Introduction
The epithelial barrier is important in maintaining
homeostasis. Epithelial barrier dysfunction is associated with the
pathogenesis of a number of inflammatory diseases, such as allergic
asthma (1), intestinal allergy
(2), inflammatory bowel diseases
(3) and allergic dermatitis
(4). The causes of epithelial
barrier dysfunction are not fully understood.
The epithelial layer serves as a barrier between the
deep tissue and the external environment. Water and a number of
small molecules, such as nutrients, are able to permeate the
epithelial layer. However, large molecular substances, such as
proteins with strong antigenicity, are prevented from permeating
the epithelial barrier. A number of factors are associated with
epithelial barrier dysfunction, such as psychological stress
(5), neural factors (6) and thermal events (7).
Two permeability pathways that are associated with
epithelial barrier function have been identified. In the
paracellular pathway, substances permeate the paracellular space,
and in the transcellular pathway, substances are endocytosed into
the cytoplasm and degraded by enzymes. A number of factors may
affect the endocytic substance in the cytoplasm. For example,
psychological stress may result in incomplete degradation of
endocytic antigens in the cytoplasm. Subsequently, these antigens
may induce aberrant immune responses, which result in immune
disorders (8).
Dicaine is used as a local anesthetic during eye and
upper airway surgery. Previous reports have suggested that a number
of local anesthetics may exhibit cytotoxic effects on epithelial
cells (9), although whether
dicaine affects epithelial barrier function, remains to be
investigated. Apoptosis-linked gene 2 (ALG-2)-interacting protein X
(Alix) is a ubiquitous adaptor protein initially described for its
capacity to bind to the calcium-binding protein, ALG-2. ALG-2 is
involved in apoptosis initiation (10) and protein-protein binding (11), and it may facilitate infection by
human immunodeficiency virus (12). Decreases in Alix expression levels
may be involved in the pathogenesis of colitis (13). The results of a previous study
suggested that Alix is associated with epithelial barrier function
(14). It is, therefore,
hypothesized that Alix expression enables the maintenance of
healthy epithelial barrier function.
Materials and methods
Reagents
Antibodies (H-270, polyclonal; 1:300) and an Alix
small hairpin RNA (shRNA) kit were purchased from Santa Cruz
Biotechnology Inc. (Shanghai, China). Reagents for quantitative
reverse transcription-polymerase chain reaction (qRT-PCR) were
purchased from Invitrogen Life Technologies (Shanghai, China).
Ovalbumin (OVA) was purchased from Sigma-Aldrich (Shanghai, China).
An OVA ELISA kit was purchased from Antibodies Online (Atlanta, GA,
USA). Molecular cloning reagents were purchased from Invitrogen
Life Technologies. The immune cell isolation kits were purchased
from Miltenyi Biotec, Inc. (Shanghai, China).
Mice
A total of 24 DO11.10 mice (8–10 weeks old) were
purchased from the Xian Experimental Animal Center (Xian, China).
Mice were maintained in a pathogen-free environment. The
experimental procedures using mice were approved by the Ethics
Committee of Wuhan Central Hospital (Wuhan, China).
Cell culture
The RPMI 2650 human airway epithelial cell line
(Rpc) was obtained from the American Type Culture Collection (ATCC,
Manassas, VA, USA). Rpc cells were cultured in Dulbecco’s modified
Eagle’s medium (DMEM; Sigma-Aldrich), supplemented with 10% fetal
bovine serum (Sigma-Aldrich), 100 U/ml penicillin, 0.1 mg/ml
streptomycin and 200 mM L-glutamine at 37°C at 5% CO2
(Sigma-Aldrich). Cells were seeded in Transwells (0.4 μm
pore size; Corning Costar, Corning, NY, USA). Once the cells had
reaching confluence, Rpc cell monolayers were used for subsequent
experiments.
qRT-PCR
Alix mRNA expression was analyzed using qRT-PCR.
Total RNA was extracted using TRIzol® reagent
(Invitrogen Life Technologies). cDNA was synthesized using a
reverse transcription kit (Invitrogen Life Technologies). β-actin
was used as a standard. The following primer sequences were used:
Forward: AAGGAACGTTGGCAAAGGAC and reverse: GAAGGGATGGCAGCATTCAG for
Alix (AF151793), and forward: CGCAAAGACCTGTATGCCAA and reverse:
CACACAGAGTACTTGCGCTC for β-actin (HQ154074). qRT-PCR was performed
using a MiniOpticon qPCR system (Bio-Rad Laboratories, Shanghai,
China) with the SYBR Green Master mix (Invitrogen Life
Technologies). The cycling conditions included 10 min at 95°C
followed by 40 cycles at 95°C for 15 sec, 60°C for 30 sec and 72°C
for 30 sec. The 2−ΔΔCt method was used to calculate
changes in Alix gene expression relative to that of β-actin.
Western blot analysis
Protein extracts (50 μg) from the Rpc cells
were dissolved in a sample buffer, heated for 5 min, separated on a
10% sodium dodecyl sulfate (SDS) polyacrylamide gel (Invitrogen
Life Technologies) and then transferred onto a nitrocellulose
membrane (Invitrogen Life Technologies). The blots were blocked for
1 h in Tris-buffered saline with 0.05% Tween-20® (TBST;
Invitrogen Life Technologies), containing 5% non-fat milk, at room
temperature, and washed three times with TBST. Subsequently, they
were incubated at room temperature for 1 h with an anti-Alix
antibody in TBST, containing 5% non-fat dried milk. The blots were
then washed three times with TBST and incubated for 1 h with
horseradish peroxidase-conjugated rabbit anti-mouse Immunoglobulin
G (cat. no. sc-358920; 1:5,000), at room temperature. After washing
three times with TBST, they were visualized using an enhanced
chemiluminescence detection system (Invitrogen Life Technologies).
The results were recorded using X-ray films. The integrated density
of the immune blots was assessed and quantified using Image J
software (version 5.1; National Institutes of Health, Bethesda, MD,
USA).
Transepithelial electric resistance
(TER)
The TER of the Rpc cell monolayers was measured
using the Millicell ERS apparatus (EMD Millipore, Billerica, MA,
USA) at 0 and 24 h after the addition of dicaine.
Assessment of epithelial monolayer
permeability
Rpc cells were cultured for 2 weeks in Transwell
inserts, until they reached confluence (TER>100
ohm/cm2). Subsequently, OVA (10 μg/ml) was added
to the inserts. Samples were removed from the basal chambers 24 h
later and OVA levels were determined using ELISA.
ELISA
The concentration of OVA in the culture supernatant
was determined by ELISA using a commercial reagent kit according to
the manufacturer’s instructions.
Alix RNA interference (RNAi)
The Alix gene was knocked down in Rpc cells using an
Alix-specific shRNA and a nonspecific shRNA was used as a positive
control, according to the manufacturer’s instructions. Lentiviral
vectors carrying the shRNA of Alix or control vectors were added to
the inserts.
Overexpression of Alix
Genomic DNA was extracted from Rpc cells, and the
Alix gene was amplified by PCR. The PCR product was then cloned
into the pTZ57R/T vector and transformed into Escherichia
coli (TG1 strain; Invitrogen Life Technologies).
In order to subclone Alix into the pcDNA3 plasmid,
the gene was cloned with linkers to join it to the HindIII
and EcoRI sites of pcDNA3, in order to produce the
recombinant eukary-otic expression plasmid, pcAlix. The upstream
primer for the thiol-specific antioxidant antigen gene contains a
HindIII site and the start codon, ATG. The downstream primer
contains an EcoRI site and the stop codon, TAA. Competent
E. coli cells (TG1 strain) were transformed using a ligation
mixture (Invitrogen Life Technologies) and heat shock by placing
the bottom half of the tube into a 42°C water bath for 45 sec. The
plasmid was purified using a plasmid extraction kit (Invitrogen
Life Technologies) according to the manufacturer’s instructions and
then sequenced. The purified PCR products were sequenced using the
BigDye Terminator kit (Invitrogen Life Technologies) and separated
by capillary electrophoresis (Sequencer model 3730XL; Applied
Biosystems, Foster City, CA, USA). The plasmid recovered from the
recombinant bacterial colony was sequenced by Jier Biotech
(Guangzhou, China), in order to confirm the presence of the Alix
gene.
Rpc cells were grown to 60–70% confluence in 5%
CO2 in DMEM, at 37°C. The cells were washed using a
serum-free medium. Transfection was performed using a transfection
kit (Invitrogen Life Technologies) according to the manufacturer’s
instructions. The cells were incubated overnight, at 37°C.
Serum-free DMEM medium was mixed with the Genejuice reagent
(Invitrogen Life Technologies) and incubated for 10 min at 37°C.
Subsequently, the recombinant plasmid was added to the mixture and
incubated for 15 min at 37°C. FCS (Sigma-Aldrich) was added to the
DMEM medium, which was then added to the cells and incubated
overnight, at 37°C.
Isolation of immune cells
Dendritic cells (DCs) and CD4+
CD25− T effector cells (Teff cells) were isolated from
DO11.10 mouse spleen using commercial reagent kits (Miltenyi
Biotec, Shanghai, China), according to the manufacturer’s
instructions. Isolated cells were analyzed using flow cytometry;
the level of purity was >96%. The cells were then cultured in
RPMI-1640 medium (Sigma-Aldrich) for subsequent experiments.
Assessment of Teff cell
proliferation
Isolated OVA-specific Teff cells [labeled with
carboxyfluorescein diacetatesuccinimidyl ester (CFSE)] were
cultured, for 3 days, in the presence of DCs (T cell:DC ratio =
5:1), in the culture supernatant collected from the basal chambers
of transwells (containing OVA transported across the Rpc
monolayers). Cells were then collected and analyzed using flow
cytometry.
Statistical analysis
The data are presented as the mean ± standard
deviation. Differences between groups were assessed using analysis
of variance. P<0.05 was considered to indicate a statistically
significant difference.
Results
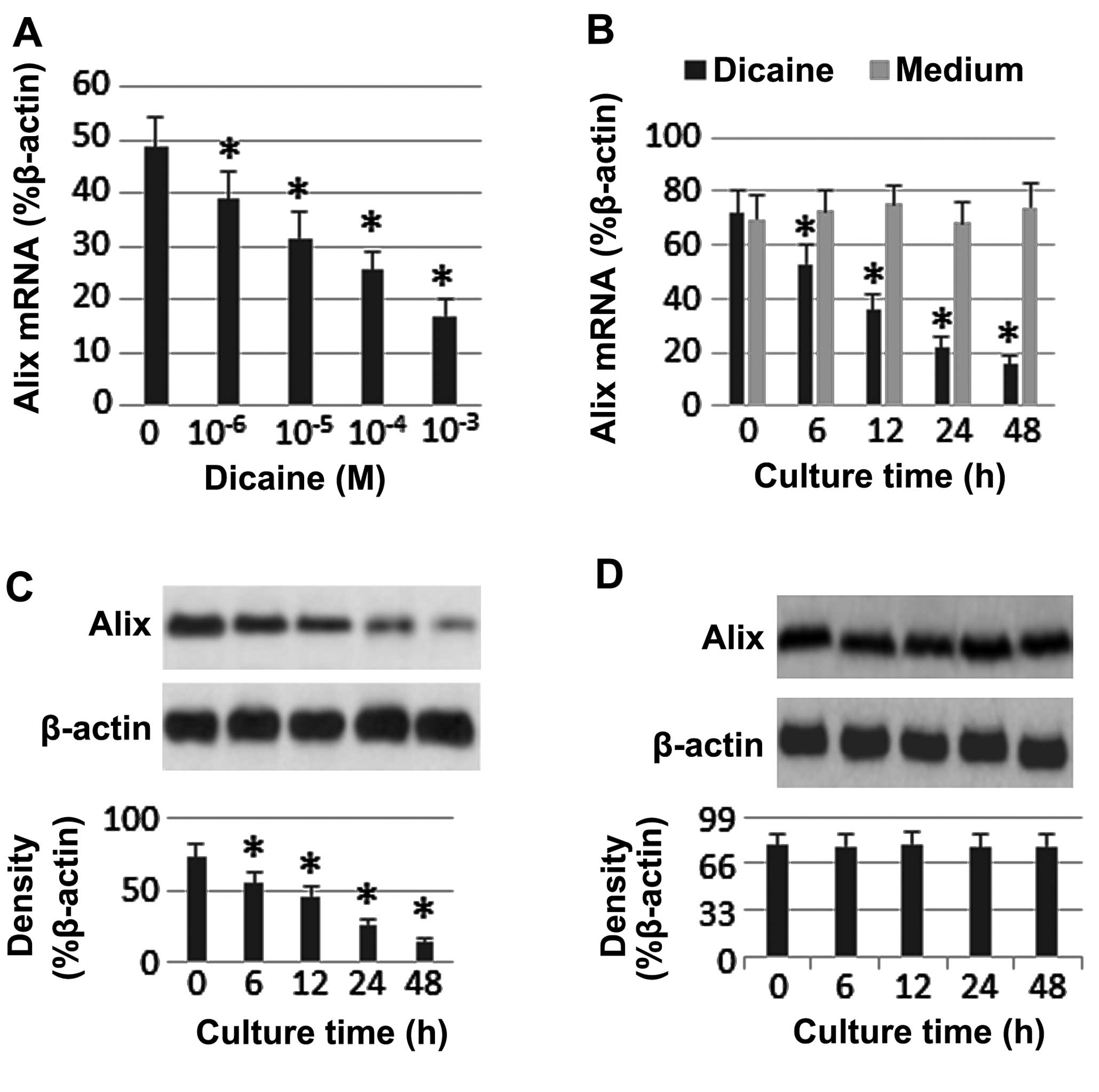
Dicaine suppresses Alix expression in
airway epithelial cells
In the present study, the effect of dicaine on the
expression of Alix in Rpc cells was assessed. Alix mRNA and protein
expression was detected in the Rpc cells. Dicaine treatment led to
a decrease in Alix expression levels in a time-dependent manner
(Fig. 1).
Alix is associated with the
dicaine-induced upregulation of airway epithelial barrier
permeability
The effects of dicaine and Alix expression on
epithelial barrier functions were assessed. Rpc cell monolayers
were treated with different concentrations of dicaine for 24 h. The
results suggested that treatment with dicaine did not affect the
TER in the Rpc monolayers. However, treatment with dicaine led to
an increase in the level of permeability to OVA in the Rpc
monolayers, in a dose-dependent manner. Alix expression was
subsequently knocked down in the Rpc cells, using RNAi. Following
Alix expression knock-down, no difference was observed between the
level of permeability to OVA in the Rpc monolayers treated with
dicaine, compared with that in the control monolayers. By contrast,
overexpression of Alix led to a marked increase in the level of
permeability to OVA, and no difference in TER, in Rpc monolayers
treated with Rpc, compared with the control monolayers, (Fig. 2). The results of the present study
indicated that Alix expression may increase the transcellular
permeability in Rpc monolayers.
 | Figure 2Assessment of Rpc cell monolayer
barrier function. Rpc cell monolayers were treated with dicaine in
Transwells with at different concentrations for 24 h. (A) TER of
Rpc monolayers. (B) Levels of OVA in the basal chambers of
transwells. a, Alix-knockdown Rpc monolayers; b, rpc monolayers
treated with control shRNA; c, rpcs with Alix overexpression; d,
rpcs treated with control plasmids. *P<0.01, compared
with group 0. (C) Alix gene. (D) Alix over-expression. The data
represent three separate experiments. Rpc cells, RPMI2650 human
airway epithelial cell line; Alix, apoptosis-linked gene
2-interacting protein X; TER, transepithelial electric resistance;
OVA, ovalbumin; shRNA, small hairpin RNA. |
Dicaine compromises antigen degradation
by airway epithelial cells
The antigenicity of OVA in dicaine-treated Rpc
monolayers was measured. Teff cells were isolated from DO11.10
mouse spleens. In the dicaine-treated group 34.3% Teff cell
proliferation was observed, whereas in the control group only 6.5%
Teff cell proliferation was observed (P<0.01; Fig. 3). These results indicated that
antigens maintained strong antigenicity and were more capable of
permeating the dicaine-treated Rpc monolayers, compared with
monolayers that had not been treated with dicaine.
Discussion
The results of the present study suggest that
dicaine, a local anesthetic, may compromise epithelial barrier
functions. Using Rpc monolayers as an in vitro epithelial
barrier model, the current study demonstrated that dicaine
treatment may lead to an increase in Alix expression in Rpc cells.
Treatment with dicaine caused an increase in the transcellular
permeability of the Rpc monolayers, and this effect was reversed
following Alix gene knock-down. Alix gene overexpression
compromised epithelial barrier function and led to an increase in
the capability of antigens to permeate the Rpc monolayer barrier.
These antigens were shown to induce antigen-specific T cell
proliferation.
Epithelial barrier dysfunction is a common
pathological condition that is observed in a number of types of
inflammatory disorders. However, the pathogenesis of epithelial
barrier dysfunction remains poorly understood. Yang et al
(15) reported that CD23
expression leads to an increase in specific antigen transcellular
transport across the epithelial barrier, which interferes with the
efficiency of antigen degradation in the cytoplasm (16,17).
The results of the present study are in accordance with previous
studies (18,19) and suggest that dicaine may affect
the epithelial barrier function by increasing the transcellular
transport. Yang et al (18)
demonstrated that stress-induced intestinal epithelial barrier
dysfunction induced typical intestinal T helper 2 type
inflammation. Saatian et al (19) indicated that Th2-cytokine
expression induces epithelial barrier dysfunction, which may
contribute to airway inflammation in allergic asthma.
In the present study, dicaine treatment led to the
upregulation of Alix expression in Rpc cells. Alix expression has
multiple functions, such as facilitating apoptosis by activating
caspase 9 (10) and interacting
with endosomal sorting complexes required for transport (20). The results of the present study
indicated that dicaine treatment leads to an increase in the
expression of Alix in Rpc cells, which increases the level of
transcellular permeability. The antigens transported across the Rpc
monolayers maintained strong levels of antigenicity. This
observation indicates that the endocytic antigens were not degraded
after permeating the Rpc cytoplasm. Alix over-expression induces
caspase activation and cell apoptosis (21). Furthermore, Alix expression is
involved in the endolysosomal system, including in regulating
endosomal trafficking (21). The
results of the present study, therefore, suggest that Alix
expression may compromise the process of endocytic antigen
degradation within the cytoplasm. The mechanisms underlying this
process require further investigation.
In conclusion, the present study demonstrates that
dicaine treatment increases the expression of Alix in Rpc cells.
Alix expression increases the transcellular permeability of Rpc
monolayers. The transported antigens maintain their
antigenicity.
References
|
1
|
Leino MS, Loxham M, Blume C, et al:
Barrier disrupting effects of alternaria alternata extract on
bronchial epithelium from asthmatic donors. PLoS One. 8:e712782013.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Suzuki T: Regulation of intestinal
epithelial permeability by tight junctions. Cell Mol Life Sci.
70:631–659. 2013. View Article : Google Scholar
|
|
3
|
McCole DF: Regulation of epithelial
barrier function by the inflammatory bowel disease candidate gene,
PTPN2. Ann NY Acad Sci. 1257:108–114. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sevilla LM, Latorre V, Sanchis A and Pérez
P: Epidermal inactivation of the glucocorticoid receptor triggers
skin barrier defects and cutaneous inflammation. J Invest Dermatol.
133:361–370. 2013. View Article : Google Scholar
|
|
5
|
Yu Y, Liu ZQ, Liu XY, et al:
Stress-derived corticotropin releasing factor breaches epithelial
endotoxin tolerance. PLoS One. 8:e657602013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zheng PY, Feng BS, Oluwole C, et al:
Psychological stress induces eosinophils to produce corticotrophin
releasing hormone in the intestine. Gut. 58:1473–1479. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu T, Wang BQ, Wang CS and Yang PC:
Concurrent exposure to thermal stress and oral Ag induces
intestinal sensitization in the mouse by a mechanism of regulation
of IL-12 expression. Immunol Cell Biol. 84:430–439. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yang PC, Jury J, Söderholm JD, et al:
Chronic psychological stress in rats induces intestinal
sensitization to luminal antigens. Am J Pathol. 168:104–114. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kobayashi K, Ohno S, Uchida S, Amano O,
Sakagami H and Nagasaka H: Cytotoxicity and type of cell death
induced by local anesthetics in human oral normal and tumor cells.
Anticancer Res. 32:2925–2933. 2012.PubMed/NCBI
|
|
10
|
Strappazzon F, Torch S, Chatellard-Causse
C, et al: Alix is involved in caspase 9 activation during
calcium-induced apoptosis. Biochem Biophys Res Commun. 397:64–69.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fisher RD, Chung HY, Zhai Q, et al:
Structural and biochemical studies of ALIX/AIP1 and its role in
retrovirus budding. Cell. 128:841–852. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhai Q, Fisher RD, Chung HY, et al:
Structural and functional studies of ALIX interactions with YPXnL
late domains of HIV-1 and EIAV. Nat Struct Mol Biol. 15:43–49.
2008. View
Article : Google Scholar
|
|
13
|
Hoffmann M, Kim SC, Sartor RB and Haller
D: Enterococcus faecalis strains differentially regulate Alix/AIP1
protein expression and ERK 1/2 activation in intestinal epithelial
cells in the context of chronic experimental colitis. J Proteome
Res. 8:1183–1192. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yan H, Yi H, Xia L, et al: Staphylococcal
enterotoxin B suppresses Alix and compromises intestinal epithelial
barrier functions. J Biomed Sci. 21:292014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yang PC, Berin MC, Yu LC, et al: Enhanced
intestinal transepithelial antigen transport in allergic rats is
mediated by IgE and CD23 (FcepsilonRII). J Clin Invest.
106:879–886. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yu LC, Yang PC, Berin MC, et al: Enhanced
transepithelial antigen transport in intestine of allergic mice is
mediated by IgE/CD23 and regulated by interleukin-4.
Gastroenterology. 121:370–381. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yu LC, Montagnac G, Yang PC, et al:
Intestinal epithelial CD23 mediates enhanced antigen transport in
allergy: evidence for novel splice forms. Am J Physiol Gastrointest
Liver Physiol. 285:G223–G234. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yang PC, Jury J, Söderholm JD, Sherman PM,
McKay DM and Perdue MH: Chronic psychological stress in rats
induces intestinal sensitization to luminal antigens. Am J Pathol.
168:104–114. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Saatian B, Rezaee F, Desando S, Emo J,
Chapman T, Knowlden S and Georas SN: Interleukin-4 and
interleukin-13 cause barrier dysfunction in human airway epithelial
cells. Tissue Barriers. 1:e243332013. View Article : Google Scholar
|
|
20
|
Mahul-Mellier AL, Strappazzon F,
Chatellard-Causse C, Blot B, Béal D, Torch S, Hemming F, Petiot A,
Verna JM, Fraboulet S and Sadoul R: Alix and ALG-2 make a link
between endosomes and neuronal death. Biochem Soc Trans.
37:200–203. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Trioulier Y, Torch S, Blot B, Cristina N,
Chatellard-Causse C, Verna JM and Sadoul R: Alix, a protein
regulating endosomal trafficking, is involved in neuronal death. J
Biol Chem. 279:2046–2052. 2004. View Article : Google Scholar
|