Introduction
Non-small-cell lung cancer (NSCLC) is the leading
cause of cancer-related mortality worldwide (1). Lung adenocarcinoma is the most common
form of NSCLC (1,2). Owing to its complex tumorigenesis,
lung adenocarcinoma is difficult to treat and its course in
individual patients is hard to predict. In order to identify
therapeutic targets and prognostic biomarkers, research on lung
adenocarcinoma has focused on a number of key molecules, in
particular, certain growth factor receptors, such as epidermal
growth factor receptor and insulin-like growth factor 1 receptor
(3,4). Recent studies have reported that
binding of estrogen to estrogen receptors (ERs) in NSCLC may affect
progression of this disease, thus offering a potential target for
treatment (5,6).
Estrogens regulate a number of biological processes,
including cell differentiation and cell proliferation, by binding
to two receptors, ERα and ERβ. It has been postulated that the
latter may be the key estrogen receptor in NSCLC progression
(7,8). Recent studies have shown that ERβ has
a number of subtypes (5,9). In a recent study, we found that
overexpression of ERβ2 was observed in NSCLC cell lines, and may
indicate the earlier stage of tumor development in prostate cancer
progression (10). However, the
mechanism by which ERβ2 influences NSCLC progression remains
unclear. A number of the most recent studies suggest that p38MAPK
signaling may be an important mediator of the effect of ERβ2 on
NSCLC (11,12). Further research is required in
order to investigate the mechanism by which ER activates nuclear
transcription of certain genes.
The biological functions of human interleukin 12
(IL-12) are known to be mediated by the IL-12 receptor (IL-12R),
which is composed of β1 and β2 subunits, that possess high affinity
and responsiveness to IL-12. The β2 subunit is hypothesized to be
the primary molecule involved in IL-12 signal transduction, and may
function as a tumor suppressor protein (13,14).
A number of studies have investigated the importance of IL-12Rβ2 in
lung adenocarcinoma (15,16). IL-12Rβ2-deficient mice were shown
to develop lung adenocarcinoma with a poor prognosis. However, the
mechanism through which IL-12Rβ2 influences NSCLC progression is
unclear. Other studies (17–22)
have shown that IL-12 activates downstream molecules via binding to
IL-12R. These molecules include p38MAPK, indicating that there may
be an interaction between p38MAPK and IL-12R. A recent study
provided further evidence that IL-12Rβ2 and ERβ2 are co-expressed
in NSCLC (22).
Despite evidence demonstrating a correlation between
IL-12Rβ2 and ERβ2 expression in NSCLC, the mechanisms by which
these molecules affect progression of this disease remains obscure.
This study aimed to explore the association between IL-12Rβ2 and
ERβ2 in vitro, and to investigate whether p38MAPK affects
the expression of IL-12Rβ2
Materials and methods
Cell lines and reagents
The human NSCLC cell lines (A549, LTEP-a2 and H358)
and human normal bronchial epithelial cells (HBE, and NC004) were
obtained from the Shanghai Institute of Cell Biology (Shanghai,
China). They were cultured in RPMI-1640 medium (Invitrogen Life
Technologies, Carlsbad, CA, USA) supplemented with 10% fetal bovine
serum (FBS) at 37̊C in a humidified atmosphere of 5%
CO2. Cells were cultured in a phenol-red free medium
supplemented with 5% charcoal stripped FBS (Sangon Biotech Co.,
Ltd, Shanghai, China) for at least 18 h prior to transfection. The
ERβ2-specific mouse anti-human monoclonal antibody (57/3; Serotec,
Kidlington, UK) and p38MAPK-specific rabbit anti-human monoclonal
antibody (4511; Cell Signaling Technology Inc., Danvers, MA USA)
were conserved in our laboratory. The p38MAPK inhibitor (SB203580)
was purchased from Calbiochem (San Diego, CA, USA).
Plasmid construction and
transfection
ERβ2-, p38MAPK- and IL-12Rβ2- (9,14)
expressing plasmids (p3xFlag-ERβ2, p3XFlag-p38MAPK and
pXJ40-Myc-IL-12Rβ2) were produced through the ligation of
polymerase chain reaction (PCR)-generated inserts into
p3xFlag-CMV-7.1–2 or pXJ40-Myc-SOX4, as appropriate. The small
hairpin (sh) IL-12Rβ2 plasmids were purchased from the national
RNAi core Facility located at the Institute of Molecular
Biology/Genomic Research Center (Academia Sinica, Taipei,
China).
The purified p3xFlag- ERβ2/p38MAPK and p
XJ40-Myc-IL-12Rβ2 plasmids were transfected into 70% confluent
A549, LTEP-a2 and H358 cells, using Lipofectamine® 2000
(Invitrogen Life Technologies) reagents in a total volume of 1 ml
of Opti-MEM (Invitrogen Life Technologies), as described in a
previous study (23). A549,
LTEP-a2 and H358 cells were co-transfected with 1 mg
p3xFlag-p38MAPK, pXJ40-Myc-IL-12Rβ2, p3xFlag-empty or
pXJ40-Myc-empty plasmids.
Cell proliferation and Transwell invasion
assays
After transfection (12 h), cells were seeded in
triplicate at a density of 5×103 cells per 96-well
plates. The following day, cells were treated with 10 mM estrogen
(E2) dissolved in ethanol. At 24, 48 and 72 h after E2 treatment,
10 μl of a modified
3-(4,5-dimethylthiazol-2-yl)2,5-diphenyl-tetrazolium bromide
solution (MTT; Dojindo, Kumamoto, Japan) was added to the culture
and reaction mixtures were incubated at 37°C for 2 h. In order to
detect migration, cells were placed in transwell chambers (Forma™;
Thermo Fisher Scientific, Waltham, MA, USA) at 2×104
cells/well. The lower transwell chamber contained 10% FBS as a
chemoattractant. For the invasion assay, the bottom of the culture
inserts (8-mm pores) were coated with 30 μl of the mixture
containing serum-free RPMI-1640 and Matrigel™ (1:8; BD Biosciences,
Bedford, MA, USA). This was allowed to solidify at 37°C overnight.
After 24 h, cells that had migrated or invaded through the membrane
were fixed with 95% alcohol and stained with crystal violet. The
number of migrated or invaded cells was quantified by counting five
independent symmetrical visual fields under an Olympus BX51
microscope at 200× magnification (Olympus Corp., Tokyo, Japan).
Scratch wound-healing assay
Cells were seeded onto six-well tissue culture
dishes (4×106 cells/well) and grown to 95% confluence.
Each confluent monolayer was wounded linearly using a 200 μl
pipette tip and washed three times with phosphate-buffered saline
(PBS). Thereafter, cell morphology and movement was observed and
photographed at 200× magnification [Olympus E-P5 (14–42mm II R)
Olympus Corp.] at 0, 12 and 24 h.
Immuocytochemical detection
NSCLC cells not treated with E2 were initially fixed
in 1.5% agarose and incubated for 15 min at room temperature (RT).
Sections (3 μm) from the paraffin block were placed on to
adhesive-coated slides. In a heated antigen retrieval process
(24), the slides were placed in
an EDTA buffer (pH 8.0) and heated for 2 min in a steamer. The
slides were incubated overnight at 4°C with monoclonal mouse
anti-human anti-ERβ2 (Serotec) and polyclonal goat anti-human
anti-IL-12 Rβ2 (Santa Cruz Biotechnology, Inc., Dallas, TX, USA)
antibodies (25) in bovine serum
albumin prior to incubating with the secondary antibodies rabbit
anti-mouse immunoglobulin G (IgG) k-chain specific antibody
(SAB3701212; 1:100, Sigma-Aldrich) or rabbit anti-goat IgG F(ab’)2
(SAB3700244; 1:100; Sigma-Aldrich) at RT for 20 min. Color was
developed in 3,3′-diaminobenzidine (DAB) solution for 10 min
followed by counterstaining with Harris hematoxylin. Cells were
dehydrated, coverslipped and reviewed under an Olympus BX51 light
microscope (400×; Olympus Corp.), and the mean percentage of ERβ2
and IL-12 Rβ2 positive cells was counted in 10 high power fields in
each group.
Western blot analysis
Cells were homogenized in a radioimmunoprecipitation
assay (RIPA) buffer containing 10% protease inhibitor
(Sigma-Aldrich, St. Louis, MO, USA), and protein concentrations
were then quantified using a BioRad protein assay (Bio-Rad
Laboratories, Hercules, CA, USA). Equal quantities of proteins were
separated by sodium dodecyl sulfate-polyacrylamide gel
electrophoresis (SDS-PAGE) and transferred to polyvinylidene
fluoride membranes (Millipore, Bedford, MA, USA). Primary goat
anti-human polyclonal anti-IL-12Rβ2 antibodies (E-20; Santa Cruz
Biotechnology Inc.) were then applied to the membranes according to
the manufacturer’s instructions. The membranes were washed and
treated with the appropriate horseradish peroxidase-conjugated
secondary antibodies (rabbit anti-goat IgG F(ab’)2; 1:100;
Sigma-Aldrich). A similar process was conducted for β-actin using
the mouse monoclonal anti-actin antibody (A3854; Sigma-Aldrich).
The results were visualized with chemiluminescence (West Dura;
CPS160; Sigma-Aldrich).
Semiquantitative reverse transcription
(RT-qPCR) analysis
RNA was prepared with TRIzol (Life Technologies,
Rockville, MD, USA). For semiquantitative RT-qPCR, complementary
(c)DNA was reverse-transcribed from total RNA with oligo(dT)16
primers and murine leukemia virus reverse transcriptase
(PerkinElmer, Wellesley, MA, USA). The quantities of cDNA were
adjusted by quantifying the level of actin DNA. Then, the same
quantities of cDNA normalized to the actin were amplified for
IL-12Rβ2, using the following primers: Forward:
5′-ATCCATGCGCCTGCTAAC-3′ and reverse:
5′-GAGTGTTTGAGAGGCCTTTTCTG-3′.
Co-immunoprecipitation
After transfection (12 h), each group of cells was
treated with mock (ethanol) for 24 h. Protein (500 μg) from
the cell lysates was incubated with 2 μg anti-Myc antibody
or normal rabbit IgG (Santa Cruz Biotechnology Inc.) for 16 h at
4°C. To each sample, 20 μl of protein A/G-agarose beads was
added (Santa Cruz Biotechnology), incubated for 1 h and washed
three times with RIPA buffer. Then, the complex was resolved on 10%
SDS-PAGE, transferred to the membrane and blotted with anti-Flag (1
μg/ml, Sigma-Aldrich) or anti-Myc antibody. Membranes were
incubated with enhanced chemiluminescence reagent (Super Signal
West Pico; Pierce, Rockford, IL, USA) and exposed to
autoradiographic film (Kodak, Rochester, NY, USA).
Statistical analysis
Data are expressed as the mean ± standard deviation.
The quantitative data were analyzed using Student’s t-test or
one-way analysis of variance. All statistical tests were two-sided.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Expression of ERβ2 and IL-12Rβ2 is
increased in NSCLC
To investigate the association between ERβ2 and
IL-12Rβ2, their expression and distribution was analyzed with
immunocytochemical technology. ERβ2 was predominantly found in the
cytoplasm and the nucleus in all three NSCLC cell lines as shown in
Fig. 1A. IL-12β2 expression was
largely confined to the nucleus. Compared with the normal bronchial
cell line, the percentage of cells positive for ERβ2 and IL-12 Rβ2
was increased by 82.23% and 82.0%, respectively (Fig. 1B), demonstrating that ERβ2 and
IL-12Rβ2 protein are overexpressed in NSCLC cell lines, which is in
agreement with previous studies (5,8,16,22).
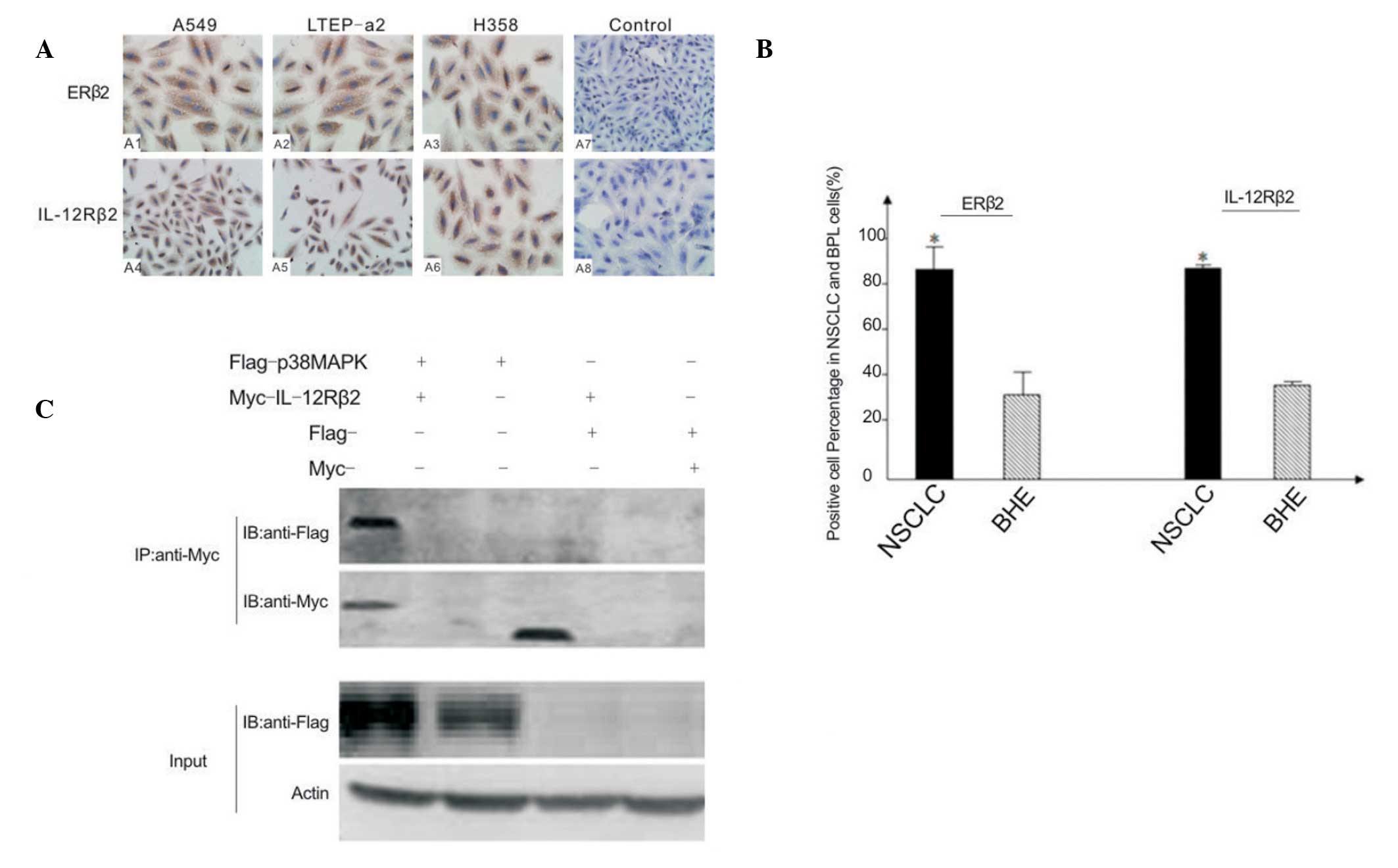 | Figure 1(A) Expression of ERβ2 and IL-12Rβ2 in
human NSCLC cell lines A549, LTEP-a2 and H358, and human normal
bronchial epithelial cells (control). Transfected cells were
incubated with antibodies against ERβ2 and IL-12Rβ2 overnight. (B)
Quantification of immunocytochemical detection of ERβ2 and
IL-12Rβ2. The mean percentage of ERβ2 and IL-12Rβ2 positive cells
in the NSCLC and BHE groups were counted in 10 high power fields in
each group. *P<0.05 compared with BHE. (C) Coimmunoprecipitation
assay. Input rows indicate the positive control (IgG as negative
control, not shown). Via construction of Flag-tagged p38MAPK and
Myc-tagged IL-12Rβ2, coimmunoprecipitation confirmed that there was
an interaction between p38MAPK and IL-12Rβ2 specifically. ERβ2,
estrogen receptor-β2; IL-12Rβ2, interleukin-12 receptor-β2; NSCLC,
non-small-cell lung cancer, BHE, bronchial (human) epithelium;
p38MAPK, p38 mitogen-activating protein kinase; IL-12Rβ2,
interleukin-12 receptorβ2. |
ERβ2 regulates IL-12Rβ2 expression via
p38MAPK signaling
To investigate the roles and mechanism of ERβ2
further, the correlation between ERβ2 and IL-12Rβ2 was
investigated. In the co-immunoprecipitation assay, certain proteins
were constructed and groups were set, i.e., Flag-p38MAPK and
Myc-IL-12Rβ2. Through the analyses, an interaction between p38MAPK
and IL-12Rβ2, rather than a mixture, was observed, indicated due to
the fact that the interaction molecular weight was slightly greater
than the sum of their weights (Fig.
1C).
Western blotting and RT-qPCR analysis were performed
to assess IL-12Rβ2 expression in the three NSCLC cell lines, each
containing p3x-ERβ2, p3x-38MAPK, p3x-ERβ2+ SB203580, blank or empty
vectors. Expression of the IL-12Rβ2 protein was increased in the
p3x-ERβ2 and p3x-p38MAPK groups compared with the other groups
(Fig. 2A and B). Fig. 2B shows that there was a significant
increase in IL-12Rβ2 gene expression in the p3x-ERβ2 and
p3x-38MAPK-containing cells compared with p3x-ERβ2+ SB203580-,
blank- or empty vector-containing cells. This suggests that p38MAPK
is required to enable the upregulation of IL-12Rβ2 by ERβ2.
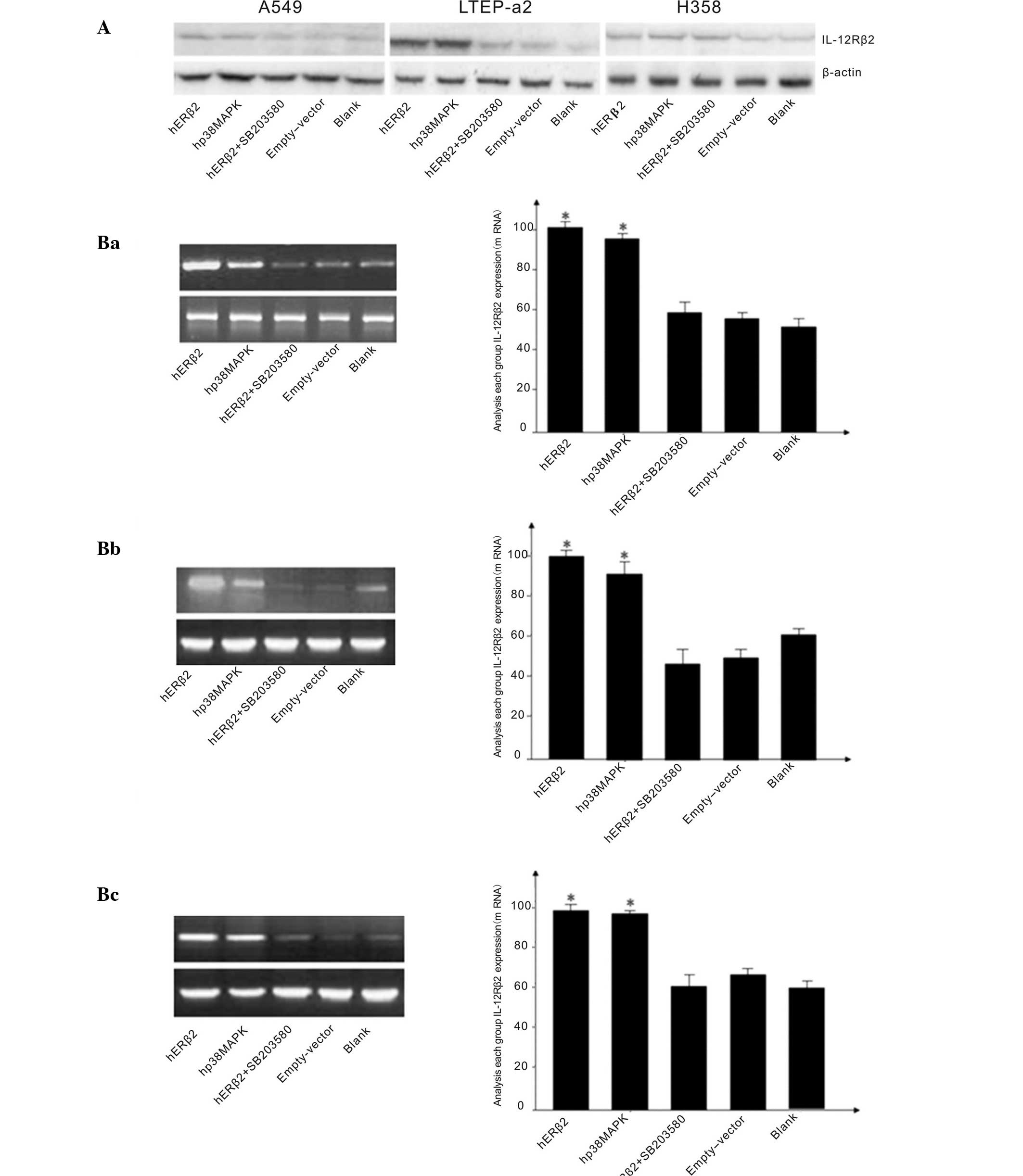 | Figure 2(A) Western blotting showing
expression of IL-12Rβ2 in human NSCLC cell lines A549, LTEP-a2 and
H358, with β-actin as a control. Expression shown for cells
containing p3x-ERβ2, p3x-p38MAPK, psx-ERβ2+SB203580, blank and
empty vectors. (B) Levels of IL-12Rβ2 expression in the NSCLC cell
lines with the vectors indicated. (Ba) cell line A549, (Bb) cell
line LTEP-a2, (Bc) cell line H358. Levels of IL-12Rβ2 cDNA were
quantified using semiquantitative reverse transcription-polymerase
chain reaction. *P<0.05 vs. empty-vector control
group. IL-12Rβ2, interleukin-12 receptorβ2; p38MAPK, p38
mitogen-activating protein kinase; ERβ2, estrogen receptor β2;
SB203580, p38MAPK inhibitor; NSCLC, non-small-cell lung cancer. |
ERβ2 inhibits NSCLC progression via
IL-12Rβ2
There has been controversy over the role of ERβ2 in
certain cancers, for example a number of studies have reported that
E2 may promote lung adenocarcinoma development as well as
insulin-like growth factor type-1 (3,4,7,8).
This study demonstrated a potentially protective effect of ERβ2 in
NSCLC, but showed that this effect did not persist, and indeed, was
reversed, in the absence of IL-12Rβ2. An MTT assay was used to
detect cell proliferation in each group. It was found that compared
with the blank group, cell proliferation in the p3x-ERβ2 and
pXJ40-IL-12Rβ2 groups was reduced. This effect was only apparent
after 48 h, and persisted at 72 h. However, there was only minimal
inhibition of proliferation in the p3x-ERβ2 and pXJ40-IL-12Rβ2
groups compared with the blank and empty vector groups at 24 h
(Fig. 3B). Notably, an opposite
effect was observed in the p3x-ERβ2+sh-IL-12Rβ2 group, where
proliferation was observed to increase significantly compared with
the blank and empty vector groups.
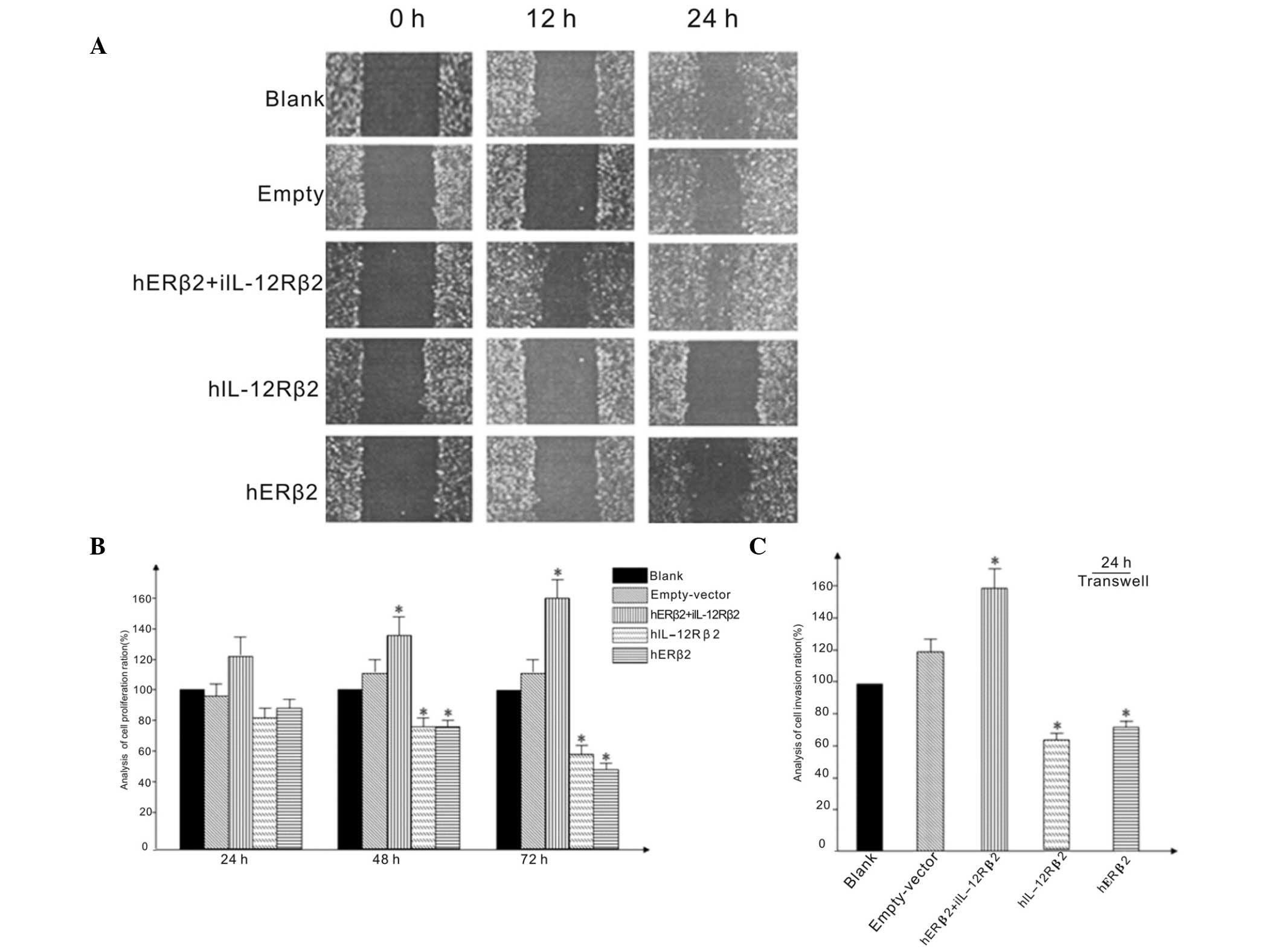 | Figure 3(A) Scratch wound-healing assay.
Effect of altering levels of expression of ERβ2 and IL-12Rβ2 in
NSCLC cell lines. Cell layers were wounded with a pipette tip, and
morphology and movement was noted and photographed at 0, 12 and 24
h. (B) Levels of cell proliferation at 24, 48 and 72 h in NSCLC
cell lines transfected with hERβ2+IL-12Rβ2, hIL-12Rβ2, hERβ2, blank
and empty vectors. Cell proliferation was measured using a
3-(4,5-dimethylthiazol-2-yl) 2,5-diphenyl-tetrazolium bromide
assay. (C) Extent of cell migration in cell transfected with the
plasmids noted. Transwell invasion assay was used to assess cell
invasiveness. Numbers of migrated cells were counted in five fields
in each group. *P<0.05 vs. empty-vector control
group. ERβ2, estrogen receptorβ2; IL-12Rβ2, interleukin-12
receptorβ2; NSCLC, non-small-cell lung cancer. |
The transwell assay was used to measure invasiveness
of the cell lines. Invasiveness was observed to be significantly
reduced in the p3x-ERβ2 and pXJ40-IL-12Rβ2 groups compared with the
p3x-ERβ2+sh-IL-12Rβ2, blank and empty vector groups (Fig. 3C). By contrast, the
p3x-ERβ2+sh-IL-12Rβ2 12Rβ2 group showed greater invasiveness
compared with the blank and empty vector groups. This suggests that
ERβ2/IL-12Rβ2 signaling may be important in regulating the
progression of NSCLC.
Discussion
Estrogen is a hormone secreted predominantly by the
ovaries to promote the development of the female reproductive
system and the proliferation of the endometrium as part of the
menstrual cycle (26,27). The biological effect of estrogen is
achieved through binding to ERs, which are comprised of two
subtypes, ERα and ERβ. Through them, E2 activates downstream
molecules, such as MAPK (28). In
certain studies (9,10,11),
ERβ has been shown to be a key protein involved in multiple
functions, including as a ligand (E2, or specific estrogen receptor
β agonists), activation of ERβ (ERβ1, 2 or 5), regulation of
nuclear proteins (AF-1/2, SRC-1, NF-κB, CyclinE and c-Myc) and
expression via MAPK signaling (6,9,10).
The ERβ isoform has been extensively investigated in certain types
of cancer, including lung and breast cancer. Studies have
demonstrated that there are three ERβ isoforms that are
overexpressed in NSCLC, and ERβ2 appears to be particularly
important (22,10). The correlation between p38MAPK and
IL-12R was investigated in other studies (21,22,29–31),
which demonstrated that IL-12 induces the activation of certain
downstream molecules and that its functions are mediated through
IL-12R, which is known to be activated by ERK and p38MAPK. ERβ2 and
IL-12Rβ2 appear to be co-expressed in NSCLC tissue (22).
Immunocytochemical technology identified that ERβ2
and IL-12Rβ2 are overexpressed in NSCLC cell lines compared with a
normal bronchial epithelia tissue cell group. This is in agreement
with previous studies (22). In
order to investigate the ERβ2 mechanism and the correlation between
them, certain interfering protein expression methods were used. The
present study showed that high expression of ERβ2 or IL-12Rβ2
protein led to a significant reduction in NSCLC cell proliferation
and invasiveness. Furthermore, when IL-12Rβ2 protein expression was
eliminated and ERβ2 expression remained high this effect was
reversed, such that proliferation and invasiveness were
significantly increased compared with the blank and empty vector
groups. These results suggest that ERβ2 may alter NSCLC progression
via its effect on IL-12Rβ2. Through the observation and analysis of
the results, further assays were performed. The interaction between
IL-12Rβ2 and p38MAPK was confirmed. In addition, IL-12Rβ2
expression appeared to be correlated with p38MAPK expression, such
that the effect of ERβ2 on the upregulation of IL-12Rβ2 was
inhibited by administration of a p38MAPK inhibitor.
The present study demonstrated that ERβ2 may
regulate downstream molecules via p38MAPK signaling, one of which
may be IL-12Rβ2. Further studies are required to elucidate the
details of the interaction between p38MAPK and IL-Rβ2 and identify
other molecules involved in this pathway in the context of NSCLC.
The results suggest that ERβ2 acts via p38MAPK/IL-12Rβ2 signaling,
which may indicate that the co-expression of IL-12Rβ2 and ERβ2 may
be associated with a more favorable prognosis.
In conclusion, the current study provides support
for further research into the role of ERβ2 in NSCLC, including the
correlation between ERβ2 and IL-12Rβ2, and further investigation
into the importance of p38MAPK in this interaction.
Acknowledgments
The authors would like to thank Mr. He-Xiao Tang
(Department of Thoracic Surgery, Tongji Hospital, affiliated to
Tongji Medical College of Hua Zhong University of Science and
Technology) who provided partial data, and Dr Chun-Hua Su
(Department of Thoracic Surgery, The First Hospital of Sun-Yat sen
University) who conducted the statistical analysis.
References
|
1
|
Pietras RJ, Márquez DC, Chen HW, et al:
Estrogen and growth factor receptor interactions in human breast
and non-small cell lung cancer cells. Steroids. 70:372–381. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Stabile LP, Davis AL, Gubish CT, et al:
Human non-small cell lung tumors and cells derived from normal lung
express both estrogen receptor alpha and beta and show biological
responses to estrogen. Cancer Res. 62:2141–2150. 2002.PubMed/NCBI
|
|
3
|
Rikova K, Guo A, Zeng Q, et al: Global
survey of phosphotyrosine signaling identifies oncogenic kinases in
lung cancer. Cell. 131:1190–1203. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Orlova A, Hofström C, Strand J, et al:
[99mTc(CO)3]+-(HE)3-ZIGF1R:4551, a new Affibody conjugate for
visualization of insulin-like growth factor-1 receptor expression
in malignant tumours. Eur J Nucl Med Mol Imaging. 40:439–449. 2013.
View Article : Google Scholar
|
|
5
|
Nose N, Uramoto H, Iwata T, et al:
Expression of estrogen receptor beta predicts a clinical response
and longer progression-free survival after treatment with EGFR-TKI
for adenocarcinoma of the lung. Lung Cancer. 71:350–355. 2011.
View Article : Google Scholar
|
|
6
|
Torres-Arzayus MI, Zhao J, Bronson R and
Brown M: Estrogen-dependent and estrogen-independent mechanisms
contribute to AIB1-mediated tumor formation. Cancer Res.
70:4102–4111. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Davydov MI, Bogush TA, Polotskiĭ BE and
Tiuliandin SA: Estrogen receptors beta - new target in cellular
lung cancer treatment. Vestn Ross Akad Med Nauk. 2:16–22. 2012.In
Russian.
|
|
8
|
Nose N, Sugio K, Oyama T, et al:
Association between estrogen receptor-beta expression and epidermal
growth factor receptor mutation in the postoperative prognosis of
adenocarcinoma of the lung. J Clin Oncol. 27:411–417. 2009.
View Article : Google Scholar
|
|
9
|
Dey P, Jonsson P, Hartman J, et al:
Estrogen receptors β1 and β2 have opposing roles in regulating
proliferation and bone metastasis genes in the prostate cancer cell
line PC3. Mol Endocrinol. 26:1991–2003. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu Z, Liao Y, Tang H and Chen G: The
expression of estrogen receptors β2, 5 identifies and is associated
with Prognosis in non-small cell lung cancer. Endocrine.
44:517–524. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
King AE, Collins F, Klonisch T, et al: An
additive interaction between the NFkappaB and estrogen receptor
signalling pathways in human endometrial epithelial cells. Hum
Reprod. 25:510–518. 2010. View Article : Google Scholar :
|
|
12
|
Duan R, Ginsburg E and Vonderhaar BK:
Estrogen stimulates transcription from the human prolactin distal
promoter through AP1 and estrogen responsive elements in T47D human
breast cancer cells. Mol Cell Endocrinol. 281:9–18. 2008.
View Article : Google Scholar
|
|
13
|
Airoldi I, Cocco C, Di Carlo E, et al:
Methylation of the IL-12Rbeta2 gene as novel tumor escape mechanism
for pediatric B-acute lymphoblastic leukemia cells. Cancer Res.
66:3978–3980. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Airoldi I, Di Carlo E, Banelli B, et al:
The IL-12Rbeta2 gene functions as a tumor suppressor in human B
cell malignancies. J Clin Invest. 113:1651–1659. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Airoldi I, Di Carlo E, Cocco C, et al:
IL-12 can target human lung adenocarcinoma cells and normal
bronchial epithelial cells surrounding tumor lesions. PLoS One.
4:e61192009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Suzuki M, Iizasa T, Nakajima T, et al:
Aberrant methylation of IL-12Rbeta2 gene in lung adenocarcinoma
cells is associated with unfavorable prognosis. Ann Surg Oncol.
14:2636–2642. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li H, Cao MY, Lee Y, et al: Virulizin, a
novel immunotherapy agent, activates NK cells through induction of
IL-12 expression in macrophages. Cancer Immunol Immunother.
54:1115–1126. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lecocq M, Detry B, Guisset A and Pilette
C: FcαRI-mediated inhibition of IL-12 production and priming by
IFN-γ of human monocytes and dendritic cells. J Immunol.
190:2362–2371. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kontoyiannis D, Kotlyarov A, Carballo E,
et al: Interleukin-10 targets p38 MAPK to modulate ARE-dependent
TNF mRNA translation and limit intestinal pathology. EMBO J.
20:3760–3770. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fahmi A, Smart N, Punn A, et al:
P42/p44-MAPK and PI3K are sufficient for IL-6 family
cytokines/gp130 to signal to hypertrophy and survival in
cardiomyocytes in the absence of JAK/STAT activation. Cell Signal.
25:898–909. 2013. View Article : Google Scholar :
|
|
21
|
Korhonen R, Huotari N, Hömmö T, et al: The
expression of interleukin-12 is increased by MAP kinase
phosphatase-1 through a mechanism related to interferon regulatory
factor 1. Mol Immunol. 51:219–226. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu ZG, Lei YY, Li WW and Chen ZG: The
co-expression of ERβ2 and IL-12Rβ2 is better prognostic factor in
non-small-cell lung cancer progression. Med Oncol. 30:5922013.
View Article : Google Scholar
|
|
23
|
Fauconnier M, Palomo J, Bourigault ML, et
al: IL-12Rβ2 is essential for the development of experimental
cerebral malaria. J Immunol. 188:1905–1914. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xu J, Zhou M, Quyang J, et al: Gambogic
acid induces mitocheondria-dependent apoptosis by modulation of
Bcl-2 and Bax in mantle cell lymphoma JeKo-1 Cells. Chin J Cancer
Res. 25:183–191. 2013.PubMed/NCBI
|
|
25
|
Pan SH, Chao YC, Hung PF, et al: The
ability of LCRMP-1 to promote cancer invasion by enhancing
filopodia formation is antagonized by CRMP-1. J Clin Invest.
121:3189–3205. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Drummond AE, Britt KL, Dyson M, et al:
Ovarian steroid receptors and their role in ovarian function. Mol
Cell Endocrinol. 191:27–33. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yager JD and Davidson NE: Estrogen
carcinogenesis in breast cancer. N Engl J Med. 354:270–282. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hua S, Kittler R and White KP: Genomic
antagonism between retinoic acid and estrogen signaling in breast
cancer. Cell. 137:1259–1271. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kondadasula SV, Roda JM, Parihar R, et al:
Colocalization of the IL-12 receptor and FcgammaRIIIa to natural
killer cell lipid rafts leads to activation of ERK and enhanced
production of interferon-gamma. Blood. 111:4173–4183. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jana M, Dasgupta S, Pal U and Pahan K:
IL-12 p40 homodimer, the so-called biologically inactive molecule,
induces nitric oxide synthase in microglia via IL-12R beta 1. Glia.
57:1553–1565. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li X, Chaudry IH and Choudhry MA: ERK and
not p38 pathway is required for IL-12 restoration of T cell IL-2
and IFN-gamma in a rodent model of alcohol intoxication and burn
injury. J Immunol. 183:3955–3962. 2009. View Article : Google Scholar : PubMed/NCBI
|

















