Introduction
Radiation therapy is an important approach for
cancer treatment worldwide. It was reported by The American Cancer
Society that ~4 million new cases of cancer were recorded in the
USA in 2006, and ~50% of these patients received radiation therapy
as treatment (1). Radiation
therapy has been important in the-improving survival rates of
cancer patients. Currently 62% of adults and >75% of patients
with pediatric cancer survive over five years in the United States
(2). However, early and late
toxicity limits the deliverable intensity of radiotherapy and may
affect a patient’s long-term quality of life. The present study
investigated the effects on bone loss, which is an area of clinical
concern. The improved survival rates of cancer patients receiving
radiotherapy increases the importance of understanding the causal
mechanisms and possible effects of radiation-induced bone loss
(2).
Radiotherapy may induce bone loss, which can result
in pathological fractures. In a previous study of >6,000 females
aged >65 years, treated for cervical, rectal and anal cancer, it
was reported that radiation therapy significantly increased the
risk of pelvic fracture by a relative risk of 1.66, 1.65 and 3.16,
respectively (3). Abe et al
(4) reported that pelvic
insufficiency fractures were identified in 27 of 80 (34%) Asian
patients with uterine cancer. Blomlie et al (5) reported prospectively that 16 of 18
(89%) advanced cervical carcinoma patients exhibited findings
compatible with radiation-induced insufficiency fractures through
magnetic resonance imaging. Therefore, radiation compromises bone
health and can have a severe impact on the functional capabilities
of patients. The rate and severity of radiation-induced bone mass
loss is greater compared with bone damage due to postmenopausal
hormone changes or due to treatment with glucocorticoids (6,7).
However, there remains no consensus on the mechanism
underlying bone damage from radiation exposure, based on the
anatomical location of the bone relative to the radiation exposure.
While Baxter et al (3)
found that the incidence of arm or spine fractures, outside the
treatment volume, was similar in irradiated and non-irradiated
groups, Zuppinger and Minder (8)
demonstrated a non-targeted effect of radiation on bone metabolism.
In another study, the unirradiated tibiae of animals that had
received 800 rad to another hind extremity exhibited significant
differences in tibial lengths compared with the bones of control
animals, indicating significant abscopal growth retardation
(9). Jia et al (10) also provided evidence that
post-radiotherapy fractures in the unirradiated skeleton may result
from the abscopal impact of local irradiation. Decreases in serum
levels of a bone formation marker, the rate of in vitro
osteoblast differentiation and bone mineral density following
administering male C57BL/6 mice with a single dose of 15 Gy x-rays
to the abdomen (10). In addition,
clinical investigations involving local radiotherapy have revealed
that a femoral fracture rate of 1,716 breast cancer patients 1 year
after treatment to the chest wall is >20 times higher compared
with the overall annual hip fracture rate (11).
The direct effect of γ-irradiation on osteoblastic
cells was examined in our previous study and the osteoblasts were
significantly affected (12).
However, the mechanism of the abscopal effect remains to be
elucidated. The aim of the present study was to investigate the
cellular mechanisms underlying the abscopal degradation of the
unexposed skeleton, which remains essentially undefined. The
elucidation of these mechanisms may have important clinical
implications as these mechanisms may define improved preventive or
curative solutions for bone loss. Thus, the radiation-induced
bystander effect is an attractive target for investigation as
alterations in osteoblast function may be, in part, associated with
abscopal effects of bone loss. Using a medium transfer procedure,
the present study aimed to investigate the complex interplay
between radiotherapy and bone loss.
Materials and methods
Radiation exposure
For irradiation, osteoblasts (5×105) were
plated in 25 cm2 flasks, cultured for one day at 37°C in
an atmosphere containing 5% CO2 and then exposed to
γ-rays generated by a 137Cs source (Gammacell 40
Exactor; Nurdion International Inc., Kanata, ON, Canada) at a dose
rate of 0.76 Gy/min. The irradiation doses were selected as 0, 1,
2, 5 and 10 Gy, which are biologically equivalent doses of clinical
relevance.
Chemicals
Minimum essential medium (MEM) and fetal bovine
serum (FBS) were purchased from Gibco-BRL (Gaithersburg, MD, USA).
Penicillin-G was purchased from Shanghai Asia Pioneer
Pharmaceutical Co., Ltd. (Shanghai, China). Streptomycin was
purchased from North China Pharmaceutical Group Corporation
(Shijiazhuang, China). Oligonucleotide primer sets were synthesized
by Sangon Biological Engineering Technology and Services Co., Ltd.
(Shanghai, China). An Alexa Fluor 488 Annexin V/dead cell apoptosis
kit was purchased from Invitrogen Life Technologies, (Carlsbad, CA,
USA).
Animals
Sprague-Dawley (SD) male rats at 8 weeks old were
obtained from the Department of Laboratory Animal Science, Fudan
Univeristy (Shanghai, China) weighing 180–200 g. The rats were fed
ad libitum and were housed in specific pathogen free animal
facilities under controlled conditions (temperature 21±1°C;
relative humidity 50±10% and 12:12 h light-dark cycle).
Cell isolation and primary culture of
osteoblasts
The procedures used in the present study were
reviewed and approved by the Committee for Ethical Use of
Experimental Animals at Fudan University. Osteoblasts were prepared
from the calvarias of 1 or 2 day old SD rats using a sequential
enzymatic digestion method, as described previously (12). Briefly, the calvarias were
incubated at 37°C for 10 min with 0.1% collagenase and 0.25%
trypsin (Sigma-Aldrich, St. Louis, MO, USA) in calcium- and
magnesium-free phosphate-buffered saline (PBS). After 10 min the
supernatant was discarded, more enzyme solution was added and the
incubation continued at 37°C for a further 10 min. This process was
repeated four times. The cells obtained during the last four
digestions were pooled in MEM supplemented with 10% FBS and
antibiotics (100 U/ml penicillin G and 100 μg/ml
streptomycin). The cells were then centrifuged at 250 × g for 5 min
and the pellets were suspended in MEM containing 10% FBS. The cells
(1×105cells/ml) were grown in MEM supplemented with 10%
FBS, 100 U/ml penicillin-G and 100 μg/ml streptomycin in
flasks. The cells were maintained in an incubator at 37°C in an
atmosphere of 5% CO2. Subculture was routinely performed
using a 1:1 solution of 0.25% trypsin and 0.02% EDTA (Sinopharm
Chemical Reagent Co., Ltd., Shanghai, China) at 37°C. The medium
was replaced every 2 days. All assessments were performed at the
first subculture of osteoblasts. The osteoblastic cells were
observed and images were captured using an inverted microscope
(DMI3000B; Leica Microsystems GmbH, Wetzlar, Germany). The
phenotype was identified via alkaline phosphatase (ALP) staining
using a nitro blue tetrazolium (NBT)/5-bromo-4-chloro-3-indolyl
phosphate (BCIP) staining kit (Beyotime Institute of Biotechnology,
Haimen, China).
Collection of irradiated cell conditioned
medium (ICCM)
Culture flasks (25 cm2, 40 ml flasks;
Corning, Inc., Acton, MA, USA) containing ~5×105 cells
were seeded and irradiated at room temperature using a
137Cs instrument delivering ~0.76 Gy/min during the
period of the experiment. Control flasks were sham-irradiated and
the medium collected was considered the control medium. The cells
were returned to the 37°C incubator immediately following
irradiation. The medium was removed from the donor flasks 24 h
following irradiation and the cells were washed twice with PBS. The
osteoblasts were then cultured in 5.0 ml serum-free MEM for an
additional 24 h at 37°C in an atmosphere containing 5%
CO2. The serum-free culture medium was collected and
filtered through a 0.22 μm filter to sterilize the solutions
and to ensure that no cells remained in the transferred medium. The
medium was then divided into 1 ml aliquots. No intact cells were
present, as detected by the examination of the aliquots of the
medium under a microscope (DMI3000B; Leica Microsystems GmbH). The
medium was then divided into aliquots in 1 ml volumes, stored at
−20°C and thawed only once when required for subsequent
experiments. The donor medium generated was referred to as
ICCM.
Cellular viability of osteoblasts
Osteoblasts were incubated in 96 well plates at
4×103 cells/well and cultured in medium for 24 h at 37°C
in an atmosphere containing 5% CO2. The cells were
treated, as described in Table I.
The extent of cellular viability in response to different doses of
the conditioned medium was determined using an MTT assay (Amresco
LLC, Solon, OH, USA), as described previously (13). The culture medium was removed and
110 μl fresh culture medium (without serum) containing 10
μl MTT solution (5 mg/ml in PBS) was added to each well. The
cells were then incubated for 4 h at 37°C and the insoluble
formazan crystals were dissolved in 100 μl 10% sodium
dodecyl sulfate (Sigma-Aldrich). After 2 h incubation at 37°C, the
optical density was measured at 570 nm using a Multiskan FC reader
(Thermo Scientific, Rockford, IL, USA). The results are expressed
as the mean ± standard deviation of 8 wells for each group.
 | Table IExperimental groups of osteoblasts
treated with different irradiation doses and concentrations of
ICCM. |
Table I
Experimental groups of osteoblasts
treated with different irradiation doses and concentrations of
ICCM.
| Irradiation dose
(Gy) | Concentration
(%ICCM+%MEM)
|
|---|
| 10% ICCM group | 20% ICCM group | 40% ICCM group |
|---|
| 0 | 10+90 | 20+80 | 40+60 |
| 1 | 10+90 | 20+80 | 40+60 |
| 2 | 10+90 | 20+80 | 40+60 |
| 5 | 10+90 | 20+80 | 40+60 |
| 10 | 10+90 | 20+80 | 40+60 |
Flow-cytometric analysis
Apoptosis was measured by analyzing the membrane
redistribution of phosphatidyl serine (PS), based on the binding
properties of annexin V to PS and the DNA-interacting capabilities
of propidium iodide (PI). The percentage of apoptotic cells was
determined by flow cytometry, using an Annexin V fluorescein
isothiocyanate (FITC) and PI staining kit (Invitrogen Life
Technologies). Briefly, the osteoblasts (7.5×104
cells/well) were cultured in 6-well culture plates in a humidified
5% CO2 incubator at 37°C for 24 h. Following the
addition of 20% or 40% conditioned medium or control medium, the
cells were cultured for a further 48 h. Following incubation, the
cells were harvested by centrifugation at 1,000 x g for 5 min.
Subsequently, the cell suspension was obtained and annexin V-FITC
and PI staining was performed, according to the manufacturer’s
instructions.
The cells were resuspended in 100 μl 1X
binding buffer supplemented with 1 μl annexin V-FITC and 5
μl PI and maintained at room temperature in the dark for 15
min. Following the incubation period, 400 μl 1X binding
buffer was added and the stained cells were maintained on ice and
assessed using a FACSCanto flow cytometer (Beckman Coulter, Miami,
FL, USA) for flow cytometric analysis.
ALP activity of osteoblasts
Osteoblasts (4×103 cells/well) were
plated into 96-well plates and the ALP activity and protein content
were examined, as described previously (14). The cells were lysed in 0.05% Triton
X-100 for 1 h at 4°C and subsequently by sonication (FS-600;
U&STAR Ultrasonic Technology Co., Ltd., Hangzhou, China). The
total cellular ALP activity was measured in
2-amino-2-methyl-1-propanol buffer, with p-nitro phenyl phosphate
(Fluka, Milwaukee, WI, USA) as a substrate, at 37°C. A total of 0.5
mmol/l NaOH was added to terminate the reactions. Absorbance of the
reaction was measured at 405 nm using a Multiskan FC reader. The
total protein content in the lysates was measured using a
bicinchoninic acid assay (Beyotime Institute of Biotechnology).
Subsequently, ALP activity was adjusted to the cell protein content
and expressed as U/mg protein.
Mineralized nodule formation in
osteoblasts
Mineralization of the nodules in the cultures was
assessed by Alizarin red S (ARS; Sinopharm Chemical Reagent Co.,
Ltd.) staining. A total of six wells were analyzed for each group
to determine the area of the mineralized matrix nodules. The
osteoblasts (5×104 cells/well) were plated into 48-well
plates and cultured in MEM (1.0 ml/well) containing 15% FBS for 24
h at 37°C in an atmosphere containing 5% CO2.
Subsequently, the matrix was replaced by MEM culture medium mixed
with 20% ICCM of 0, 2, 5 and 10 Gy. The medium was replaced every 2
days. After 7 days, the medium was aspirated and the cells were
treated with mineralization medium [MEM supplemented with 15% FBS,
50 μg/ml ascorbic acid and 10 mmol/l β-glycerophosphate
(Sigma-Aldrich)] for 21 days. Subsequently, cells were rinsed with
PBS and the mineralization nodules were visualized by fixing the
cells in 0.25% glutaraldehyde and staining with ARS for 10 min at
room temperature. Mineralization was measured by visual counting
under an optical microscope (magnification, ×100). All six wells
from each group were visualized, and the sizes of the nodules were
analyzed for each group. Simple PCI (Compix Inc., Cranberry
Township, PA, USA) imaging software was used to analyze the area of
the mineralization nodes.
Reverse transcription quantitative
polymerase chain reaction (RT-qPCR)
Osteoblasts (5.0×104 cells/well) were
plated into 6-well plates and cultured in medium for 24 h at 37°C
in an atmosphere containing 5% CO2. The cells were
cultured with 20% ICCM from osteoblasts irradiated with 0, 2 or 10
Gy γ-rays. The total RNA was isolated using TRIzol reagent (Tiangen
Biotech, Beijing, China) and the first-strand cDNA was generated
using Transcript First-Strand cDNA Synthesis Supermix (Tiangen
Biotech), according to the manufacturer’s instructions. The primer
sequences used are shown in Table
II. The RT-qPCR was performed using SYBR Green supermix (Takara
Bio Inc. Ohtsu, Japan) using a Light Cycler 2.0 (Roche Diagnostics,
Mannheim, Germany). The relative mRNA expression of the target gene
was quantified by measuring the threshold cycle (Ct) and normalized
against the mRNA expression of β-actin. Each sample was assessed in
triplicate. The fold change was determined using the
2−ΔΔCT method (15).
 | Table IIPrimer sequences. |
Table II
Primer sequences.
| Gene | Forward primer
(5′-3′) | Reverse primer
(5′-3′) | Amplicon size
(bp) |
|---|
| β-actin |
agccatgtacgtagccatcc |
ctctcagctgtggtggtgaa | 228 |
| ALP |
ctgagcgcacgcgagcaac |
ggcgtggttcacccgagtgg | 116 |
| BGP |
gaacagacaagtcccacac |
gagctcacacacctccctg | 270 |
| OPG |
tgggaatgaagatcctccag |
gaggaaggaaagggcctatg | 109 |
| RANKL |
agccgagactacggcaagta |
gcgctcgaaagtacaggaac | 208 |
| Caspase 3 |
gccctggcacacgggacttg |
gcacagacgccctgatgggg | 106 |
Statistical analysis
Statistical analysis was performed using SPSS 16
(SPSS, Inc, Chicago, IL, USA). All data are expressed as the mean ±
standard deviation. A one-way analysis of variance, followed by
Dunnett’s T3 or Dunnett’s test were used to analyze the data.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Effects of ICCM on the viability of
osteoblasts
An MTT assay was performed to identify the cell
survival in cells cultured with ICCM (10%), or ICCM (20%) for 5
days. As shown in Fig. 1, no
significant differences were identified between the responses to 0,
2 and 5 Gy ICCM. However, the viability of cells was significantly
decreased in the 10 Gy ICCM group compared with the control
cultures in the 10 and 20% groups. The MTT absorbance of the
control cells was 0.809±0.031 and 0.757±0.026 in the 10 and 20%
groups, respectively, but only 0.705±0.040 and 0.690±0.030 in the
10 Gy ICCM-treated cells in the 10 and 20% groups,
respectively.
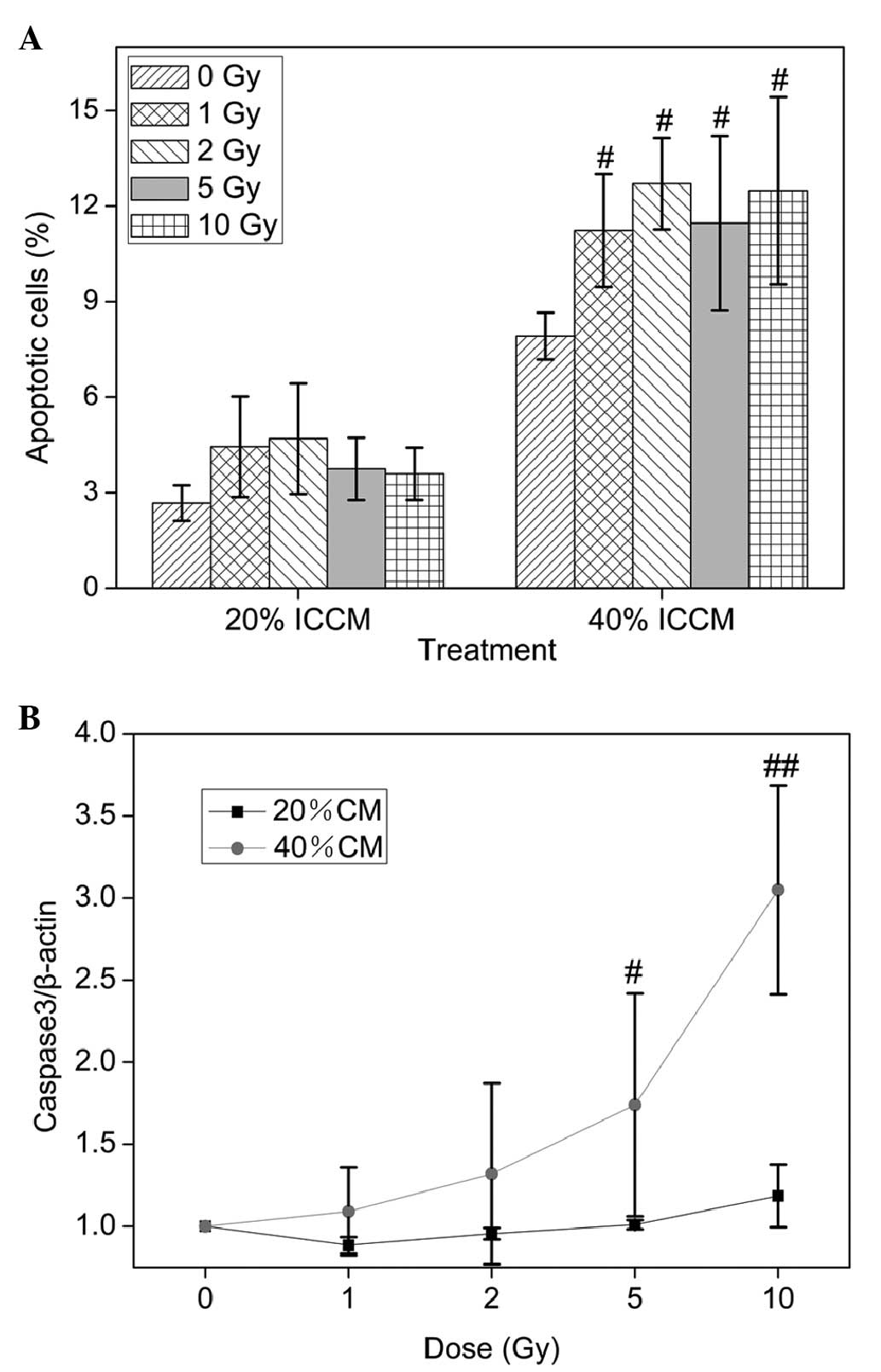
ICCM-induced cell apoptosis in
osteoblasts
The present study subsequently investigated whether
ICCM induces cell death through an apoptotic mechanism. The number
apoptotic and/or necrotic cells were assessed by measuring the
binding of annexin V-FITC to PS expressed at the membrane (early
apoptosis) and by staining the nuclei with impermeable fluorescence
PI to indicate lost plasma membrane integrity (late apoptotic
and/or necrotic cells). These fluorescence-stained cells were
analyzed by flow cytometry. A significant increase of apoptosis in
the osteoblastic cells was found in the 40% ICCM-treated cultures
(P<0.05; Fig. 2A). These data
suggested that ICCM induced apoptosis in the osteoblasts.
In addition, it was observed that the expression of
caspase 3 was upregulated in the conditioned medium treated cells
(Fig. 2B). The ICCM (40%) from
osteoblasts irradiated with 5 and 10 Gy upregulated the mRNA
expression of caspase 3 compared with the 0 Gy ICCM group and were
elevated in a dose-independent manner.
Effects of ICCM on the ALP activity of
osteoblasts
Following 5 days of incubation, the production of
ALP was evaluated using an NBT-BCIP assay. Compared with the
vehicle-treated group, no significant differences were identified
in the enzyme production when the osteoblasts were incubated in the
presence of ICCM irradiated by 2 Gy γ-ray in the 10 and 20% group.
In the groups treated with 5 and 10 Gy, the ICCM attenuated the
activity of ALP (Fig. 3).
Effects of ICCM on the mineralization
capacity of osteoblasts
The mineralized nodules were stained with ARS
(Fig. 4A–4D) and the areas were
analyzed using Simple PCI imaging software. As shown in Fig. 4E, 20% ICCM inhibited the formation
of the mineralized matrix (P<0.05) and had a significant effect
at all doses compared with the control with inhibitory rates of 34,
41 and 49%, respectively. Quantization of the ARS deposition areas
revealed that ICCM suppressed the mineralization of osteoblasts at
all doses.
ICCM-induced mRNA expression changes in
osteoblasts
To better understand the effect of ICCM in
osteoblastogenesis, its effect on the expression of several
osteoblast markers was examined using RT-qPCR analysis. The results
are presented as histograms in Fig.
5. The osteoblasts cultured with 1 or 10 Gy ICCM exhibited
different gene expression patterns compared with the controls. ALP
is an early osteoblastic marker and a single dose of x-rays (4 Gy)
can induce significant changes in osteoblast differentiation 6 days
after radiation (16). As shown in
Fig. 5A, incubation of the cells
with ICCM inhibited the mRNA expression of ALP. In addition, ICCM
induced a more marked downregulation of the mRNA expression of
osteocalcin (BGP; Fig. 5B).
Furthermore, the mRNA expression levels of osteoprotegerin (OPG)
and receptor activator of nuclear factor-κB ligand (RANKL) were
decreased markedly following ICCM administration. However, no
significant difference was identified between the responses to 1 or
10 Gy ICCM.
Discussion
Radiotherapy-associated bone complications have been
recognized in patients since the early application of ionizing
radiation almost a century ago. It has continued to be one of the
treatment-associated side-effects, which considerably compromises
the quality of life of the patient (17). Non-malignant bone complications,
frequently in the form of bone loss and often only diagnosed
following the occurrence of fractures, are a common complication
post-irradiation. The etiology of non-malignant bone complications
in cancer patients treated with radiotherapy remains to be
elucidated and appears to be multifactorial. Cumulative evidence
suggests abscopal effects are involved in the development of
injuries in normal tissues, including the skeleton, following local
radiotherapy (11,17). Jia et al (18) and Travis (19) observed potent effects on bone
density and other parameters even when single organs, including the
gut, are irradiated. These abscopal effects further highlight the
importance of developing a better understanding of the complete
mechanism by which direct and indirect radiation exposure affects
bone quality.
Bone damage occurs following exposure to low linear
energy transfer ionizing radiation (γ and x-rays) and is
hypothesized to be a result of physiological changes that occur to
vasculature and bone cells, including bone-forming osteoblasts and
resorbing osteoclasts (20). To
date, whether ICCM affects bone metabolism by affecting the
proliferation, differentiation, and mineralization function of
primary osteoblasts in vitro has not been reported.
Osteoblasts are specialized, terminally differentiated products of
mesencyhmal stem cells. They synthesize very dense, cross-linked
collagen, and various additional specialized proteins in smaller
quantities, including osteocalcin and osteopontin, which comprise
the organic matrix of bone (21).
Osteoblasts cultured from newborn mouse skulls retain more somatic
cell functional characteristics compared with cell lines and
simulate biological changes in vivo (21). The present study investigated the
γ-ray-induced ICCM-bystander-type effects on osteoblasts at the
cellular level at clinically therapeutic doses. It has been
established that irradiated cells release cytotoxic factor(s) into
the surrounding medium, which causes the death of unirradiated
cells (22,23). The present study demonstrated that
10 Gy ICCM exposure significantly inhibited osteoblast
proliferation. ALP activity, which is an early characteristic
marker of osteogenic differentiation, was also significantly
inhibited in cells treated with 5 and 10 Gy ICCM. Mineralization is
a necessary condition of osteoblasts to form bone calcification
(14), and the present study
observed that 2, 5 and 10 Gy ICCM exposure suppressed the
mineralization function of osteoblasts. Radiation is a well-known
inducer of apoptosis in numerous cell lines and there is evidence
that apoptotic death is a prominent feature of cultures
demonstrating bystander effects (22,24).
This is supported by data presented in the present study. Analysis
of the cells revealed that ICCM increased the percentages of
osteoblasts processing apoptosis in a dose-independent manner. In
addition, upregulation in the mRNA expression of caspase 3was
observed. Caspase 3 is a frequently activated protease in mammalian
cell apoptosis, essential for certain characteristic changes in
cell morphology and biochemical events associated with the
execution and completion of apoptosis (25). In the present study, RT-qPCR was
performed on the samples to investigate the effect of ICCM on the
expression of a series of osteoblast markers. As expected, ICCM
also significantly suppressed the expression levels of ALP, BGP,
OPG and RANKL to various degrees. These data, although preliminary,
are indicative that ICCM has potential deleterious effects on
osteoblasts.
In terms of the implications for radiotherapy, there
is a body of data that remains to be fully understood concerning
the abscopal effects of radiation, including the response of an
organ or tissue that was not in the field of treatment to
experimental irradiation or radiotherapy (9,26–31).
Radiation-induced bystander effects may provide an explanation for
certain abscopal effects. Previous studies using a range of
epithelial cells, including normal and tumor cells, indicated a
wide variation in the production of the signal and the expression
of the effect (24,32–36),
therefore, a ‘holistic’ approach to understanding the process. may
be required For example, there are multiple inducers, which have
multiple possible consequences in different cell systems, and the
precise consequence of a specific trigger may depend on numerous
factors. Lyng et al (37)
hypothesized that irradiated cells release toxic factors other than
reactive oxygen species (ROS) into the medium; ROS appear to be
involved in the signaling pathway for specific end points, however,
the existence of other signaling mechanisms is indicated,
particularly for cell death. It has been demonstrated that specific
long-lived signaling factors may cause apoptosis in unirradiated
cells exposed to ICCM by triggering calcium fluxes and the
mitogen-activated protein kinase signaling pathway (38). Increases in transforming growth
factor β1 and interleukin-8 have been demonstrated previously in
bystander cell supernatants (39–41).
Mothersill et al (42)
suggested that a signal transduction mechanism may control cell
death or survival via the bystander effect rather than by release
of a directly cytotoxic factor. While Mothersill et al
demonstrated that serum has the ability to produce bystander
factors and that serotonin in serum is important in the mechanism
of signal production, this possibility is excluded in the present
study, in which the ICCM is serum-free (43). These conflicting experimental
results imply that there are complex mechanisms underlying these
effects, which remain to be elucidated. Whether radiation-induced
abscopal damage to the skeleton is associated with the systemic
effects of radiation injury, where damage to a critical organ may
have consequences for another organ, and its precise mechanism are
not fully understood and require further investigation.
The morbidity of bone irradiation and its attendant
complications are significant. Subsequent investigations aim to
identify irradiation-induced changes in the expression of
cytokines, which are involved in inhibitory effects, and to include
co-culture and an in vivo model. In conclusion, the present
study has improved current understanding of the skeletal
complications associated with localized radiotherapy and, if
preventing or minimizing post-radiation-induced bone loss is a
valid investigative goal, further understanding of in-field and out
of-field damage is required. A potentially important outcome may be
the identification of treatments, which protect the bone from
irradiation and/or facilitate bone repair.
Acknowledgments
The present study was supported by the Shanghai
Municipal Health Bureau (grant nos. 2009003 and 12GWZX0401).
References
|
1
|
Bentzen SM: Preventing or reducing late
side effects of radiation therapy: radiobiology meets molecular
pathology. Nat Rev Cancer. 6:702–713. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Robbins ME and Zhao W: Chronic oxidative
stress and radiation-induced late normal tissue injury: a review.
Int J Radiat Biol. 80:251–259. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Baxter NN, Habermann EB, Tepper JE, Durham
SB and Virnig BA: Risk of pelvic fractures in older women following
pelvic irradiation. JAMA. 294:2587–2593. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Abe H, Nakamura M, Takahashi S, Maruoka S,
Ogawa Y and Sakamoto K: Radiation-induced insufficiency fractures
of the pelvis: evaluation with 99mTc-methylene diphosphonate
scintigraphy. AJR Am J Roentgenol. 158:599–602. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Blomlie V, Rofstad EK, Talle K, Sundfør K,
Winderen M and Lien HH: Incidence of radiation-induced
insufficiency fractures of the female pelvis: evaluation with MR
imaging. AJR Am J Roentgenol. 167:1205–1210. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Van Staa TP, Leufkens HG, Abenhaim L,
Zhang B and Cooper C: Use of oral corticosteroids and risk of
fractures. June, 2000. J Bone Miner Res. 20:1487–1494. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nelson HD, Helfand M, Woolf SH and Allan
JD: Screening for postmenopausal osteoporosis: a review of the
evidence for the U.S. Preventive Services Task Force. Ann Intern
Med. 137:529–541. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zuppinger A and Minder W: Calcium uptake
in irradiated bone. United Nations International Conference on the
Peaceful Uses of Atomic Energy; United Nations, Geneva,
Switzerland. 22. pp. p2471959
|
|
9
|
Pappas AM and Cohen J: The abscopal effect
of X-irradiation on bone growth in rats. J Bone Joint Surg.
45A:765–772. 1963.
|
|
10
|
Jia D, Gaddy D, Suva LJ and Corry PM:
Rapid loss of bone mass and strength in mice after abdominal
irradiation. Radiat Res. 176:624–635. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen Z, Maricic M, Aragaki AK, et al:
Fracture risk increases after diagnosis of breast or other cancers
in postmenopausal women: results from the Women’s Health
Initiative. Osteoporos Int. 20:527–536. 2009. View Article : Google Scholar
|
|
12
|
Hinoi E, Fujimori S, Takemori A and Yoneda
Y: Cell death by pyruvate deficiency in proliferative cultured
calvarial osteoblasts. Biochem Biophys Res Commun. 294:1177–1183.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hansen MB, Nielsen SE and Berg K:
Re-examination and further development of a precise and rapid dye
method for measuring cell growth/cell kill. J Immunol Methods.
119:203–210. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kirsch T, Nah HD, Shapiro IM and Pacifici
M: Regulated production of mineralization-competent matrix vesicles
in hypertrophic chondrocytes. J Cell Biol. 137:1149–1160. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Schmittgen TD and Livak KJ: Analyzing
real-time PCR data by the comparative C(T) method. Nat Protoc.
3:1101–1108. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dare A, Hachisu R, Yamaguchi A, Yokose S,
Yoshiki S and Okano T: Effects of ionizing radiation on
proliferation and differentiation of osteoblast-like cells. J Dent
Res. 76:658–664. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Aksnes LH and Bruland ØS: Some
musculo-skeletal sequelae in cancer survivors. Acta Oncol.
46:490–496. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jia D, Koonce NA, Griffin RJ, Jackson C
and Corry PM: Prevention and mitigation of acute death of mice
after abdominal irradiation by the antioxidant N-acetyl-cysteine
(NAC). Radiat Res. 173:579–589. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Travis EL: The sequence of histological
changes in mouse lungs after single doses of x-rays. Int J Radiat
Oncol Biol Phys. 6:345–347. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hopewell JW: Radiation-therapy effects on
bone density. Med Pediatr Oncol. 41:208–211. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhou G, Gu G, Li Y, et al: Effects of
cerium oxide nanoparticles on the proliferation, differentiation,
and mineralization function of primary osteoblasts in vitro. Biol
Trace Elem Res. 153:411–418. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lyng FM, Seymour CB and Mothersill C:
Early events in the apoptotic cascade initiated in cells treated
with medium from the progeny of irradiated cells. Radiat Prot
Dosimetry. 99:169–172. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lehnert BE, Goodwin EH and Deshpande A:
Extracellular factor(s) following exposure to alpha particles can
cause sister chromatid exchanges in normal human cells. Cancer Res.
57:2164–2171. 1997.PubMed/NCBI
|
|
24
|
Lyng FM, Seymour CB and Mothersill C:
Production of a signal by irradiated cells which leads to a
response in unirradiated cells characteristic of initiation of
apoptosis. Br J Cancer. 83:1223–1230. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li P, Nijhawan D, Budihardjo I, et al:
Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9
complex initiates an apoptotic protease cascade. Cell. 91:479–489.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Collett WK, Watson JA and Wald N: Abscopal
and direct effects on calcium mobilization, alkaline phosphatase
levels, and dentin formation following x-irradiation of either the
rat incisor or the thyroid-parathyroid region. J Dent Res.
45:1529–1538. 1966. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Montour JL: Abscopal radiation damage to
chick thymus and bursa of Fabricius. Acta Radiol Ther Phys Biol.
10:150–160. 1971. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Nobler MP: The abscopal effect in
malignant lymphoma and its relationship to lymphocyte circulation.
Radiology. 93:410–412. 1969. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Van der Meeren A, Monti P, Vandamme M,
Squiban C, Wysocki J and Griffiths N: Abdominal radiation exposure
elicits inflammatory responses and abscopal effects in the lungs of
mice. Radiat Res. 163:144–152. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kurnick NB and Nokay N: Abscopal effects
and bone-marrow repop-ulation in man and mouse. Ann N Y Acad Sci.
114:528–537. 1964. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Conard RA: Indirect Effect of X-radiation
on bone growth in rats. Ann N Y Acad Sci. 114:335–338. 1964.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mothersill C, Rea D, Wright EG, et al:
Individual variation in the production of a ‘bystander signal’
following irradiation of primary cultures of normal human
urothelium. Carcinogenesis. 22:1465–1471. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Azzam EI, de Toledo SM, Gooding T and
Little JB: Intercellular communication is involved in the bystander
regulation of gene expression in human cells exposed to very low
fluences of alpha particles. Radiat Res. 150:497–504. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Azzam EI, de Toledo SM, Waker AJ and
Little JB: High and low fluences of alpha-particles induce a G1
checkpoint in human diploid fibroblasts. Cancer Res. 60:2623–2631.
2000.PubMed/NCBI
|
|
35
|
Ghandhi SA, Yaghoubian B and Amundson SA:
Global gene expression analyses of bystander and alpha particle
irradiated normal human lung fibroblasts: synchronous and
differential responses. BMC Med Genomics. 1:632008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Belyakov OV, Folkard M, Mothersill C,
Prise KM and Michael BD: A proliferation-dependent bystander effect
in primary porcine and human urothelial explants in response to
targeted irradiation. Br J Cancer. 88:767–774. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lyng FM, Howe OL and McClean B: Reactive
oxygen species-induced release of signalling factors in irradiated
cells triggers membrane signalling and calcium influx in bystander
cells. Int J Radiat Biol. 87:683–695. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lyng FM, Maguire P, McClean B, Seymour C
and Mothersill C: The involvement of calcium and MAP kinase
signaling pathways in the production of radiation-induced bystander
effects. Radiat Res. 165:400–409. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Shao C, Folkard M and Prise KM: Role of
TGF-beta1 and nitric oxide in the bystander response of irradiated
glioma cells. Oncogene. 27:434–440. 2008. View Article : Google Scholar
|
|
40
|
Facoetti A, Ballarini F, Cherubini R, et
al: Gamma ray-induced bystander effect in tumour glioblastoma
cells: a specific study on cell survival, cytokine release and
cytokine receptors. Radiat Prot Dosimetry. 122:271–274. 2006.
View Article : Google Scholar
|
|
41
|
Facoetti A, Mariotti L, Ballarini F, et
al: Experimental and theoretical analysis of cytokine release for
the study of radiation-induced bystander effect. Int J Radiat Biol.
85:690–699. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Mothersill C and Seymour CB: Cell-cell
contact during gamma irradiation is not required to induce a
bystander effect in normal human keratinocytes: evidence for
release during irradiation of a signal controlling survival into
the medium. Radiat Res. 149:256–262. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Mothersill C, Saroya R, Smith RW, Singh H
and Seymour CB: Serum serotonin levels determine the magnitude and
type of bystander effects in medium transfer experiments. Radiat
Res. 174:119–123. 2010. View Article : Google Scholar : PubMed/NCBI
|