuroprotective effect of resveratrol prophylaxis on experimental
retinal ischemic injury. Exp Eye Res. 108:72–75. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Busch F, Mobasheri A, Shayan P, Lueders C,
Stahlmann R and Shakibaei M: Resveratrol modulates
interleukin-1β-induced phosphatidylinositol 3-kinase and nuclear
factor κB signaling pathways in human tenocytes. J Biol Chem.
287:38050–38063. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang ZX, Wang YS, Shi YY, et al: Hypoxia
specific SDF-1 expression by retinal pigment epithelium initiates
bone marrow-derived cells to participate in Choroidal
neovascu-larization in a laser-induced mouse model. Curr Eye Res.
36:838–849. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Carbajo-Pescador S, Ordoñez R, Benet M, et
al: Inhibtion of VEGF expression through blockade of Hif1α and
STAT3 signalling mediates the anti-angiogenic effect of melatonin
in HepG2 liver cancer cells. Br J Cancer. 109:83–91. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Semenza GL: HIF-1 and mechanisms of
hypoxia sensing. Curr Opin Cell Biol. 13:167–171. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Piret JP, Mottet D, Raes M and Michiels C:
CoCl2V, a chemical inducer of hypoxia-inducible factor-1
and hypoxia reduce apoptotic cell death in hepatoma cell line
HepG2. Ann N Y Acad Sci. 973:443–447. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jin F, Hagemann N, Schäfer ST, Brockmeier
U, Zechariah A and Hermann DM: SDF-1 restores angiogenesis
synergistically with VEGF upon LDL exposure despite CXCR4
internalization and degradation. Cardiovasc Res. 100:481–491. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sun X, Charbonneau C, Wei L, Yang W, Chen
Q and Terek RM: CXCR4-targeted therapy inhibits VEGF expression and
chon-drosarcoma angiogenesis and metastasis. Mol Cancer Ther.
12:1163–1170. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Manu KA, Shanmugam MK, Rajendran P, et al:
Plumbagin inhibits invasion and migration of breast and gastric
cancer cells by downregulating the expression of chemokine receptor
CXCR4. Mol Cancer. 10:1072011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Manu KA, Shanmugam MK, Ramachandran L, et
al: First evidence that γ-tocotrienol inhibits the growth of human
gastric cancer and chemosensitizes it to capecitabine in a
xenograft mouse model through the modulation of NF-κB pathway. Clin
Cancer Res. 18:2220–2229. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Shanmugam MK, Manu KA, Ong TH, et al:
Inhibition of CXCR4/CXCL12 signaling axis by ursolic acid leads to
suppression of metastasis in transgenic adenocarcinoma of mouse
prostate model. Int J Cancer. 129:1552–1563. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang G, Chen C, Yang R, et al: p55PIK-PI3K
stimulates angiogenesis in colorectal cancer cell by activating
NF-κB pathway. Angiogenesis. 16:561–573. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Arora S, Bhardwaj A, Singh S, et al: An
undesired effect of chemotherapy: gemcitabine promotes pancreatic
cancer cell invasiveness through reactive oxygen species-dependent,
nuclear factor κB- and hypoxia-inducible factor 1α-mediated
up-regulation of CXCR4. J Biol Chem. 288:21197–21207. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Weekes CD, Song D, Arcaroli J, et al:
Stromal cell-derived factor 1α mediates resistance to mTOR-directed
therapy in pancreatic cancer. Neoplasia. 14:690–701. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kunimasa K, Ohta T, Tani H, et al:
Resveratrol derivative-rich melinjo (Gnetum gnemon L.) seed extract
suppresses multiple angiogenesis-related endothelial cell functions
and tumor angiogenesis. Mol Nutr Food Res. 55:1730–1734. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Fouad MA, Agha AM, Merzabani MM and
Shouman SA: Resveratrol inhibits proliferation, angiogenesis and
induces apoptosis in colon cancer cells: calorie restriction is the
force to the cytotoxicity. Hum Exp Toxicol. 32:1067–1080. 2013.
View Article : Google Scholar : PubMed/NCBI
|