Introduction
Cysticercosis, which is caused by the larva of
Taenia solium, has affected humans in numerous developing
regions worldwide (1,2), and even in certain developed
countries (3,4). Cysticercosis is a serious public
health problem as well as a great threat to the animal industry,
which may result in tremendous economic damages. This disease has
been identified by the World Health Organization as one to be
eliminated worldwide (5). In the
life-cycle of Taenia (T.) solium, swine serve
as the intermediate host, while humans are intermediate as well as
definitive hosts. Humans contract cysticercosis through ingesting
raw or poorly cooked meat contaminated with T. solium
larvae. These larvae mature to become tapeworms, which are
parasites of the small intestine. The gravid proglottids of
tapeworms, which are filled with eggs, are discharged with the
feces of the hosts; under circumstances of poor hygiene, swine and
humans may intake food and water polluted by eggs, which may lead
to cysticercosis. Efforts, including drug therapy, environmental
sanitation, enhanced management of feces and quarantine measures,
have achieved only minimally positive results in disease control
(6). Vaccination has been
confirmed as a potentially valuable novel method for the prevention
of T. solium transmission (7,8). The
TSOL18 gene, which encodes the T. solium-specific
TSOL18 antigen, is a homologue of T. ovis To18
(9) and T. saginata TSA18
(10), and along with other
oncosphere antigens, it was shown to contain a fibronectin type III
domain (11). A previous study has
shown that the TSOL18 antigen may be the most immunogenic
and protective protein ever reported against cysticercosis
(12–15). A number of different expression
systems have previously been used to create proteins; however,
species-specific variations in codon usage often serve as one of
the primary influences of the results (16,17).
A study has demonstrated that highly expressed genes ‘prefer’
optimal codons (18) and the
expression levels of optimized codons were markedly higher compared
with those of non-optimal codons (16,19,20).
In order to increase immunogenicity of TSOL18 through higher
recombinant protein production, it is necessary to optimize the
codon in the expression host due to codon bias.
Therefore, the aim of the present study was to use
codon optimization for the gene encoding TSOL18 of T.
solium, construct an optimized plasmid vector (optimized
pVAX1/TSOL18) for transfection and then evaluate the
expression of the optimized gene in vivo and in vitro
in order to determine its immunogenicity.
Materials and methods
Codon optimization, design and synthesis
of the TSOL18 gene
According to the codon usage in mouse cells, 79 out
of 130 amino acids of TSOL18 were modified into the most
preferred ones, based on codon preference in host species, for
mammalian using GeneOptimizer software (Geneart AG, Regensburg,
Germany), without changing the amino acid sequence; then, the
optimized full-length TSOL18 gene was synthesized by Jinsite
Biotechnology Co. (Nanjing, China). The target fragments were
amplified using polymerase chain reaction (PCR; S100 Thermal
Cycler, Bio-Rad Laboratories, Inc., Hercules, CA, USA) and the
following pair of 19 bp primers: P1, 5′-ATGGTGTGCAGGTTCGCCC-3′ and
P2, 5′-GGATCCTCAGCTTCTCCTC-3′, were constructed. Restriction
endonucleases HindIII and BamHI (Fermentas, Vilnius,
Lithuania) were added at the N-terminus and PCR was performed using
the primers self-complemented through 30 cycles of pre-denaturizing
(95°C for 5 min), denaturizing (94°C for 30 sec), annealing (55°C
for 30 sec) and extension (72°C for 2 min). Reaction products were
purified and preserved, then the optimized TSOL18 gene and
expression vector (pVAX1) were double digested with HindIII
and BamHI, respectively, and ligated with T4 DNA Ligase
(Fermentas) to construct the optimized expression vector
pVAX1/TSOL18. Following transfection into E. coli
DH5α, the positive transfectants were selected, cultured on 5 ml
lysogeny broth medium (Oxoid Limited, Basingstoke, UK)
(kanamycin+10 μg/ml) and confirmed through
HindIII/BamHI double digestion. The DNA sequence of
the clones was confirmed by Shanghai Sheng Gong Biological
Engineering Co., Ltd (Shanghai, China).
Chinese hamster ovary (CHO)-K1 cell
culture and transfection
CHO-K1 cells (cat. no. CCL-61; American Type Culture
Collection, Rockville, MD, USA) were cultured at 37°C in a
humidified atmosphere with 5% CO2 in F12K medium
(Gibco-BRL, Carlsbad, CA, USA) with 10% fetal calf serum
(Gibco-BRL), 2 mM l-glutamine and 1.5 g/l NaHCO3. For
the experiment 5×105 cells/ml were cultured per plate
and medium was replaced every 24 h prior to transfection. CHO-K1
cells floating in F12K medium containing 10% fetal calf serum were
seeded (3.5×105 cells/well) onto six-well cell culture
plates and anchorage-dependent cells reached 50–70% confluence.
Subsequently, 100 μl Sofast™ lipofection
transfection reagent (Sunma Biotechnology Co., Ltd., Xiamen, China)
diluent was added to 100 μl (1 μg/ml) optimized
plasmid pVAX1/TSOL18 and 100 μl (1 μg/ml)
plasmid pVAX1 (Invitrogen Life Technologies, Carlsbad, CA, USA).
The mixtures were then added to the wells of a CHO-K1 cell culture
plate with mixing. Following transfection, plasmid-transfected
CHO-K1 cells were cultivated at 37°C in a humidified atmosphere
with 5% CO2 in F12K medium with 10% fetal calf serum for
48 h, with replacement of the medium every 24 h. Transfected cells
were harvested and preserved at −80°C.
Reverse transcription (RT)-PCR analysis
of optimized pVAX1/TSOL18 mRNAs
Total RNA was extracted from CHO-K1 cells using
TRIzol (Invitrogen Life Technologies) 48 h following transfection,
according to the manufacturer’s instructions. The RNA precipitate
was pelleted through centrifugation at 11,500 × g for 10 min and
washed with 70% ethanol (Sigma-Aldrich, St. Louis, MO, USA).
Following an additional centrifugation under identical conditions,
the precipitate was dissolved in 20–30 μl 0.1%
diethylpyrocar-bonate (Sigma-Aldrich)-treated water and preserved
at −80°C. cDNA was synthesized using Revert Aid™ M-Mulv
Reverse Transcriptase (Fermentas) with oligo (dT) primer (1
μl) from the total RNA (4 mg) in a final volume of 20
μl, according to the manufacturer’s instructions under the
following conditions: 25°C for 5 min, 42°C for 60 min, 70°C for 5
min and then maintained at 4°C. PCR was performed using 0.2
μl cDNA obtained from the RT-reaction, described above, as a
template. The primer used for the optimized TSOL18 gene was
the same as above. The reaction mixture (25 μl) contained
0.2 μg cDNA template, 10 mM deoxyribonucleotide, 0.5
μl forward primer, 0.5 μl reverse primer and 0.5
μl Taq polymerase (PrimeSTAR HS PCR kit; Takara Bio, Inc.,
Tokyo, Japan). PCR was performed under the following conditions:
Preheating at 95°C for 3 min, denaturing at 94°C for 45 sec,
annealing at 52°C for 45 sec and extension at 72°C for 1 min.
Following 30 cycles of amplification, the PCR products were
analyzed using agarose gel electrophoresis.
Western blot analysis of optimized
pVAX1/TSOL18 protein
Transfected cells (1×107) were added to
100 μl 1X lysis buffer (1% NP-40, 0.5% deoxycholate and 0.1%
SDS; Sigma-Aldrich). Following boiling for 5 min, centrifugation at
8,944 × g at 4°C for 5 min was performed. The whole suspension was
then applied to 12% SDS-PAGE at 100 V for 120 min, and the proteins
were transferred to a nitrocellulose membrane (Millipore Corp.,
Bedford, MA, USA). Following blocking with 5% skimmed milk (Oxoid
Limited) in phosphate-buffered saline (PBS) at 4°C for 12 h, the
membranes were probed with 1:2,000 dilutions of the human
anti-oncosphere polyclonal antibody at 37°C for 2 h, followed by
the addition of horseradish peroxidase (HRP)-conjugated goat
anti-human polyclonal immunoglobulin G (1:1,000; Wuhan Boster
Biological Technology, Ltd., Wuhan, China) at 37°C for 2 h.
Reactive bands were visualized using diaminobenzidine (Wuhan Boster
Biological Technology, Ltd.). Meanwhile, standard protein markers
were cut off from the nitrocellulose membrane and dyed with
Ponceaux (Amresco LLC, Solon, OH, USA) for 10–15 min.
Immunohistochemical analysis
A total of 12 eight-week-old wild-type female BALB/c
mice were purchased from Anhui Medical University (Hefei, China),
and the experiment was approved by the Institutional Ethical Review
Committee of Bengbu Medical College (Bengbu, China). The mice were
fed with food and water ad libitum in a specific
pathogen-free environment maintained at 23±1°C, in a 12 h
light/dark cycle. The mice were randomly assigned to four groups
(n=3/group) and intramuscularly injected in the hind legs with 50
μl Sofast™ lipofection transfection reagent
diluent. In order to improve the efficiency of gene transfer,
bupivacaine (80 μl, 6.7 mg/ml; Sigma-Aldrich) was injected
at the same sites to regenerate muscle 24 h prior to the injection
of the plasmids. The groups were vaccinated as follows: Group A,
empty control, 10 μl 5% glucose (Sigma-Aldrich); group B,
empty vector, 100 μg pVAX1; group C, 100 μg
pVAX1/TSOL18; and group D, 100 μg optimized
pVAX1/TSOL18.
The 12 mice were sacrificed by CO2
inhalation 48 h following immunization to examine histological
changes within the injection site. Injected muscle tissues were
removed and fixed in 10% formalin (Sigma-Aldrich), embedded in
paraffin (Polysciences, Inc., Warrington, PA, USA) and sectioned
for microscopic examination. Muscle tissue sections were incubated
at 4°C for 12 h with human anti-oncosphere polyclonal antibody at
dilutions of 1:200, 1:300, 1:400 and 1:500, followed by the
addition of alkaline phosphatase (ALP)-conjugated rabbit
immunoglobulin G secondary antibody for immunohistochemistry.
Binding of antibodies to the muscle sections was evaluated using
light microscopy (BX41; Olympus Corporation, Tokyo, Japan) at
magnifications of ×100 and ×400. Slides were reviewed to evaluate
the staining result of the protein by two pathologists who were
blinded to the experiment at the First Affiliated Hospital of
Bengbu Medical College (Anhui, China). Scores were determined by
combining the proportion of positively stained muscle cells and the
intensity of staining. Mice muscle cell proportions were scored as
follows: 0, no positive cells; 1, <10% positive cells; 2, 10–35%
positive cells; 3, 35–75% positive cells; and 4, >75% positive
cells. Staining intensity was graded on a four-point scale: 1, no
staining; 2, light yellow; 3, yellow brown; and 4, brown. Total
scores of ≤1 were regarded as negative (−), 2 as low expression
(+), 3 as moderate expression (++) and ≥4 were taken as high
expression (+++).
Proliferation measurement by
3H-thymidine
Eight-week-old wild-type female BALB/c mice were
randomly assigned to four groups (n=10/group): Optimized
pVAX1/TSOL18 (150 μg/injection), pVAX1/TSOL18
(150 μg/injection), pVAX1 empty vector (150
μg/injection) and PBS (150 μg/injection). The four
groups were immunized intramuscularly in the hind legs for a total
of three immunizations administered at two-week intervals. In order
to improve the efficiency of gene transfer, 6.7 mg/ml bupivacaine
was injected at the same sites to regenerate muscle 1 h prior to
plasmid injection. This study was carried out in strict accordance
with the recommendations in the Guide for the Care and Use of
Laboratory Animals of the National Institutes of Health. The
protocol was approved by the Committee on the Ethics of Animal
Experiments of the Institute for Bengbu Medical College (permit no.
LAEC-2009-027). All surgery was performed under sodium
pentobarbital anesthesia, and all efforts were made to minimize
suffering.
Two weeks following the final immunization, mice
were sacrificed, spleens were excised and single viable cell
suspensions were isolated. Erythrocytes were lysed by ammonium
chloride (155 mM; Sigma-Aldrich) and splenocyte cultures from
individual animals were prepared using a syringe plunger, pressing
spleen tissue through a cell strainer. Following washing in RPMI
1640 media (Gibco-BRL), supplemented with 10% fetal bovine serum, 2
mmol/l glutamine, 1 mmol/l sodium pyruvate, 100 U/ml penicillin
(Sigma-Aldrich), 100 μg/ml streptomycin (Sigma-Aldrich) and
12.5 U/ml nystatin (Sigma-Aldrich), lymphocytes were counted using
trypan blue to identify viable cells and were resuspended to a
concentration of 1×106 cells/ml. Cells (2×105
in 200 μl) were plated in 96-well plates and stimulated in
triplicate using the corresponding TSOL18 protein (1
μg/μl, 20 μl per well; as previously prepared
in our lab). The positive control was conA. Following 48 h of
incubation at 37°C with 5% CO2, cells were pulsed with 1
μCi 3H-thymidine for 24 h to measure
proliferation. Cells were then harvested and thymidine
incorporation was determined using a liquid scintillation counter
(FJ-2107P; Xi’an Nuclear Instrument Factory, Xi’an, China) at 72
h.
Statistical analysis
Values are presented as the mean ± standard error of
the mean. All data were analyzed using SPSS 17.0 software
(International Business Machines, Armonk, NY, USA). Comparisons
between the means were made using a one-way analysis of variance.
P<0.05 was considered to indicate a statistically significant
difference between values.
Results
Codon optimization, design and synthesis
of the TSOL18 gene
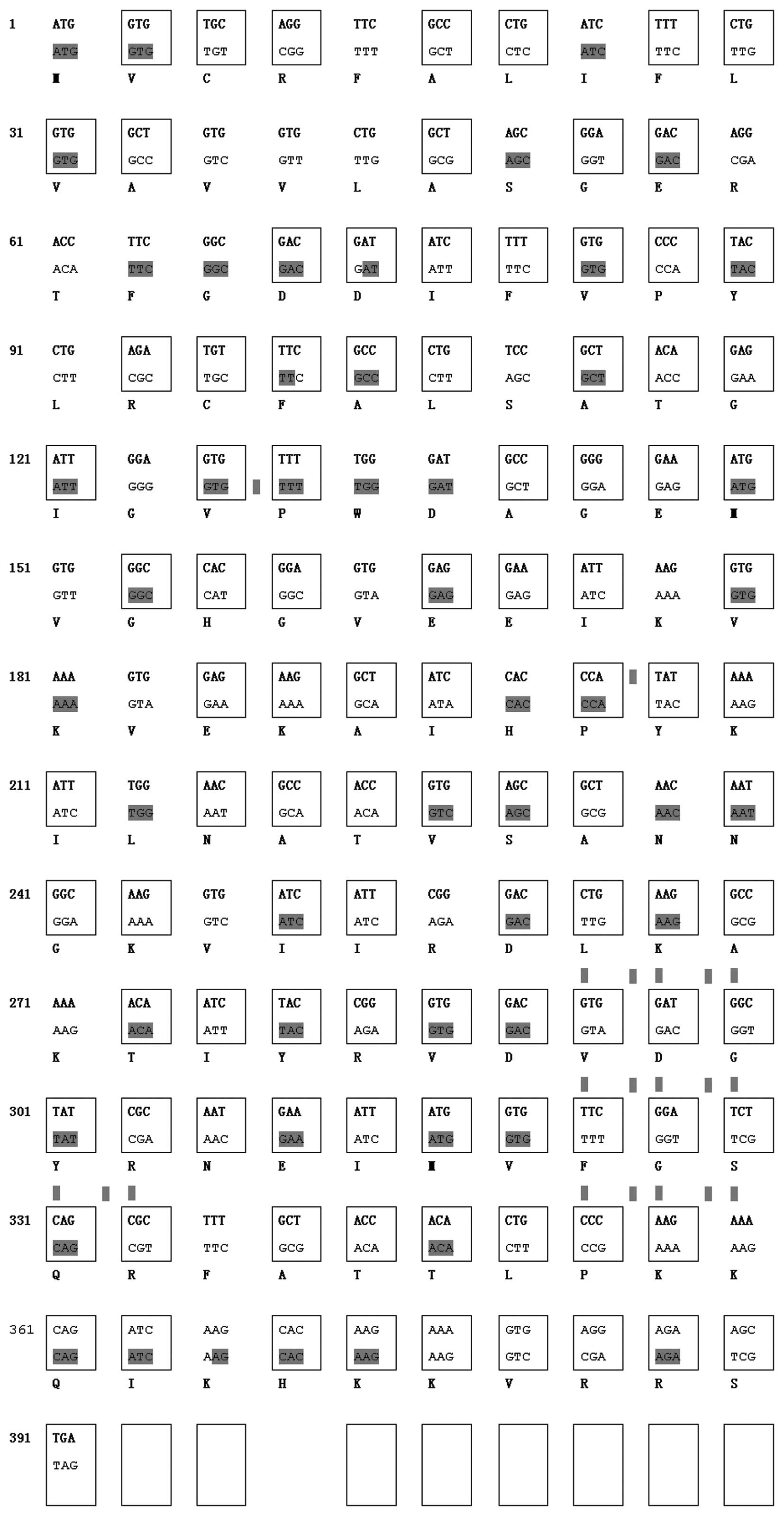
A comparison of the TSOL18 gene and
codon-optimized TSOL18 gene is shown in Fig. 1. A total of 79 out of 130 amino
acids of TSOL18 were modified, and identical residues are
marked in yellow.
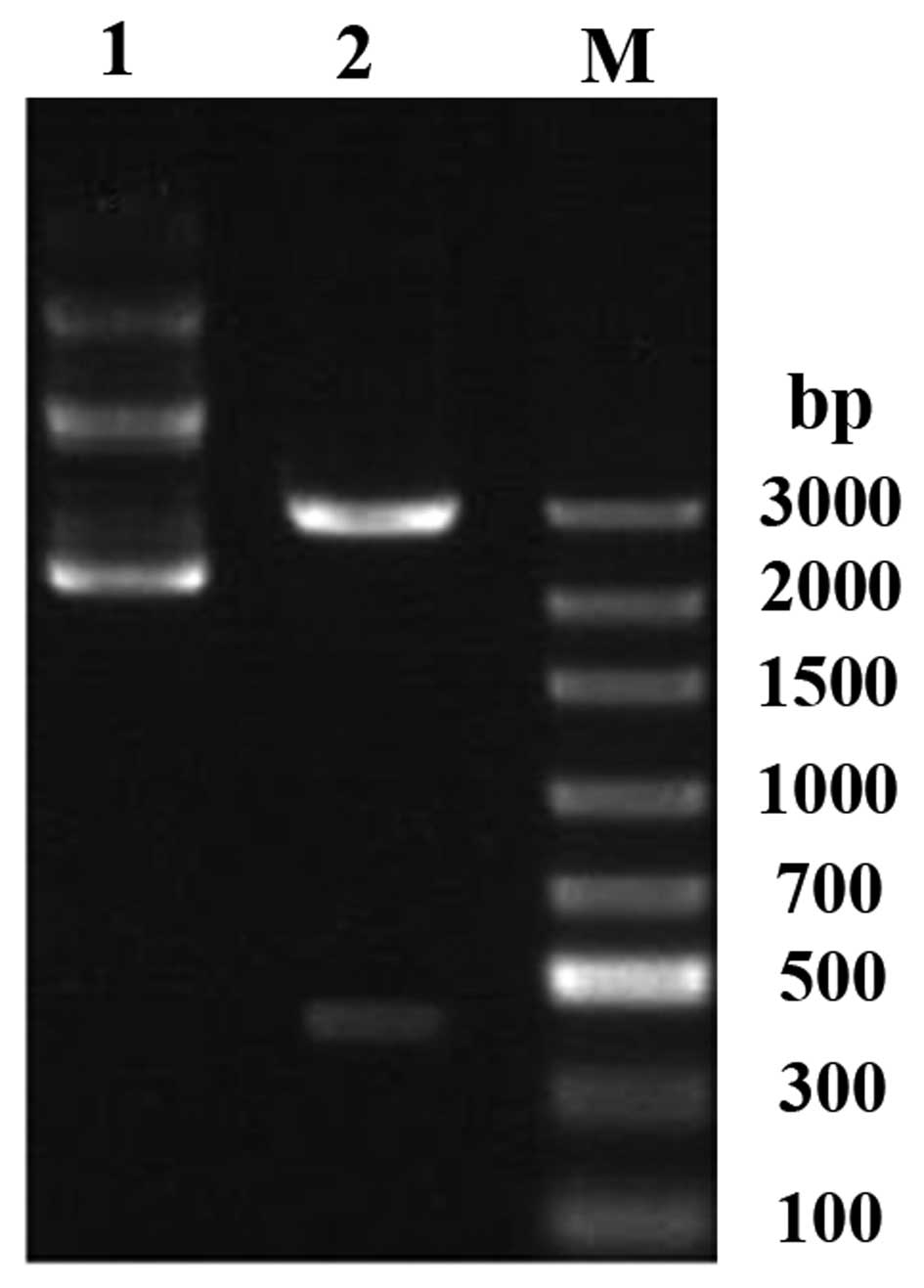
Fig. 2 shows the
results of the electrophoresis of optimized pVAX1/TSOL18 and
recombinant plasmid-optimized pVAX1/TSOL18 following
digestion with HindIII and BamHI. Following
electrophoresis, two different sets of DNA fragments were detected.
The band at ~3,000 bp indicated the full-length recombinant plasmid
vector DNA and the band at ~414 bp indicated the optimized
pVAX1/TSOL18 DNA. These results were consistent with the
theoretical base-pair calculation and the fragments corresponding
to the predicted DNA length. In addition, the inserted fragment
(414 bp) was confirmed to be recombinant plasmid-optimized
pVAX1/TSOL18, as determined using directed sequencing.
Expression of optimized TSOL18 in
eukaryotic cells
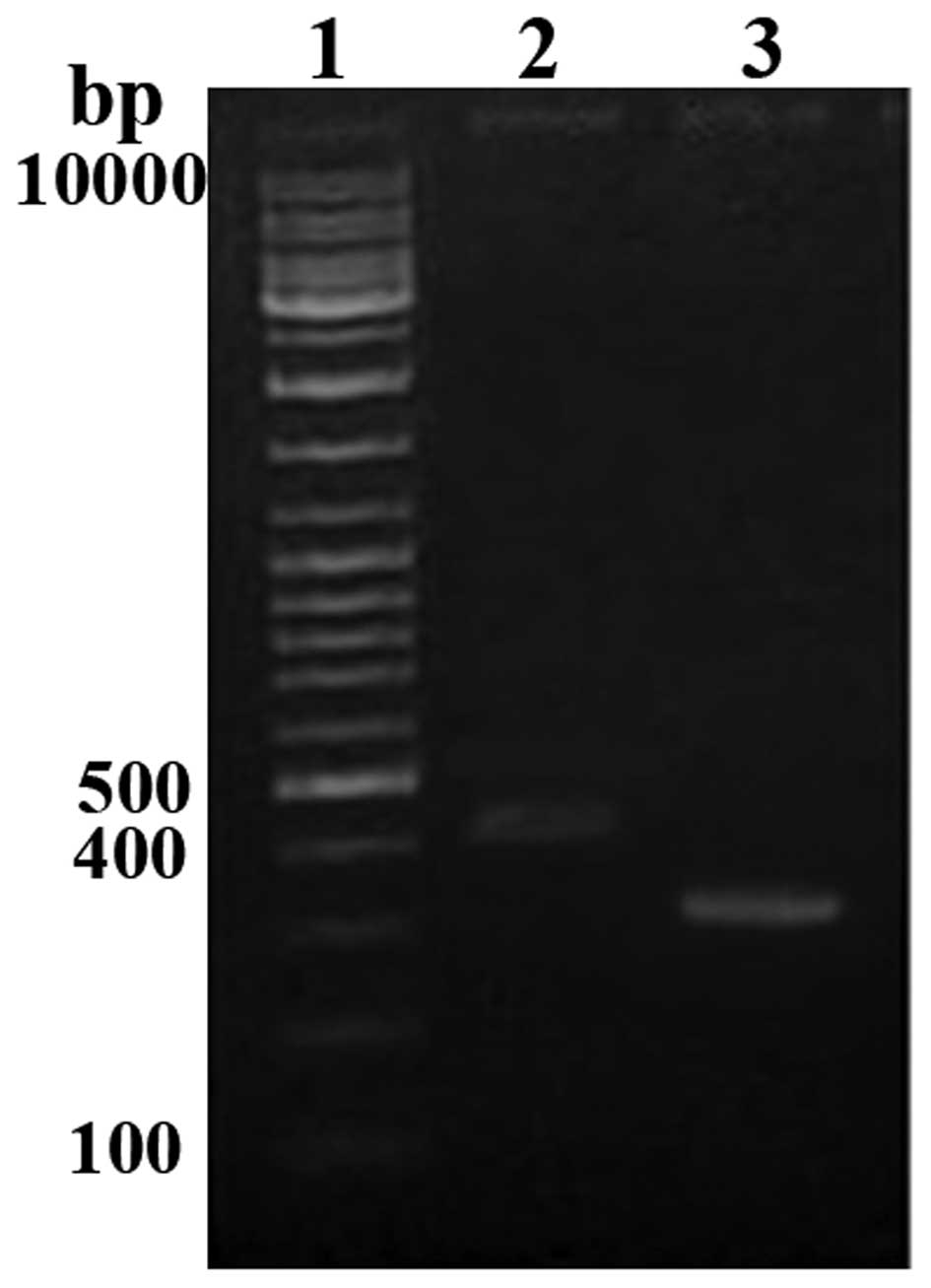
The functions of optimized pVAX1/TSOL18 gene
vaccine rely on its expression of a certain amount of protein. To
verify whether the optimized pVAX1/TSOL18 gene could be
cultured in the eukaryotic cell system, CHO-K1 cells were
transfected and the mRNA expression of optimized TSOL18 was
analyzed using RT-PCR. The results showed that the size of product
was in accordance with the expected size (~414 bp) (Fig. 3); the internal control β-actin was
detected at similar levels (~300 bp). These results demonstrated
that the recombinant plasmid DNA vaccine expressed TSOL18 in
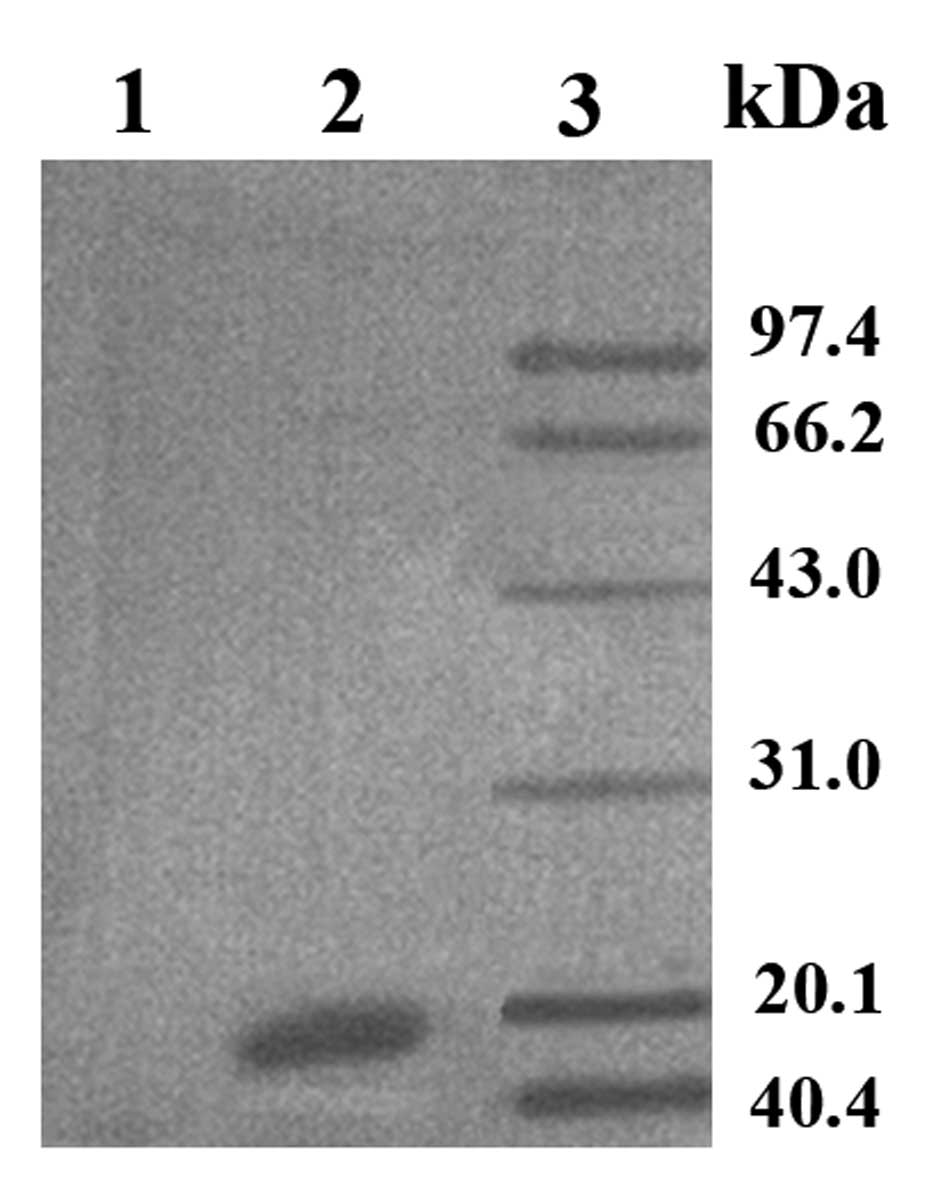
eukaryotic cells. The cell lysate was then analyzed by western
blotting, which revealed an obvious strong protein band at 18 kDa
in the optimized pVAX1/TSOL18 group, whereas the empty
vector pVAX1 group had no band (Fig.
4). These results demonstrated that the recombinant plasmid was
able to express protein with immunocompetence in CHO-K1 cells.
Expression of optimized TSOL18 in
vivo
In order to determine whether optimized codon usage
enhances TSOL18 gene expression in vivo,
immunohistochemical analysis was performed to observe its
expression in the hind leg muscles of mice (Fig. 5). The results revealed that
immunostaining in the muscle of the optimized pVAX1/TSOL18
group was positive (+++), with brown-yellow color pellets in the
muscle fibers and more pellets were present in the optimized
pVAX1/TSOL18 group compared with that of the
pVAX1/TSOL18 group (++). In addition, the pVAX1 group muscle
was negative for TSOL18 expression (-~+).
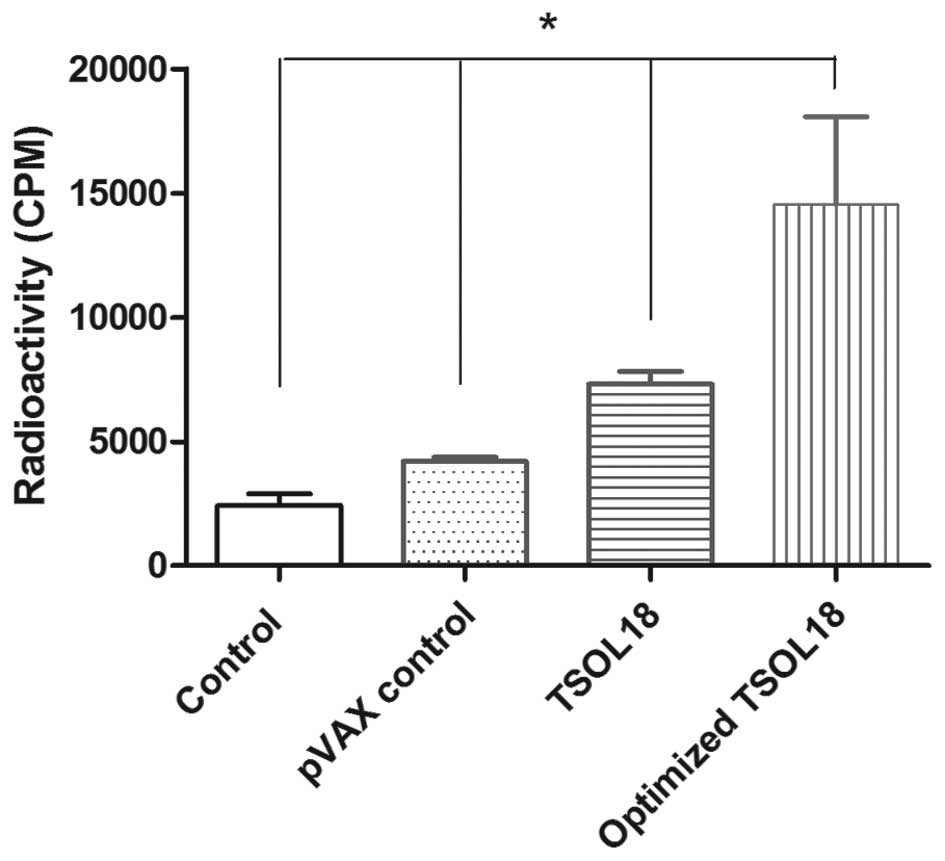
Cell proliferation effect induced by the
TSOL18 protein
In order to further verify whether the optimized
pVAX1/TSOL18 induced a good immunity response, the cell
proliferation rate was determined using 3H-thymidine. As
shown in Fig. 6,
3H-thymidine incorporation was significantly greater in
the optimized pVAX1/TSOL18 group compared with that in the
pVAX1/TSOL18, pVAX1 and blank control groups (P<0.01).
This suggested that the number of spleen cells in the optimized
pVAX1/TSOL18 group stimulated by the TSOL18 protein
was significantly increased compared with that in the
pVAX1/TSOL18 group, indicating that the optimized
pVAX1/TSOL18 demonstrated enhanced immunogenicity compared
with that of pVAX1/TSOL18.
Discussion
Numerous factors may impact the expression of a
protein, including the suitability of the promoter, codon bias, the
position of the Shine-Dalgarno sequence and the stability of mRNA
(18,21). If a gene experiences strong
selection pressure (such as high expression), it may have more
usage codon bias and thus, a higher translation efficiency
(22). Using codon modification,
Zheng et al (23)
successfully enhanced the expression of the glycoprotein gene in
E. coli. In addition, Mani et al (24) reported that the codon adaptation
index and guanine-cytosine content of the genes in optimized DNA
was significantly enhanced.
In the present study, in order to improve
TSOL18 expression, the codon optimization method was used to
match codon frequencies in animal (mouse) organisms, modify
ribosome binding sites and mRNA degradation sites, as well as
adjust translational rates to allow various domains of the protein
to fold properly (25). The codon
usage for TSOL18 was mapped. RT-PCR amplification and gel
electrophoresis revealed an obvious band at ~414 bp in the
recombinant plasmid optimized pVAX1/TSOL18 transfected cells,
indicating that the constructed plasmid had been successfully
transfected into the CHO-K1 cells. It has been reported that the
induction of a specific immune response only occurs when the gene
accessing the host body expresses a certain amount of protein
(26,27). Western blot analysis of the cell
lysates revealed an obvious strong protein band at 18 kDa in the
optimized pVAX1/TSOL18 group, whereas the empty vector pVAX1
group had no band. These results demonstrated that the recombinant
plasmid was able to express protein with immunocompetence in
eukaryotic cells.
In the present study, pVAX1 was selected as the
carrier vaccine expression vector, the safety of which has been
approved by the Food and Drug Administration; in addition, CHO-K1
cells were previously found to be suitable for the expression of
the pVAX1 plasmid (28). In the
present study, the cationic polymer Sofast™ was applied
in order to insert the reconstruction plasmid pVAX1/TSOL18
into CHO-K1 cells. Due to the high transfection efficiency and low
cytotoxicity of Sofast™, the procedure is simple and
quick (29).
DNA vaccines combine pathogen genes, encoding
effective immunogens, and plasmids in order to get into the host
cells through direct immunization and express the protective
antigens (30). Thus, plasmid DNA
vaccines must be injected into animal models in order to verify
their effectiveness. In the present study, mice were selected
instead of pigs as an animal model due to their small size, clear
genetic background, easy use and exhibition of similar immune
effects. At present, gene vaccine immunization is primarily
performed using intramuscular injections, the gene gun method,
mucosal immunization or intravenous and celiac injections (31). The route and delivery method used
for genetic immunization studies have numerous implications
affecting the outcome of the immune response. In the present study,
intramuscular injection was selected as the method of immunization
as experiments have shown that the DNA is readily absorbed by the
host cells (Fig. 5D). Sheep were
immunized intramuscularly with TO45w plasmid DNA in a study by
Rothel et al (32), the
results of which demonstrated high levels of immunoglobulin G1. In
addition, BALB/c mice were immunized intramuscularly with plasmid
DNA (pCDI-K45w, pcDI-K45 sec and pcDI-K45wTR) in a study by Drew
et al (33), which reported
that the cellular localization of the DNA vaccine antigen had a
significant effect on the production of antibody.
The results of the present study revealed that
immunostaining with anti-oncosphere antibodies in plasmid-injected
muscle showed positive staining for TSOL18, whereas
immunostaining in empty pVAX1-injected muscle showed negative
results. In addition, an increased number of brown-yellow color
pellets was observed in the optimized pVAX1/TSOL18 gene
group compared with that of the pVAX1/TSOL18 group; this
therefore indicated that the optimized pVAX1/TSOL18 gene
expressed more protein compared with that of the un-optimized
gene.
3H-thymidine, as a DNA precursor, can be
used to indicate the degree of cell proliferation (34–36).
In the present study, the lymphocyte transformation degree was
determined according to 3H-thymidine radiation in the
lymphocytes. This method is objective, sensitive and accurate
(34–36). The results revealed that the number
of spleen cells stimulated in the optimized pVAX1/TSOL18
gene group was significantly increased compared with that of the
pVAX1/TSOL18 gene and empty contrast groups. These results
indicated that following codon optimization, the quantity of
protein expression increased and the active spleen cells were
stimulated to rapidly proliferate, and therefore induced improved
immunogenicity compared with that of the pVAX1/TSOL18
group.
In conclusion, the eukaryotic expression vector
containing the codon-optimized TSOL18 gene was successfully
constructed and expressed in vivo as well as in
vitro. In addition, the expression and immunogenicity of the
codon-optimized TSOL18 gene were markedly greater compared
with those of the un-optimized gene in mice. These results provided
the basis for developing an optimized TSOL18 gene vaccine
against cysticercosis.
Acknowledgments
The authors would like thank Professor Junjie Sun,
Department of Nuclear medicine, Bengbu Medical College and
Professor Baiqing Li, MD, PhD, Department of Immunology, Bengbu
Medical College, for their technical support. The present study was
supported by grants from The Natural Science Foundation of China
(no. 30600518), Anhui Provincial Natural Science Research Project
(no. KJ2011B095) and Bengbu Medical College Fund (no. BY1001).
References
|
1
|
Shoji H, Hirai T, Shirakura T, et al: A
case of cysticercosis with multiple lesions in the brain and
femoral muscles. Kansenshogaku Zasshi. 87:608–612. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rasamoelina-Andriamanivo H, Porphyre V and
Jambou R: Control of cysticercosis in Madagascar: beware of the
pitfalls. Trends Parasitol. 29:538–547. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Iacoangeli M, Moriconi E, Gladi M and
Scerrati M: Isolated cysticercosis of the cauda equina. J Neurosci
Rural Pract. 4(Suppl 1): S117–S119. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sciutto E, Fragoso G, Fleury A, et al:
Taenia solium disease in humans and pigs: an ancient parasitosis
disease rooted in developing countries and emerging as a major
health problem of global dimensions. Microbes Infect. 2:1875–1890.
2000. View Article : Google Scholar
|
|
5
|
Schantz PM, Cruz M, Sarti E and Pawlowski
Z: Potential eradicability of taeniasis and cysticercosis. Bull Pan
Am Health Organ. 27:397–403. 1993.PubMed/NCBI
|
|
6
|
García HH, Gonzalez AE, Evans CA and
Gilman RH; Cysticercosis Working Group in Peru: Taenia solium
cysticercosis. Lancet. 362:547–556. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Drew DR, Lightowlers M and Strugnell RA:
Vaccination with plasmid DNA expressing antigen from genomic or
cDNA gene forms induces equivalent humoral immune responses.
Vaccine. 18:692–702. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Solís CF, Ostoa-Saloma P, Lugo-Martínez
VH, Johnston SA and Laclette JP: vaccination against murine
cysticercosis by using a plasmid vector carrying Taenia solium
paramyosin. Infect Immun. 73:1895–1897. 2005. View Article : Google Scholar
|
|
9
|
Harrison GB, Heath DD, Dempster RP, et al:
Identification and cDNA cloning of two novel low molecular weight
host-protective antigens from Taenia ovis oncospheres. Int J
Parasitol. 26:195–204. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lightowlers MW, Rolfe R and Gauci CG:
Taenia saginata: vaccination against cysticercosis in cattle with
recombinant oncosphere antigens. Exp Parasitol. 84:330–338. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lightowlers MW, Flisser A, Gauci CG, Heath
DD, Jensen O and Rolfe R: Vaccination against cysticercosis and
hydatid disease. Parasitol Today. 16:191–196. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rosas G, Fragoso G, Garate T, et al:
Protective immunity against Taenia crassiceps murine cysticercosis
induced by DNA vaccination with a Taenia saginata tegument antigen.
Microbes Infect. 4:1417–1426. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Martinez-Ocaña J, Romero-Valdovinos M, de
Kaminsky RG, Maravilla P and Flisser A: Immunolocalization of TSOL
18 and TSOL 45-1A, the successful protective peptides against
porcine cysticercosis, in Taenia solium oncospheres. Parasit
Vectors. 4:32011. View Article : Google Scholar
|
|
14
|
Flisser A, Gauci CG, Zoli A, et al:
Induction of protection against porcine cysticercosis by
vaccination with recombinant oncosphere antigens. Infect Immun.
72:5292–5297. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cai X, Yuan G, Zheng Y, et al: Effective
production and purification of the glycosylated TSOL 18 antigen,
which is protective against pig cysticercosis. Infect Immun.
76:767–770. 2008. View Article : Google Scholar :
|
|
16
|
Zhou Z, Schnake P, Xiao L and Lal AA:
Enhanced expression of a recombinant malaria candidate vaccine in
Escherichia coli by codon optimization. Protein Expr Purif.
34:87–94. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Villalobos A, Ness JE, Gustafsson C, et
al: Gene Designer: a synthetic biology tool for constructing
artificial DNA segments. BMC Bioinformatics. 7:2852006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sharp PM and Devine KM: Codon usage and
gene expression level in Dictyostelium discoideum: highly expressed
genes do ‘prefer’ optimal codons. Nucleic Acids Res. 17:5029–5039.
1989. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gao Z, Li Z, Zhang Y, et al: High-level
expression of the Penicillium notatum glucose oxidase gene in
Pichia pastoris using codon optimization. Biotechnol Lett.
34:507–514. 2012. View Article : Google Scholar
|
|
20
|
Lee MS, Hseu YC, Lai GH, et al: High yield
expression in a recombinant E. coli of a codon optimized chicken
anemia virus capsid protein VP1 useful for vaccine development.
Microb Cell Fact. 10:562011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gustafsson C, Govindarajan S and Minshull
J: Codon bias and heterologous protein expression. Trends
Biotechnol. 22:346–353. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bulmer M: The selection-mutation-drift
theory of synonymous codon usage. Genetics. 129:897–907.
1991.PubMed/NCBI
|
|
23
|
Zheng JL, Jian GB and Guo XF: Codon
modification for improvement in prokaryotic expression of
glycoprotein gene in rabies virus. Chin J Zoonoses. 26:403–407.
2010.
|
|
24
|
Mani I, Singh V, Chaudhary DK, Somvanshi P
and Negi MP: Codon optimization of the major antigen encoding genes
of diverse strains of influenza a virus. Interdiscip Sci. 3:36–42.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Raab D, Graf M, Notka F, Schödl T and
Wagner R: The GeneOptimizer Algorithm: using a sliding window
approach to cope with the vast sequence space in multiparameter DNA
sequence optimization. Syst Synth Biol. 4:215–225. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang JX, Peng YC and Yao YX:
Stage-specific expression of antigen encoded cDNA of cysticercus
cellulosae. Chin J Parasitol Parasit Dis. 2:89–93. 1996.
|
|
27
|
Greene RM, Hancock K, Wilkins PP and Tsang
VC: Taenia solium: molecular cloning and serologic evaluation of
14- and 18-kDa related, diagnostic antigens. J Parasitol.
86:1001–1007. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hiszczyńska-Sawicka E, Li H, Xu JB, et al:
Comparison of immune response in sheep immunized with DNA vaccine
encoding Toxoplasma gondii GRA7 antigen in different adjuvant
formulations. Exp Parasitol. 124:365–372. 2010. View Article : Google Scholar
|
|
29
|
Yang TC: Study on the transfection
efficiency of the new cationic polymer transfection reagent:
Sofast. Xia men da xue xue bao. 43:572–577. 2004.
|
|
30
|
Khan KH: DNA vaccines: roles against
diseases. Germs. 3:26–35. 2013. View Article : Google Scholar
|
|
31
|
Vibanco-perez N, Jimenez L,
Mendoza-Hernandez G and Landa A: Characterization of a recombinant
mu-class glutathione stransferase from Taenia solium. Parasitol
Res. 88:394–404. 2002.
|
|
32
|
Rothel JS, Boyle DB, Both GW, et al:
Sequential nucleic acid and recombinant adenovirus vaccination
induces host-protective immune responses against Taenia ovis
infection in sheep. Parasite Immunol. 19:221–227. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Drew DR, Lightowlers M and Strugnell RA:
Humoral immune responses to DNA vaccines expressing secreted,
membrane bound and non-secreted forms of the Tania ovis 45W
antigen. Vaccine. 18:2522–2532. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Dong XX, Zhang C, Yang XW, et al:
Activities of treg cells stimulated by soluble adult worm antigen
and egg antigen of Schistosoma japonicum. Zhongguo Xue Xi Chong
Bing Fang Zhi Za Zhi. 25:146–150. 2013.PubMed/NCBI
|
|
35
|
Hwang J, Yi M, Zhang X, Xu Y, Jung JH and
Kim DK: Cytochalasin B induces apoptosis through the mitochondrial
apoptotic pathway in HeLa human cervical carcinoma cells. Oncol
Rep. 30:1929–1935. 2013.PubMed/NCBI
|
|
36
|
Weng SX, Sui MH, Chen S, et al:
Parthenolide inhibits proliferation of vascular smooth muscle cells
through induction of G0/G1 phase cell cycle arrest. Zhejiang Univ
Sci B. 10:528–535. 2009. View Article : Google Scholar
|