Introduction
Chronic progressive neurological diseases, or
neurodegenerative diseases, including Parkinson’s, Alzheimer’s and
Huntington’s diseases, are characterized by irreversible loss of
neuronal function in specific areas, apoptosis and death. Previous
studies have demonstrated that neurodegenerative diseases are
inextricably correlated with neuronal inflammation, such as tumor
necrosis factor-α (TNF-α)-induced neuronal injury (1,2). At
present, in spite of the progress which previous studies have made
in the development of therapeutic strategies for neurodegenerative
diseases (3,4), it is necessary to further elucidate
the underlying pathogenesis and to develop improved treatments.
Bone marrow stromal cells (BMSCs) are derived from
the mesoderm and are able to undergo self-renewal and
differentiation (5). Previous
studies have demonstrated that BMSCs have the potential to
differentiate into neuronal cells and that transplanted BMSCs are
able to promote neuronal regeneration (6–8).
However, the mechanisms of BMSCs in inflammatory factor-induced
neuronal cell injuries remains to be fully elucidated.
In the present study, apoptosis was induced in PC12
cells by TNF-α. PC12 cells were co-cultured with BMSCs using
Transwell chambers in order to investigate whether BMSCs protect
PC12 cells against TNF-α stimulation. Furthermore, the present
study aimed to elucidate the effects of co-culture with BMSCs on
the TNF receptor (TNFR)/caspase signaling pathway, which is
associated with cell viability.
Materials and methods
Isolation and culture of BMSCs
Three Sprague-Dawley (SD) rats (mean body weight,
150 g; 4–6 weeks-old) were purchased from the Animal Center of
China Medical University (Shenyang, China). Animal experiments in
the present study were approved by the ethical committee of China
Medical University. SD rats were anesthetized via intraperitoneal
injection of 10% chloral hydrate (Sinopharm Chemical Reagent Co.,
Ltd., Shanghai, China; 3.5 ml/kg body weight). Whole bone marrow
was isolated from SD rats, as described previously (9), and cells were isolated and seeded in
a 25-cm2 plastic bottle at a concentration of
106 cells/ml in low-glucose Dulbecco’s modified Eagle’s
medium (DMEM; GE Healthcare Life Sciences, Little Chalfont, UK)
containing 20% fetal bovine serum (FBS; GE Healthcare Life
Sciences). When adherent cells had reached 80% confluence, they
were detached using 0.2% trypsin (GE Healthcare Life Sciences) and
re-plated at a 1:2 ratio for continued passaging. Cells from
passage 3 were cultured in low-glucose DMEM containing 5% FBS.
BMSC identification
Cells were seeded onto sterilized cover slips,
washed three times with phosphate-buffered saline (PBS; GE
Healthcare Life Sciences) and fixed in 4% formaldehyde (Sinopharm
Chemical Reagent Co., Ltd.) for 15–20 min. Subsequent to washing
three times for 2 min in PBS, the cells were blocked with 1% bovine
serum albumin (Beijing Solarbio Science & Technology Co., Ltd.,
Beijing, China) for 30 min. Following blocking, DAPI (1:1,000;
Roche Diagnostics, Basel, Switzerland) and fluorescently labeled
mouse monoclonal antibodies: anti-CD44 (1:100; Cell Signaling
Technology, Inc., Danvers, MA, USA) and anti-CD45 (1:50; BD
Biosciences, Franklin Lakes, NJ, USA) were added. The samples were
subsequently analyzed using a fluorescence microscope (LX70;
Olympus Corporation, Tokyo, Japan).
Cell culture
Neuronal PC12 cells were purchased from the Cell
Bank of Chinese Academy of Sciences (Shanghai, China). PC12 cells
were cultured in RPMI 1640 medium (GE Healthcare Life Sciences)
containing 10% horse serum (Beijing Solarbio Science &
Technology Co., Ltd.) and 5% FBS.
Cell treatment
PC12 cells were divided into three groups: i)
Negative control group (NC), normal cultured PC12 cells; ii)
positive control group (PC), PC12 cells treated with 50 ng/ml TNF-α
(Peprotech, Inc., Rocky Hill, NJ, USA) for 2 h, then removal of
supernatant and addition of fresh serum-free DMEM for 24 h; iii)
co-culture group (co-culture), PC12 cells co-cultured with BMSCs
using Transwell chambers (BD Biosciences), addition of 50 ng/ml
TNF-α for 2 h, removal of the supernatant and addition of fresh
serum-free medium for 24 h.
Cell proliferation assays
Cell proliferation was monitored using the MTT cell
proliferation assay. In brief, PC12 cells and PC12 cells that had
been co-cultured with BMSCs were transferred to 96-well plates and
seeded at a density of 5,000 cells (100 μl)/well with eight
repeats for each group. Twenty hours later, 10 μl MTT (5
mg/ml) was added to each well. Following 4-h incubation at 37°C,
the medium was removed and dimethyl sulfoxide (Beijing Solarbio
Science & Technology Co., Ltd.) was added in order to dissolve
the formazan crystals. Subsequently, the absorbance was measured at
a wavelength of 570 nm with an ELISA microplate reader (Sunrise™;
Tecan Group Ltd., Männedorf, Switzerland). The percentage of cell
proliferation was calculated according to the following formula:
Survival rate (%) = {[treated group optical density
(OD)570 − blank group OD570]/(control group
OD570 − blank group OD570)} ×100%.
Apoptosis analysis
The Annexin V-fluorescein isothiocyanate (FITC)
Apoptosis Detection kit (Nanjing KeyGen Biotech. Co., Ltd.,
Nanjing, China) was used to assess the apoptosis of PC12 cells in
each group. PC12 cells were harvested subsequent to digestion with
trypsin, washed twice with PBS and then re-suspended in 300
μl Annexin V-FITC binding buffer. Cells were incubated with
10 μl Annexin V-FITC for 10 min and 5 μl propidium
iodide (PI) for 5 min. Finally, 200 μl binding buffer was
added and the samples were incubated for 15 min at room temperature
in the dark and then subjected to flow cytometric analysis (BD
FACSCalibur; BD Biosciences).
Analysis of morphological
alterations
PC12 cells were harvested following digestion with
0.25% trypsin, washed once with PBS and then fixed with 4%
glutaraldehyde (Sinopharm Chemical Reagent Co., Ltd.) for 12 h.
Subsequent to washing three times with PBS at 4°C, the samples were
fixed with 1% osmium tetroxide (J&K Scientific Ltd., Beijing,
China) for 15–30 min. Subsequently, the samples were dehydrated
using acetone (Sinopharm Chemical Reagent Co., Ltd.) and embedded
in epon resin. Following cutting semi-thin sections (50 nm) and
staining with lead citrate (J&K Scientific Ltd., Beijing,
China) and uranyl acetate (Micxy Reagent, Chengdu, China), the
sections were analyzed by transmission electron microscopy (TEM;
H-7100; Hitachi Ltd., Tokyo, Japan).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was isolated using TRIzol (Takara
Biotechnology, Co., Ltd., Dalian, China) according to the
manufacturer’s instructions. The concentration and purity of RNA
were determined by detecting the absorbance at wavelengths at 260
and 280 nm (NANO 2000; Thermo Fisher Scientific, Waltham, MA, USA).
An equal amount of RNA was reverse transcribed to synthesize
complementary DNA (cDNA) using a PrimeScript RT reagent kit (Takara
Biotechnology, Co., Ltd.). RT-qPCR was conducted using SYBR-Green
(Takara Biotechnology, Co., Ltd.) on a LightCycler 480 PCR system
(Roche Diagnostics). The sequences of the primers are presented in
Table I. β-actin was used as the
endogenous RNA control to normalize samples. Data were analyzed by
the comparative threshold cycle method.
 | Table IOligonucleotide primer sets for
reverse transcription-quantitative polymerase chain reaction. |
Table I
Oligonucleotide primer sets for
reverse transcription-quantitative polymerase chain reaction.
| Gene symbol | Sequence
(5′–3′) | Product
size
(bp) |
|---|
| TNFR1-F |
CGGGCTTACTGGATACGA | 143 |
| TNFR1-R |
GCAACGCTGGTGAATGAA | |
| TNFR2-F |
CACCTGTCTCGTCCTACCT | 333 |
| TNFR2-R |
AACAACTGGGCTCCTCTAA | |
| β-actin-F |
CGTGCGTGACATTAAAGAG | 132 |
| β-actin-R |
TTGCCGATAGTGATGACCT | |
Western blot analysis
Cells were washed with cold PBS and subsequently
harvested using lysis buffer (Beyotime Institute of Biotechnology,
Haimen, China). Following incubation on ice for 40 min, the
supernatant was centrifuged at 12,000 × g for 20 min at 4°C and the
concentrations of these protein samples were determined using the
Lowry method (10). Subsequently,
proteins were denatured in sample buffer (Beijing Solarbio Science
& Technology Co., Ltd.) for 5 min at 100°C. Equal amounts of
proteins were loaded onto each lane of 8%SDS-PAGE gel and were then
transferred onto polyvinylidene fluoride membranes (EMD Millipore,
Billerica, MA, USA). The membranes were blocked with 5% non-fat dry
milk (Yili, Inner Mongolia, China) in Tris-buffered saline (TBS)
and then incubated with rabbit polyclonal anti-caspase-8 (1:400;
BioVision, Inc., Milpitas, CA, USA) and mouse monoclonal β-actin
(1:10,000; Kangchen, Shanghai, China) antibodies at 4°C overnight.
Subsequent to washing with TBS supplemented with 0.1% Tween-20, the
membranes were incubated with secondary antibodies (1:2,000) for 2
h. Densitometric analysis was conducted with ChemiImager 5500
version 2.03 (ProteinSimple, Santa Clara, CA, USA).
Statistical analysis
Values are presented as the mean ± standard
deviation. Data were analyzed using a one-way analysis of variance.
All statistical analyses were conducted using SPSS version 13.0
software (SPSS, Inc., Chicago, IL, USA). P<0.05 was considered
to indicate a statistically significant difference.
Results
Culture and identification of BMSCs
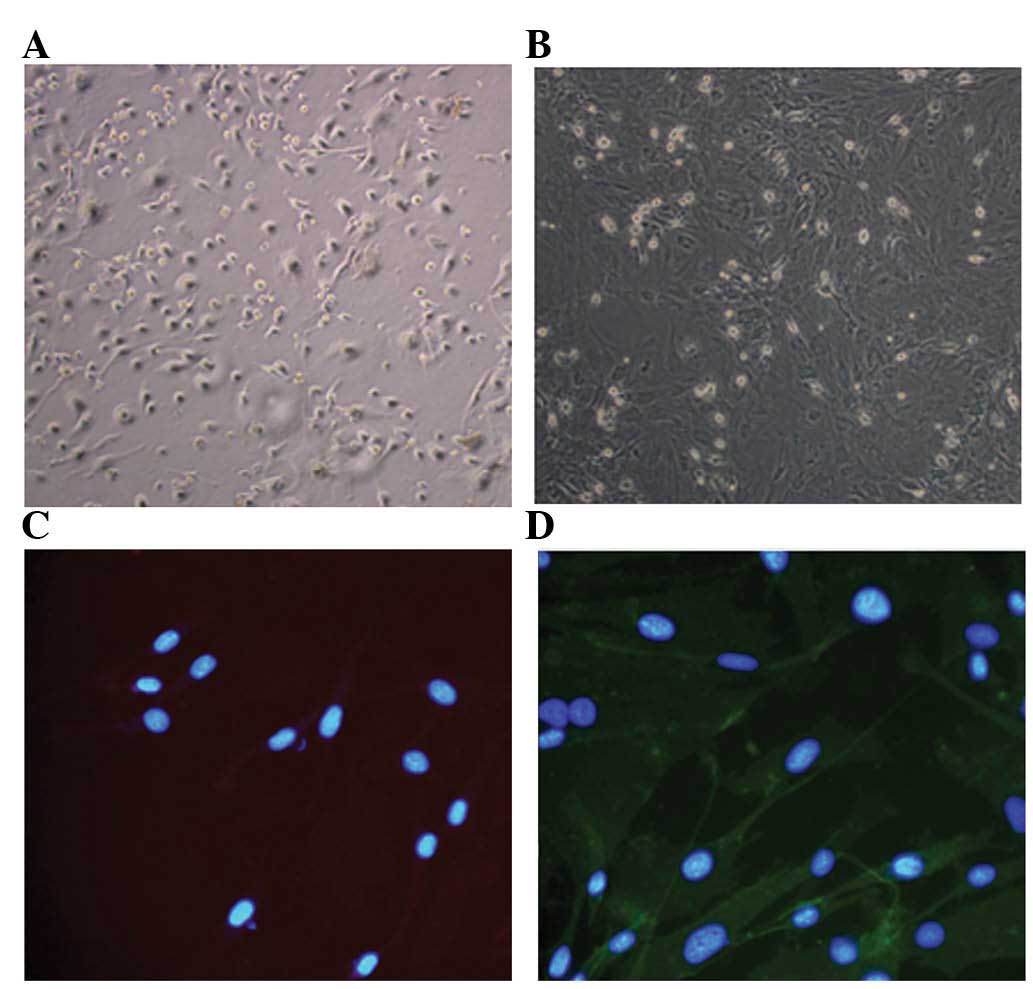
Isolation and primary culture of BMSCs were
performed as described. BMSCs appeared circular under the
microscope and grew efficiently in the medium at the second day
(Fig. 1A). Subsequent to passage
cultures, the BMSCs appeared to have a shuttle- and fibroblast-like
morphology (Fig. 1B). To assess
the purity of the isolated cells, immunofluorescence staining was
conducted and the results demonstrated that the majority of cells
were CD45-negative and CD44-positive (Fig. 1C and D). It was observed that the
BMSCs obtained in the present study were highly pure.
BMSCs reverse the TNF-α-induced decrease
in PC12 cell survival rate
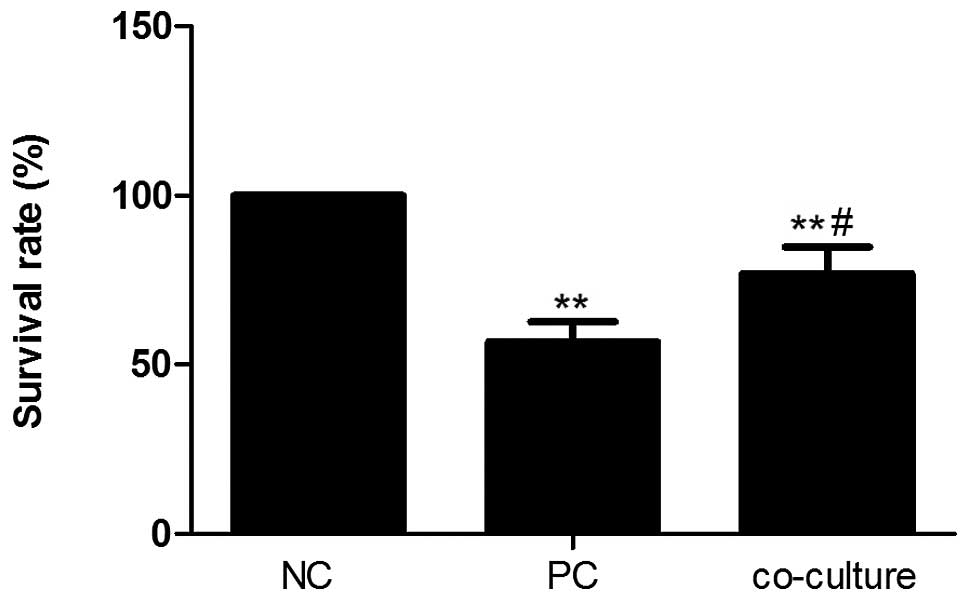
To assess the effect of BMSCs on the viability of
PC12 cells treated with TNF-α, the proliferation of PC12 cells in
each group was assessed using an MTT assay. As presented in
Fig. 2, TNF-α treatment
significantly reduced the proliferation of PC12 cells (P<0.01)
and the previous low survival rate of PC12 cells was significantly
improved by co-culture with BMSCs (56.71 and 76.86% in the PC and
co-culture groups, respectively; P<0.05). These results
suggested that BMSCs significantly increased the low survival rate
of PC12 cells stimulated with TNF-α.
BMSCs inhibit TNF-α-induced apoptosis of
PC12 cells
Cell apoptosis is known to be a key factor in the
pathogenesis of neurodegenerative diseases. As cell proliferation
was observed, an Annexin V/PI (AV/PI) staining method was used
followed by flow cytometric analysis. The results demonstrated that
TNF-α induced significant apoptosis in PC12 cells and BMSCs
(P<0.001), notably suppressing apoptosis in PC12 cells (Fig. 3A). The percentage of apoptotic
cells was 3.49% in the control group, whereas it was 40.74 and
16.97% in the PC and co-culture groups, respectively. Furthermore,
TEM was used to detect the morphological alterations in PC12 cells
in each group. Fig. 3B illustrates
that the normal PC12 cells were round, containing predominantly
euchromatin in the cell nucleus. In the PC group, PC12 cells
exhibited TNF-α-induced ultrastructural alterations that were
characteristic of apoptosis, including cytoplasmic vacuolation and
chromosome condensation at the periphery of the nucleus. These
morphological markers of apoptosis were largely abolished by
treatment with BMSCs. These observations suggested that
TNF-α-induced apoptosis in PC12 cells was diminished by co-culture
with BMSCs.
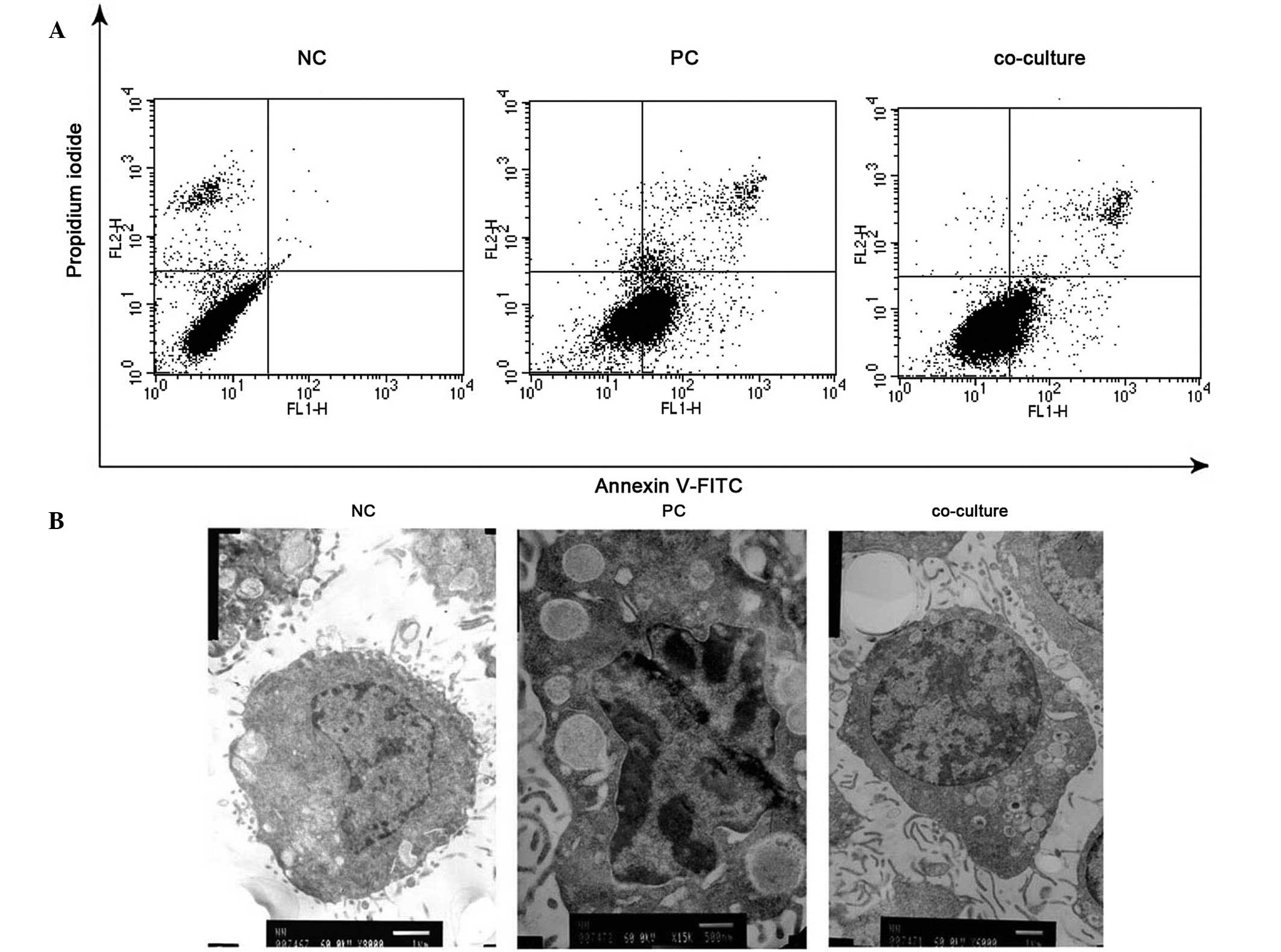 | Figure 3Effect of BMSCs on PC12 cell apoptosis
induced by TNF-α. (A) AV/PI staining for assessment of apoptosis.
PC12 cells were harvested, stained with AV/PI and then subjected to
flow cytometric analysis. (B) TEM for analysis of morphological
alterations. PC12 cells were harvested, fixed with 1% osmium
tetroxide, stained with lead citrate and uranyl acetate and then
examined by TEM (NC, magnification ×8,000; PC, magnification
×15,000; co-culture, magnification ×6,000). BMSCs, bone marrow
stromal cells; TNF-α, tumor necrosis factor-α; AV/PI, Annexin
V/propidium iodide; TEM, transmission electron microscopy; NC,
negative control; PC, positive control. |
BMSCs interact with PC12 cells via the
TNFR/caspase signaling pathway
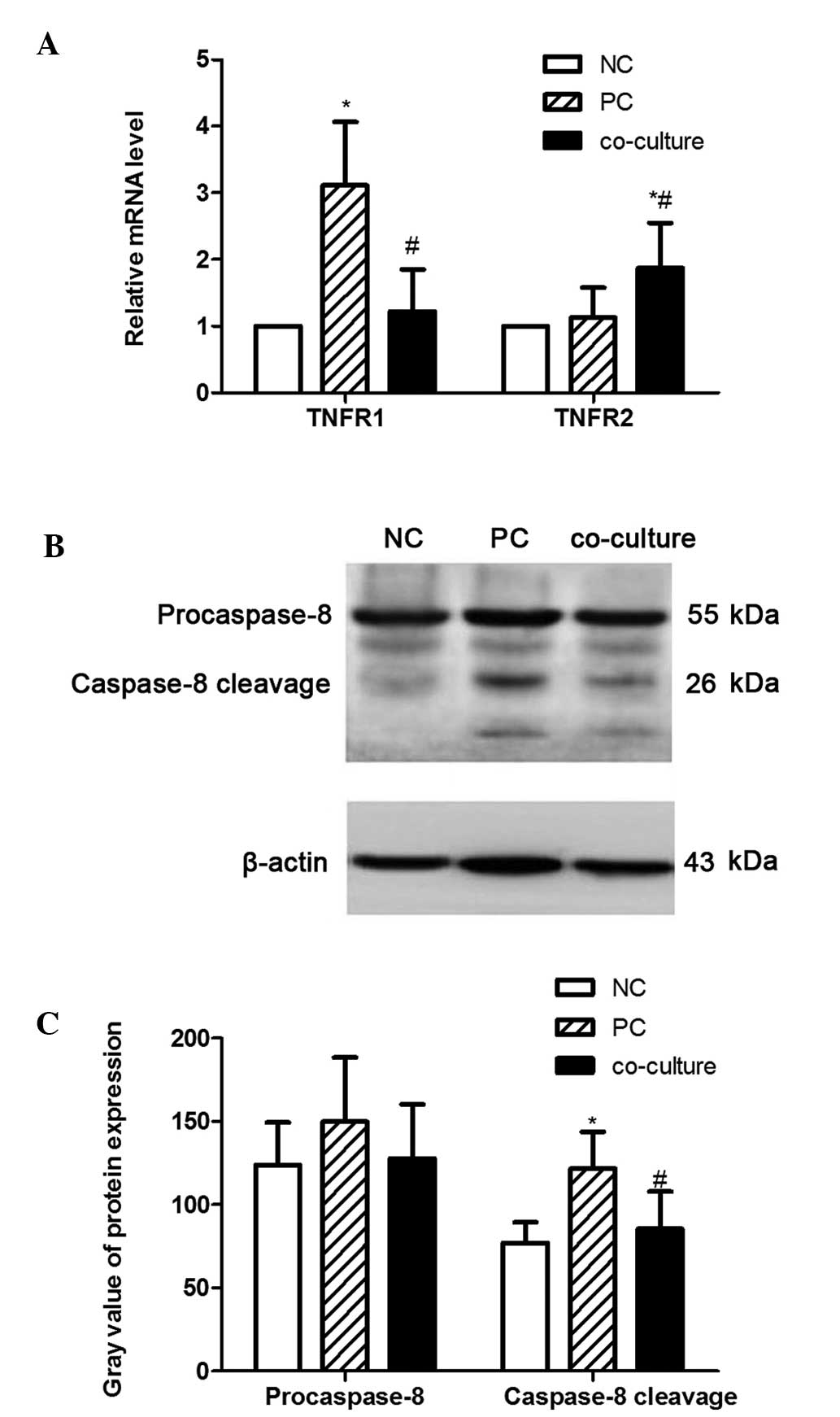
To investigate the signaling pathway activated by
BMSCs in PC12 cells, the expression levels of TNFR1, TNFR2 and
caspase-8 were examined. As presented in Fig. 4A, the mRNA levels of TNFR1 were
significantly increased in the PC group compared with those in the
NC group (P<0.05). Of note, this induction of TNFR1 mRNA
expression by TNF-α was significantly inhibited in the presence of
BMSCs (P<0.05). However, the mRNA levels of TNFR2 were observed
to be affected in a different way to those of TNFR1, as they
increased in the presence of BMSCs. Furthermore, treatment with
TNF-α significantly increased the levels of caspase-8 protein
expression, which was markedly attenuated following co-incubation
with BMSCs (Fig. 4B and C). These
results suggested that the TNFR/caspase signaling pathway was
involved in the alteration of cell apoptosis induced by TNF-α.
Discussion
The slow and progressive loss and dysfunction of
axons and neurons is commonly observed in the central nervous
system (CNS) during the pathogenesis of neurodegenerative diseases
(11). PC12 cells are commonly
used in models of neuronal differentiation in neuropharmacological
and neurophysiological studies (12). PC12 is a cell line derived from a
pheochromocytoma of the rat adrenal medulla and has similar
characteristics to those of neuroendocrine cells. Although neuronal
degeneration predominantly starts with specific neuronal
populations, there are various similarities between different
neurodegenerative diseases. These include atypical protein
oligomerization and assemblies in addition to induced cell
apoptosis and death. In addition, previous studies indicated that
inflammatory cytokines, such as TNF-α, may be associated with
neuronal cell death and neurodegenerative disorders (13). Furthermore, TNF-α has been shown to
be associated with glutamate-induced neuronal cell death (14), and is able to induce neurotoxicity
through glutamate production (15). In the present study, the function
of BMSCs in proliferation and apoptosis of PC12 cells treated with
TNF-α was investigated. The results indicated that BMSCs inhibited
the TNF-α-induced low survival rate and apoptosis of PC12 cells.
Furthermore, it was suggested that the TNFR/caspase signaling
pathway was involved in these processes.
BMSCs are important candidates for therapeutic use
in the repair of injured tissue, as they possess multilineage
differentiation potential (16).
The primary cultured BMSCs were isolated from SD rats in the
present study according to the method of a previous study (9). Subcultured BMSCs exhibited a shuttle-
and fibroblast-like shape. In addition, immunofluorescence results
demonstrated that the BMSCs expressed CD44 but not CD45, which is
in agreement with a previous study by Herzog et al (17), and the BMSCs obtained were
considered to be highly pure. Previous studies have described the
effects of BMSCs on neurodegenerative diseases; for example, Koh
et al (18) demonstrated
that the functional deficiency of BMSCs in patients with amytrophic
lateral sclerosis is proportional to the rate of disease
progression. Sun et al (19) identified that BMSCs induced by
tricyclodecane-9-yl-xanthogenate differentiated into cholinergic
neuron-like cells, which were able to promote functional recovery
and neural protein following spinal cord injury. In addition,
Pastor et al (20) reported
that glial cell line-derived neurotrophic factor expression was
upregulated in the spinal cords of mice treated with BMSCs. These
studies indicated that BMSCs not only differentiate into
neuron-like cells, but also secrete neurotrophic factor and create
a suitable environment for neural cell survival.
Through inflammatory signals from the CNS and
through removal of neurotoxic proteins and damaged tissue, the CNS
returns to its normative state; however, neuroinflammation which is
not inhibited and repaired may lead to cellular dysfunction and a
reduction in neuronal dendritic branching, and result in
neurodegenerative disease (21,22).
To assess the potential role of BMSCs in regulating the number of
PC12 cells induced by TNF-α, an MTT assay was performed in order to
detect the proliferation of PC12 cells in each group. The analysis
demonstrated that TNF-α inhibited the proliferation of PC12 cells
and resulted in a lower survival rate of PC12 cells compared with
that of the NC group. The addition of 50 ng/ml TNF-α significantly
inhibited the proliferation of PC12 cells, and this inhibition was
alleviated by BMSCs. Furthermore, co-culture with BMSCs resulted in
an improvement of the low survival rate induced by TNF-α. TNF-α is
a cytokine involved in systemic inflammation and was first
described by Carswell et al in 1975 (23). Previous studies have suggested that
TNF-α is associated with neuronal maturation and arborization.
Golan et al (24) observed
that a lack of TNF-α resulted in accelerated dentate gyrus
development, which correlated to increased levels of nerve growth
factor. Neumann et al (25)
indicated that treatment with TNF-α led to a reduction in dendritic
branching. In addition, inhibition of neuronal cell death and
promotion of regeneration by BMSCs had also been observed in a
previous study (26). Observations
of the present study suggested that BMSCs significantly suppress
TNF-α-induced inhibition of proliferation in PC12 cells.
Apoptosis of neuronal cells is a key contributor in
the pathogenesis of neurodegeneration and the inhibition of
neuronal cell apoptosis is important for the treatment of
neurodegenerative diseases (27,28).
The aim of the present study was to investigate the
anti-neurodegenerative mechanism of BMSCs in PC12 cells against
TNF-α treatment, as TNF-α acts as a stimulator in the pathogenesis
of neurodegenerative disease. In agreement with previous studies,
the results of the present study suggested that TNF-α significantly
induced PC12 cell apoptosis (21,29).
In addition, it was observed in the present study that BMSCs
attenuated TNF-α-induced PC12 cell apoptosis, as demonstrated by
AV/PI staining and TEM. These results are consistent with those of
a previous study by Guo et al (30), which indicated that BMSCs inhibit
cisplatin- and perimenopause-induced rat granulosa cell apoptosis
via a p21-, B-cell lymphoma-associated X protein and c-myc-
dependent apoptotic pathway. The results of the present study,
together with those of previous studies, suggested that BMSCs may
be useful for the treatment of neurodegenerative diseases.
The binding of TNF-α to its receptor TNFR1 is able
to induce cell survival, apoptosis or death through the formation
of two sequential complexes. Complex I triggers activation of the
transcription factor nuclear factor-κ B and prevents caspase-8
activation; however, complex II leads to caspase-dependent
apoptosis (31–33). More specifically, when PC12 cells
were stimulated with TNF-α, the TNF receptor-associated death
domain, which recruits TNFR1, can bind to the Fas-associated death
domain via a homologous sequence at the C-terminus. These binding
proteins that formed complex Ⅱ can facilitate the oligomerization
of pro-caspase-8 and then auto-cleave into activated caspase-8,
eventually resulting in a cascade leading to apoptosis (34,35).
In the present study, the mRNA levels of TNFR1 and the protein
levels of caspase-8 were increased in the PC group; however,
treatment with BMSCs inhibited the increase induced by TNF-α in
TNFR1 and caspase-8 in the co-culture group. By contrast, the TNFR2
mRNA levels were elevated in the group co-cultured with BMSCs.
Thus, it is suggested that BMSCs are able to inhibit TNFR1 to
recruit caspase-8 and ultimately suppress the apoptosis of PC12
cells. However, further studies are required to elucidate the
precise mechanisms by which BMSCs exert anti-apoptotic effects in
TNF-α-stimulated PC12 cells.
In conclusion, the results of the present study
demonstrated that BMSCs effectively suppress TNF-α-induced
proliferation inhibition and apoptosis in cultured PC12 cells. In
addition, the TNFR/caspase signaling pathway was involved in the
alterations of apoptosis and proliferation induced by TNF-α. The
observations of the present study also implied that BMSCs possess
therapeutic potential in the prevention of neurodegenerative
diseases and that their application presents a promising
therapeutic strategy.
References
|
1
|
Glass CK, Saijo K, Winner B, Marchetto MC
and Gage FH: Mechanisms underlying inflammation in
neurodegeneration. Cell. 140:918–934. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Amor S, Puentes F, Baker D and van der
Valk P: Inflammation in neurodegenerative diseases. Immunology.
129:154–169. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Deierborg T, Soulet D, Roybon L, Hall V
and Brundin P: Emerging restorative treatments for Parkinson’s
disease. Prog Neurobiol. 85:407–432. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhao J and Xu Q: Emerging restorative
treatments for Parkinson’s disease: Manipulation and inducement of
dopaminergic neurons from adult stem cells. CNS Neurol Disord Drug
Targets. 10:509–516. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mezey E: The therapeutic potential of bone
marrow-derived stromal cells. J Cell Biochem. 112:2683–2687. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cho H, Seo YK, Yoon HH, et al: Neural
stimulation on human bone marrow-derived mesenchymal stem celels by
extremely low frequency electromagnetic fields. Biotechnol Prog.
28:1329–1335. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tang Y, Cui YC, Wang XJ, et al: Neural
progenitor cells derived from adult bone marrow mesenchymal stem
cells promote neuronal regeneration. Life Sci. 91:951–958. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Polisetti N, Chaitanya VG, Babu PP and
Vemuganti GK: Isolation, characterization and differentiation
potential of rat bone marrow stromal cells. Neurol India.
58:201–208. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhao T, Yan W, Xu K, Qi Y, Dai X and Shi
Z: Combined treatment with platelet-rich plasma and brain-derived
neurotrophic factor-overexpressing bone marrow stromal cells
supports axonal remyelination in a rat spinal cord hemi-section
model. Cytotherapy. 15:792–804. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Waterborg JH and Matthews HR: The Lowry
method for protein quantitation. Methods Mol Biol. 32:1–4.
1994.PubMed/NCBI
|
|
11
|
Varvel NH, Bhaskar K, Patil AR, Pimplikar
SW, Herrup K and Lamb BT: Abeta oligomers induce neuronal cell
cycle events in Alzheimer’s disease. J Neurosci. 28:10786–10793.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jung IS, Kim HJ, Noh R, Kim SC and Kim CW:
Effects of extremely low frequency magnetic fields on NGF induced
neuronal differentiation of PC12 cells. Bioelectromagnetics.
35:459–469. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Al-Gayyar MM and Elsherbiny NM:
Contribution of TNF-α to the development of retinal
neurodegenerative disorders. Eur Cytokine Netw. 24:27–36.
2013.PubMed/NCBI
|
|
14
|
Kogo J, Takeba Y, Kumai T, et al:
Involvement of TNF-alpha in glutamate-induced apoptosis in a
differentiated neuronal cell line. Brain Res. 1122:201–208. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ye L, Huang Y, Zhao L, et al: IL-1β and
TNF-α induce neurotoxicity through glutamate production: a
potential role for neuronal glutaminase. J Neurochem. 125:897–908.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Su YR, Wang J, Wu JJ, Chen Y and Jiang YP:
Overexpression of lentivirus-mediated glial cell line-derived
neurotrophic factor in bone marrow stromal cells and its
neuroprotection for the PC12 cells damaged by lactacystin. Neurosci
Bull. 23:67–74. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Herzog EL, Chai L and Krause DS:
Plasticity of marrow-derived stem cells. Blood. 102:3483–3493.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Koh SH, Baik W, Noh MY, et al: The
functional deficiency of bone marrow mesenchymal stromal cells in
ALS patients is proportional to disease progression rate. Exp
Neurol. 233:472–480. 2012. View Article : Google Scholar
|
|
19
|
Sun C, Shao J, Su L, et al: Cholinergic
neuron-like cells derived from bone marrow stromal cells induced by
tricyclodecane-9-yl-xanthogenate promote functional recovery and
neural protection after spinal cord injury. Cell Transplant.
22:961–975. 2013. View Article : Google Scholar
|
|
20
|
Pastor D, Viso-León MC, Jones J, et al:
Comparative effects between bone marrow and mesenchymal stem cell
transplantation in GDNF expression and motor function recovery in a
motorneuron degenerative mouse model. Stem Cell Rev. 8:445–458.
2012. View Article : Google Scholar
|
|
21
|
Park KM and Bowers WJ: Tumor necrosis
factor-alpha mediated signaling in neuronal homeostasis and
dysfunction. Cell Signal. 22:977–983. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Paganelli R, Di Iorio A, Patricelli L, et
al: Proinflammatory cytokines in sera of elderly patients with
dementia: levels in vascular injury are higher than those of
mild-moderate Alzheimer’s disease patients. Exp Gerontol.
37:257–263. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Carswell EA, Old LJ, Kassel RL, Green S,
Fiore N and Williamson B: An endotoxin-induced serum factor that
causes necrosis of tumors. Proc Natl Acad Sci USA. 72:3666–3670.
1975. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Golan H, Levav T, Mendelsohn A and
Huleihel M: Involvement of tumor necrosis factor alpha in
hippocampal development and function. Cereb Cortex. 14:97–105.
2004. View Article : Google Scholar
|
|
25
|
Neumann H, Schweigreiter R, Yamashita T,
Rosenkranz K, Wekerle H and Barde YA: Tumor necrosis factor
inhibits neurite outgrowth and branching of hippocampal neurons by
a rho-dependent mechanism. J Neurosci. 22:854–862. 2002.PubMed/NCBI
|
|
26
|
Hsu SH, Kuo WC, Chen YT, et al: New nerve
regeneration strategy combining laminin-coated chitosan conduits
and stem cell therapy. Acta Biomater. 9:6606–6615. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kermer P, Liman J, Weishaupt JH and Bähr
M: Neuronal apoptosis in neurodegenerative diseases: from basic
research to clinical application. Neurodegener Dis. 1:9–19. 2004.
View Article : Google Scholar
|
|
28
|
Zhang H, Zhang YW, Chen Y, et al:
Appoptosin is a novel pro-apoptotic protein and mediates cell death
in neurodegeneration. J Neurosci. 32:15565–15576. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang C, Zhang Q, Qian Y and Zhao M:
p,p′-DDE induces apoptosis through the modulation of tumor necrosis
factor α in PC12 cells. Chem Res Toxicol. 27:507–513. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Guo JQ, Gao X, Lin ZJ, et al: BMSCs reduce
rat granulosa cell apoptosis induced by cisplatin and
perimenopause. BMC Cell Biol. 14:182013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Micheau O and Tschopp J: Induction of TNF
receptor I-mediated apoptosis via two sequential signaling
complexes. Cell. 114:181–190. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Schneider-Brachert W, Tchikov V, Neumeyer
J, et al: Compartmentalization of TNF receptor 1 signaling:
internalized TNF receptosomes as death signaling vesicles.
Immunity. 21:415–428. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Schütze S, Tchikov V and
Schneider-Brachert W: Regulation of TNFR1 and CD95 signalling by
receptor compartmentalization. Nat Rev Mol Cell Biol. 9:655–662.
2008. View
Article : Google Scholar : PubMed/NCBI
|
|
34
|
Bradley JR and Pober JS: Tumor necrosis
factor receptor-associated factors (TRAFs). Oncogene. 20:6482–6491.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Badiola N, Malagelada C, Llecha N, et al:
Activation of caspase-8 by tumour necrosis factor receptor 1 is
necessary for caspase-3 activation and apoptosis in oxygen-glucose
deprived cultured cortical cells. Neurobiol Dis. 35:438–447. 2009.
View Article : Google Scholar : PubMed/NCBI
|