Introduction
Organophosphorus pesticides have been widely used in
agricultural environments, since the ban of organochlorine
insecticides, to protect crops against a range of pests due to
their broad spectrum of insecticidal activity, effectiveness and
their non-persistence in the environment (1). Despite the benefits of pesticides,
residues may remain in crops, animal feed and environmental
substrates, leading to contamination and potential human exposure
through the food chain (2–4). Chlorpyrifos (CPF) is a major type of
organophosphorus pesticide and is currently used in the production
of >40 crops and fruits, including peaches, citrus fruits,
almonds and grapes (1). CPF and
its metabolites have been detected in farm animals, including
cattle and sheep (1,5), and CPF residues or metabolites have
also been detected in food and human urine (6). In addition, CPF has been found in the
umbilical cord blood from female individuals living in urban
environments (7). CPF has been
confirmed as a neurotoxin by inhibiting acetylcholinesterase in the
central nervous system (8). This
can damage the nervous system, resulting in dizziness, nausea,
confusion and, at a high concentration, respiratory paralysis and
mortality. Additionally, a previous study demonstrated that CPF
incurs developmental toxicity in rats at concentrations of 25
mg/kg/day, which was a maternally toxic dose (9). Similar to the majority of
environmental pollutants, CPF elicits significant cytotoxicity
through generating oxidative stress (10–12).
Cadmium (Cd) is one of the most prevalent
environmental heavy metals, and human exposure to Cd is associated
with a number of toxicities, including hepatotoxicity, DNA damage
and cell apoptosis (13–15). Notably, CPF and Cd often coexist in
the same environmental media and food chains, causing simultaneous
exposure to organisms (16,17)
and resulting in common toxicities, including carcinogenicity and
hepatotoxicity (18–20). Our previous study characterized an
interaction between Cd and CPF, and this interaction likely occurs
due to bonding between Cd and nitrogen atoms in the pyridine ring
of CPF, or the chelation between one Cd2+ and two CPF
molecules (21). The joint
hepatotoxicity of Cd and CPF to HepG2 cells was also demonstrated,
with the Cd-CPF complex increasing the level of apoptosis by
compared with its parental components (21).
Hepatic lipid metabolism is important in governing
the whole body energy metabolism, as the liver is the major site
for the storage and release of glucose and lipids (22,23).
Lipid accumulation within liver has been suggested to cause
obesity, insulin resistance and type II diabetes (22–24),
and also predisposes individuals to nutritional stresses.
Increasing evidence suggests that fatty acid synthase (FASN) is a
critical regulator of hepatic lipid homeostasis, including fat and
cholesterol synthesis (25,26).
FASN encodes one of the key enzymes involved in fatty acid
synthesis and is required for the de novo synthesis of fatty
acids (27). Upregulated
expression levels of FASN have been reported in various types of
human cancer, and has been suggested to contribute to poor
prognosis and recurrence of these types of cancer (28). Sterol regulatory element-binding
protein (SREBP) is the predominant transcriptional activator of
FASN (29,30), and previous studies have revealed
that the increased expression of SREBP-1 is significantly
associated with the extent of fatty liver in mouse models of
diabetes mellitus (31–33). The present study aimed to
investigate the synergistic effect of Cd and CPF on fat metabolism
and fat accumulation in hepatocytes.
Materials and methods
Chemicals and reagents
CPF was purchased from Shuangma Fine Chemical Co.,
Ltd. (Nantong, China) and was dissolved in dimethyl sulfoxide
(DMSO) (Solarbio Science & Technology Co., Ltd., Beijing,
China). The final concentration of DMSO was <0.1% in the culture
medium. CdCl2 was purchased from Sigma-Aldrich (St.
Louis, MO, USA). Sterile water was used to dissolve the
CdCl2, and the stock solution was filtered through a
0.45 mm membrane (Solarbio Science & Technology Co., Ltd.).
Cell culture
The HepG2 human hepatic carcinoma cell line was
purchased from Shanghai Cell Bank of Type Culture Collection of the
Chinese Academy of Sciences (Shanghai, China). The HepG2 cells were
cultured at a concentration of 5.0×103 cells/well in
RPMI-1640 medium (Gibco Life Technologies, Carlsbad, CA, USA)
supplemented with 10% fetal bovine serum (FBS; HyClone, Logan, UT.
USA) and 100 U/ml penicillin-streptomycin (HyClone) at 37°C in an
atmosphere of 5% CO2. The cell culture medium was
changed every day and the cells were passed every other day.
Cytotoxicity assessment
The cytotoxicity was initially screened using an MTT
assay (Invitrogen Life Technologies, Carlsbad, CA, USA). Briefly,
the cells were inoculated and cultured overnight at 37°C in 96-well
plates (Corning, Inc., New York, NY, USA) at a density of
8.0×103 cells/well in RPMI-1640 medium supplemented with
penicillin, streptomycin (100 mM) and 1% FBS. Following culture,
the HepG2 cells were treated with various concentrations of CPF
(10, 50, 100, 500, 1,000, 1,500, 2,000 or 2,500 μM) or Cd
(5, 10, 20, 40, 60, 80 or 100 μM) for a further 24 h at
37°C, and 20 μl MTT (5 mg/ml) was then added to each well.
Following an additional 4 h incubation at 37°C, 100 μl DMSO
was added to each well, followed by absorption assessment at 490 nm
on a microplate reader (Multiskan MK3; Thermo Fisher Scientific,
Co., Ltd., Waltham, MA, USA). The synergistic toxicity was further
determined using an Alamar Blue assay (Invitrogen Life
Technologies) and a bromodeoxyuridine (BrdU) assay (Roche
Diagnostics, Mannheim, Germany). Similar to the MTT assay,
following treatment with 10 μM CPF or Cd, 10 μl
Alamar Blue reagent (Invitrogen Life Technologies) was added to
each well and incubated for 2 h at 37°C, prior to the plates being
read on a microplate reader with an emission wavelength of 590 nm
and an excitation wavelength of 540 nm. The BrdU assay was
performed according to the manufacturer’s instructions, as
described previously (34).
High glucose exposure
The cells were exposed to high glucose, as described
previously (35). Briefly, the
HepG2 cells were seeded into 6-well plates in RPMI-1640 medium
supplemented with 10% FBS. The cells were grown to 70% confluence,
prior to being maintained in serum-free RPMI-1640 medium,
containing high concentrations of glucose (30 mmol/l) overnight.
The cells were subsequently treated with Cd and CPF, as described
above.
Determination of total cholesterol and
triglyceride levels
The intracellular concentrations of total
cholesterol and triglycerides were assayed in the HepG2 cell
lysates, according to the manufacturer’s instructions of the
Triglycerides Assay and Total Cholesterol Assay kits (Nanjing
Jiancheng Bioengineering Institute, Nanjing, China). The cell
pellet was collected and 0.3 ml Triton-X 100 (1–2%) was added. It
was lysed for 30 min prior to the start of the assays according to
the manufacturer’s instructions. The mixture was incubated at 37°C
for 5 min, followed by reading with the microplate reader at 590
nm.
Western blotting
Following the treatment with Cd and CPF, described
above, the HepG2 cells were collected and washed twice with
phosphate-buffered saline (Solarbio Science & Technology Co.,
Ltd.). The harvested cells were lysed in radioimmunoprecipitation
lysis buffer supplemented with protease inhibitor cocktail (Roche
Diagnostics) on ice for 30 min. The cells were subsequently
centrifuged at 12,000 × g for 10 min. The supernatants were
collected and were subjected to 10% SDS-PAGE (gel, Beijing ComWin
Biotech Co., Ltd., Beijing, China; SDS buffer, Solarbio Science
& Technology Co., Ltd.) and were transferred onto
nitrocellulose membranes (Solarbio Science & Technology Co.,
Ltd.). The membranes were incubated with primary antibodies against
SREBP-1 (1:200; bs-1402R), FASN (1:200; bs-1498R-PE) and β-actin
(1:1,000; bs-0061R) (Bioss, Beijing, China) in 5% milk overnight at
4°C. They were then washed 3 times in Tris-buffered saline with
Tween-20 (Solarbio Science & Technology Co., Ltd.) for 5 min,
and then incubated with anti-rabbit-horseradish peroxidase
secondary antibodies (1:8,000; ComWin Biotech Co., Ltd.) for 1 h at
37°C in 5% milk, then washed again in a similar manner. The target
proteins were colored by a chemical method. The intensity of the
protein bands were assessed using Image J software, version 1.48
(NIH, Bethesda, MD, USA).
Statistical analysis
Using SPSS software, version 17.0 (SPSS, Inc.,
Chicago, IL, USA) Student’s two-tailed t-test was performed to
analyze the experimental data between two groups, and one-way
analysis of variance was used to analyze the mean differences
between groups relative to the control. The data are expressed as
the mean ± standard deviation. P<0.05 was considered to indicate
a statistically significant difference.
Results and Discussion
The toxicity of combinations of environmental
pollutants is important when determining the health risks of each
individually. Cd and CPF have been demonstrated to have common
targets and elicit similar phenotypes, including hepatotoxicity
(11,36–38).
Our previous study revealed a novel interaction between Cd ions and
CPF, and described the cytotoxicity induced by the Cd-CPF complex
(21). The present study
hypothesized that the synergistic cytotoxicity between CPF and
Cd2+ was likely to cause metabolic disorders in
hepatocytes. Initially, a series of experiments were performed to
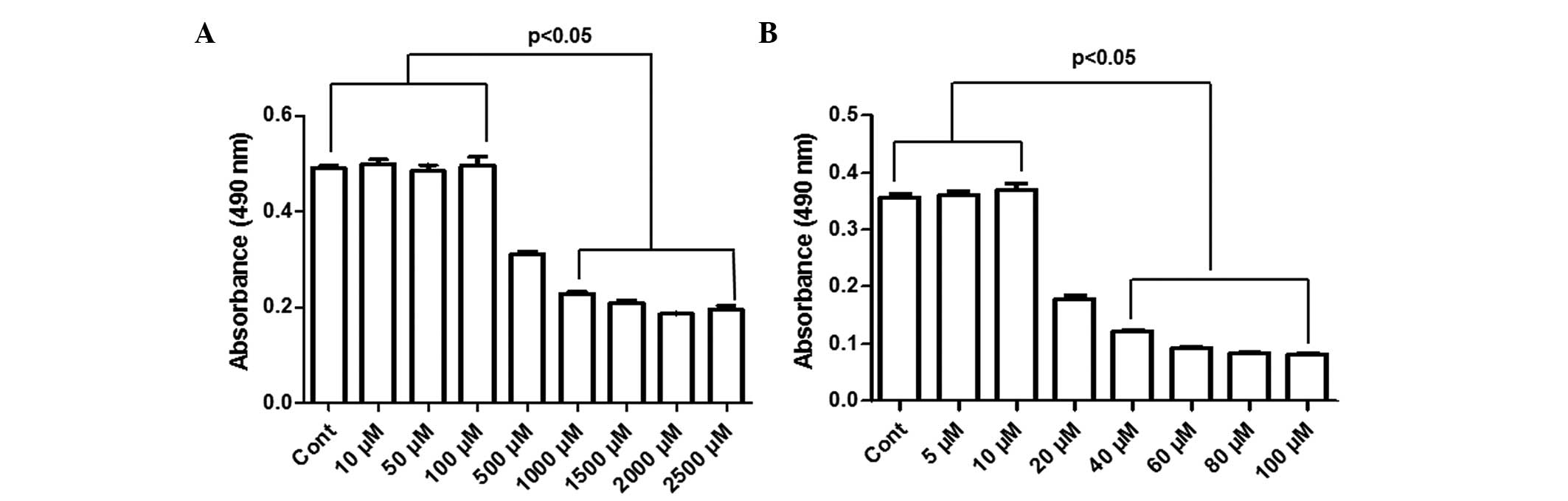
confirm the synergistic toxicity, as previously discussed (21). As shown in Fig. 1A, the MTT assay revealed that CPF
induced significant toxicity to the HepG2 cells following 24 h
treatment with 0.5, 1, 1.5, 2 and 2.5 mM, in a dose-dependent
manner, compared with the control (P<0.05). The half maximal
inhibitory concentration (IC50) was ~0.5 mM in the HepG2
cells (Fig. 1A). No cytotoxicity
was observed in the HepG2 cells at CPF concentrations ≤100
μM (P>0.05; Fig. 1A). In
addition, the cytotoxicity of Cd was determined in HepG2 cells
treated with 5, 10, 20, 40, 60, 80 and 100 μM
CdCl2. Following treatment for 24 h, no toxicity was
observed ≤10 μM (P>0.05; Fig. 1B), however, CdCl2
induced significant cell death at Cd concentrations >10
μM (P<0.05). The IC50 value for
Cd2+ was ~20 μM (Fig. 1B).
Previous studies have suggested that the formation
of a complex between chemicals alters their transport across the
cell membrane and increases intracellular localization, resulting
in enforced cytotoxicity, which may not occur with individual
chemicals (39–41). A classical interaction of CPF was
identified with methyl mercury, as the formation of this complex
significantly increased the bioaccumulation of methyl mercury,
coupled with increased toxicity (42). Our previous study demonstrated that
the combined toxicity of Cd and CPF was attributable to the Cd-CPF
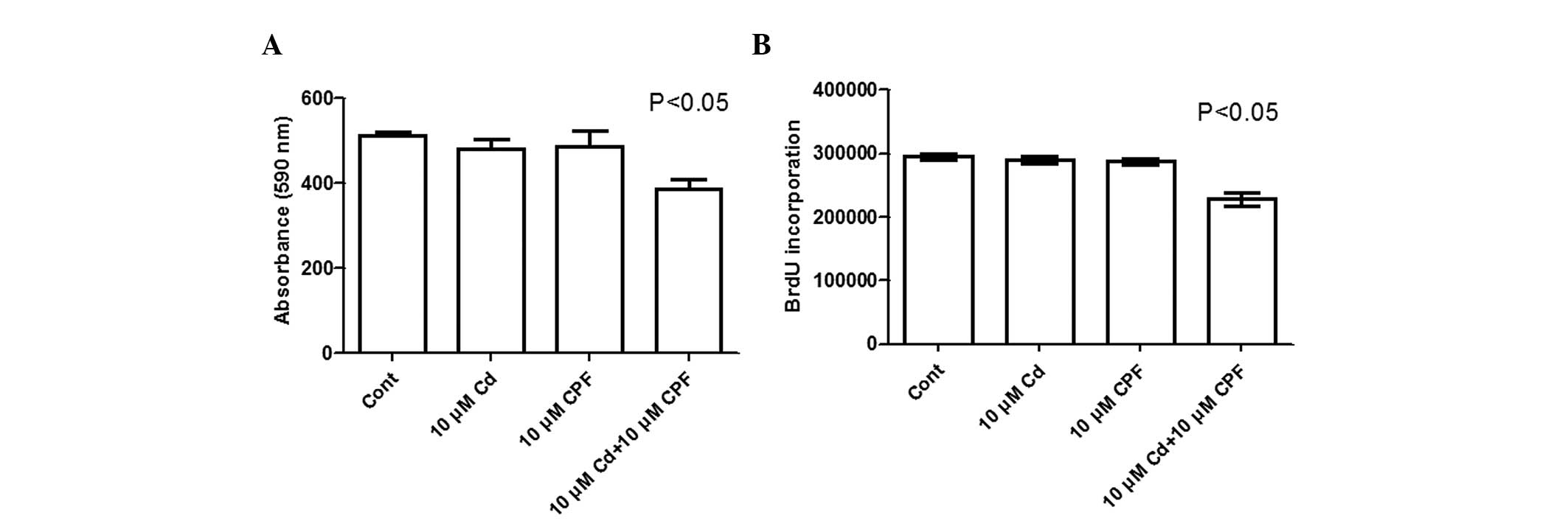
complex-facilitated intracellular transport (21). In the present study the combined
toxicity of CPF and Cd was further confirmed using Alamar Blue and
BrdU assays, and by selecting non-toxic concentrations (10
μM each) of CPF and Cd. As shown in Fig. 2A, cell viability was significantly
reduced by 21% upon concomitant exposure of Cd2+ and CPF
(P<0.05) compared with the control or following individual
treatment with either Cd2+ or CPF, as demonstrated using
an Alamar Blue assay. Similarly, in the BrdU incorporation assay,
the concomitant exposure of Cd2+ and CPF markedly
inhibited cell proliferation by 23%, relative to the control or
following individual treatment with Cd2+ or CPF
(P<0.05; Fig. 2B). In addition,
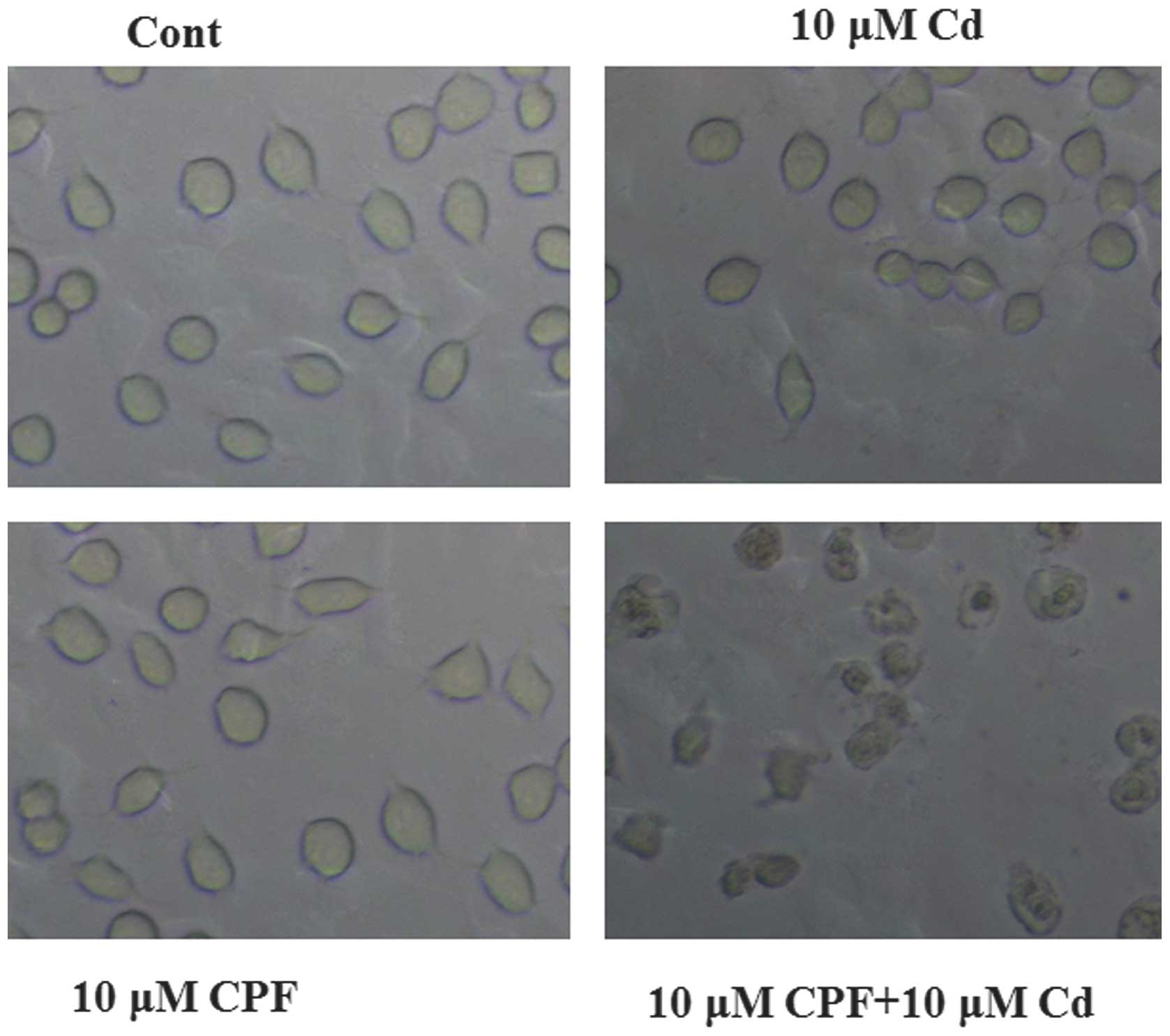
a marked morphological change, of smaller and rounder cells, was
observed in the cells simultaneously treated with CPF and Cd
compared with the cells in the control and individual treatment
groups (Fig. 3). Consistent with
the cytotoxicity results, as discussed above, these morphological
changes were indicative of cell death (Fig. 3). Taken together, these results
revealed a significant synergistic cytotoxic effect of
Cd2+ and CPF on the HepG2 cells, which was distinct from
the effects of the individual chemicals.
To investigate the potential disturbance to lipid
metabolism by the synergistic exposure of CPF and Cd, the present
study investigated the lipid concentrations in HepG2 cells
post-treatment. To improve characterization of lipid metabolism,
this was performed using a cell model treated with high glucose, as
previously described (35). As
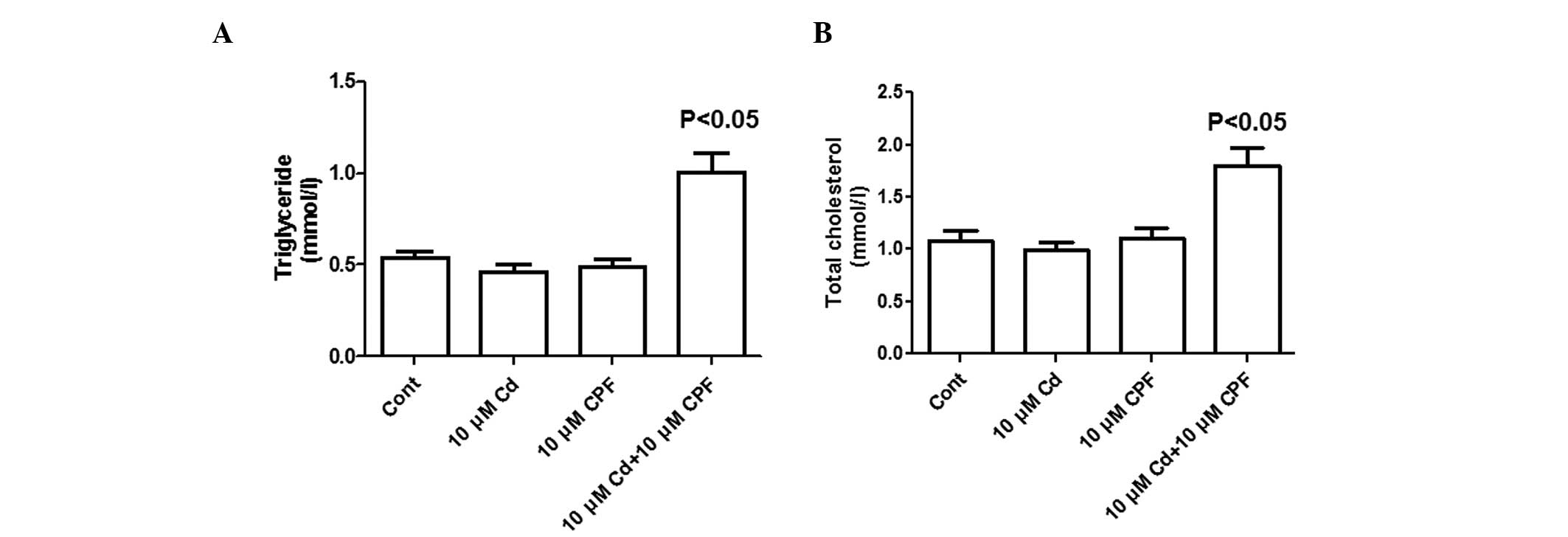
shown in Fig. 4A, the
concentration of triglycerides increased ~2-fold in cells exposed
to simultaneous treatment of CPF and Cd compared with the untreated
cells or single compound-treated cells. Similar to the changes in
triglyceride levels, the concomitant exposure to CPF and
Cd2+ (10 μM each) significantly increased the
total cholesterol concentration by 80% (P<0.05) compared with
the untreated cells or those treated with individual components
(P<0.05; Fig. 4B). These
results collectively suggested that the synergistic effect of CPF
and Cd markedly altered hepatic lipid metabolism, associated with
cholesterol and triglyceride accumulation, in the hepatocytes.
The present study subsequently aimed to investigate
the molecular mechanism underlying CPF/Cd-mediated disorders in
lipid metabolism. SREBP is a critical regulator of hepatic lipid
metabolism, including glucose transport, gluconeogenesis and
lipolysis (43). The SREBP family
consists of SREBP-1a, SREBP-1c and SREBP-2 (44), and these members are essential for
regulating the expression of lipogenic enzymes, including
acetyl-coenzyme A (CoA) carboxylase and FASN (45,46).
SREBP-1 is an important transcription factor, which stimulates
lipogenic enzymes involved in liver fatty-acid synthesis, whereas
SREBP-2 is relatively specific to the regulation of genes
responsible for cholesterol synthesis and uptake, including
low-density lipoprotein receptor and 3-hydroxy-3-methylglutaryl CoA
reductase (44). The lipid
accumulation, induced by exposure to CPF/Cd, may reside in the
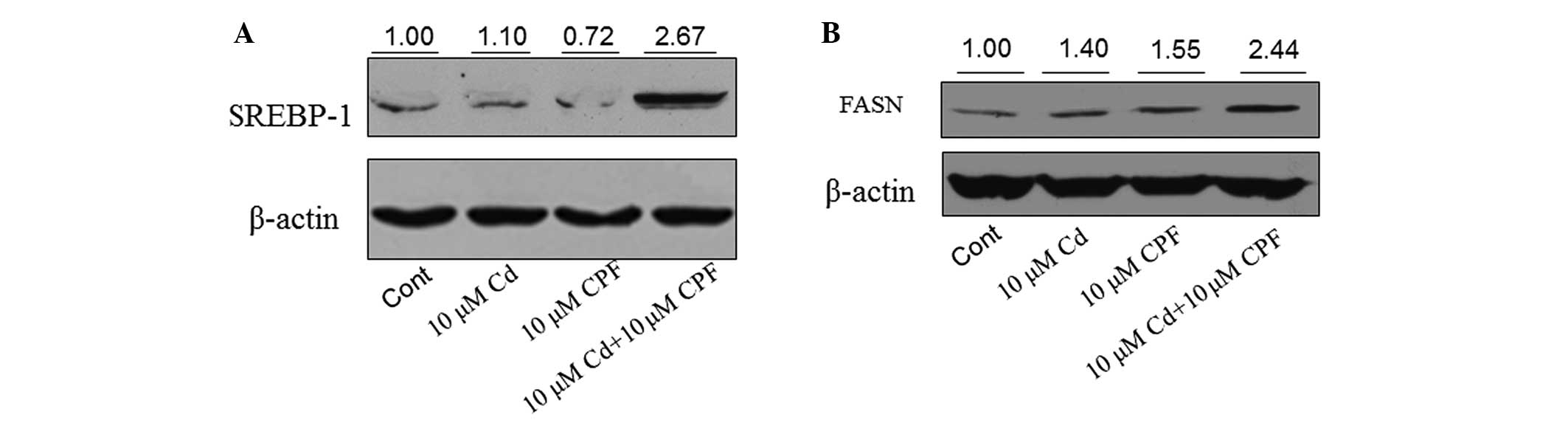
dysfunction of SREBP-1. The concentration of SREBP-1 was assessed
in HepG2 cells following various treatments. Western blot analysis
revealed that the expression of SREBP-1 was markedly increased by
>2-fold in the cells following combined treatment with CPF and
Cd compared with the untreated cells or those treated with CPF or
Cd alone (Fig. 5A). The expression
of FASN was also markedly induced following treatment with CPF/Cd
(>2-fold) compared with the untreated cells or those treated
with the individual chemicals (Fig.
5B). These results demonstrated that the concomitant exposure
of CPF and Cd induced hepatic fat accumulation through an increase
in hepatic lipogenesis by increasing the expression levels of
SREBP-1 and FASN.
Organophosphorus pesticides and heavy metals are
amongst the most serious environmental pollutants. CPF, as a broad
class of organophosphorus pesticides, is widely used throughout the
world for agricultural and non-agricultural purposes (1–4,13).
Cd is a toxic metal, commonly found in industrial workplaces and
diverse environmental substrates, which contaminates food chains,
including crops and fruits (47).
Cd is toxic to various cell types, even at low concentrations, and
has a long biological half-life in humans (10–30 years) (13,21).
However, CPF and Cd are often detected in identical environmental
substrates and food chains, and they elicit similar damage to
organisms, including hepatotoxicity (16,17).
Our previous study demonstrated the synergistic effect of CPF and
Cd on reducing the viability of HepG2 cells (21). The present study demonstrated a
novel HepG2 cell phenotype, caused by the CPF/Cd complex, with
significant cholesterol and triglyceride accumulation in the
hepatocytes. The molecular mechanism underlying hepatic lipogenesis
elicited by joint exposure of CPF+Cd was found to occur through
inducing the expression levels of SREBP-1 and FASN. Future
investigations are required to determine the detailed mechanisms
responsible for CPF/Cd-mediated action in lipogenesis.
Acknowledgments
This study was supported by grants from the Special
Fund for Forest Scientific Research in the Public Welfare (no.
201304805), the National Natural Science Foundation of China (nos.
21377159 and 31201339), the Chinese Academy of Sciences (nos.
KZCX2-EW-404 and XDB14000000) and the New Century Excellent Talents
Program in University of the Ministry of Education (no.
NCET-11-0587). The authors would like to thank the laboratory
members for their assistance with experiments and reagents.
Abbreviations:
|
CPF
|
chlorpyrifos
|
|
Cd
|
cadmium
|
|
OPPs
|
organophosphorus pesticides
|
|
FASN
|
fatty acid synthase
|
|
SREBP
|
sterol regulatory element-binding
protein
|
|
DMSO
|
dimethyl sulfoxide
|
|
FBS
|
fetal bovine serum
|
|
ACC
|
acetyl-coenzyme A carboxylase
|
|
CoA
|
coenzyme A
|
References
|
1
|
Chung SW and Chan BT: Validation and use
of a fast sample preparation method and liquid
chromatography-tandem mass spectrometry in analysis of ultra-trace
levels of 98 organophosphorus pesticide and carbamate residues in a
total diet study involving diversified food types. J Chromatogr A.
1217:4815–4824. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Stepán R, Tichá J, Hajslová J, Kovalczuk T
and Kocourek V: Baby food production chain: pesticide residues in
fresh apples and products. Food Addit Contam. 22:1231–1242. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Szpyrka E, Kurdziel A, Slowik-Borowiec M,
Grzegorzak M and Matyaszek A: Consumer exposure to pesticide
residues in apples from the region of south-eastern Poland. Environ
Monit Assess. 185:8873–8878. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Reiss R, Johnston J, Tucker K, DeSesso JM
and Keen CL: Estimation of cancer risks and benefits associated
with a potential increased consumption of fruits and vegetables.
Food Chem Toxicol. 50:4421–4427. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ivey MC and Palmer JS: Chlorpyrifos and
3,5,6-trichloro-2-pyridinol: residues in body tissues of swine
treated with chlorpyrifos for hog louse and itch mite control. J
Econ Entomol. 72:837–838. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fenske RA, Lu C, Barr D and Needham L:
Children’s exposure to chlorpyrifos and parathion in an
agricultural community in central Washington State. Environ Health
Perspect. 110:549–553. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Whyatt RM, Rauh V, Barr DB, et al:
Prenatal insecticide exposures and birth weight and length among an
urban minority cohort. Environ Health Perspect. 112:1125–1132.
2004. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Caughlan A, Newhouse K, Namgung U and Xia
Z: Chlorpyrifos induces apoptosis in rat cortical neurons that is
regulated by a balance between p38 and ERK/JNK MAP kinases. Toxicol
Sci. 78:125–134. 2004. View Article : Google Scholar
|
|
9
|
Farag A, El Okazy AM and El-Aswed AF:
Developmental toxicity study of chlorpyrifos in rats. Reprod
Toxicol. 17:203–208. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bagchi D, Bagchi M, Hassoun EA and Stohs
SJ: In vitro and in vivo generation of reactive oxygen species, DNA
damage and lactate dehydrogenase leakage by selected pesticides.
Toxicology. 104:129–140. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Das PC, Cao Y, Rose RL, Cherrington N and
Hodgson E: Enzyme induction and cytotoxicity in human hepatocytes
by chlorpyrifos and N,N-diethyl-m-toluamide (DEET). Drug Metabol
Drug Interact. 23:237–260. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ki YW, Park JH, Lee JE, Shin IC and Koh
HC: JNK and p38 MAPK regulate oxidative stress and the inflammatory
response in chlorpyrifos-induced apoptosis. Toxicol Lett.
218:235–245. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Son YO, Lee JC, Hitron JA, Pan J, Zhang Z
and Shi X: Cadmium induces intracellular Ca2+- and
H2O2-dependent apoptosis through JNK- and
p53-mediated pathways in skin epidermal cell line. Toxicol Sci.
113:127–137. 2010. View Article : Google Scholar
|
|
14
|
Nzengue Y, Steiman R, Garrel C, Lefèbvre E
and Guiraud P: Oxidative stress and DNA damage induced by cadmium
in the human keratinocyte HaCaT cell line: role of glutathione in
the resistance to cadmium. Toxicology. 243:193–206. 2008.
View Article : Google Scholar
|
|
15
|
Zhou T, Zhou G, Song W, et al:
Cadmium-induced apoptosis and changes in expression of p53, c-jun
and MT-I genes in testes and ventral prostate of rats. Toxicology.
142:1–13. 1999. View Article : Google Scholar
|
|
16
|
Mansour SA, Belal MH, Abou-Arab AA and Gad
MF: Monitoring of pesticides and heavy metals in cucumber fruits
produced from different farming systems. Chemosphere. 75:601–609.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fatta D, Canna-Michaelidou S, Michael C,
et al: Organochlorine and organophosphoric insecticides, herbicides
and heavy metals residue in industrial wastewaters in Cyprus. J
Hazard Mater. 145:169–179. 2007. View Article : Google Scholar
|
|
18
|
Tuzmen N, Candan N, Kaya E and Demiryas N:
Biochemical effects of chlorpyrifos and deltamethrin on altered
antioxidative defense mechanisms and lipid peroxidation in rat
liver. Cell Biochem Funct. 26:119–124. 2008. View Article : Google Scholar
|
|
19
|
Rai A, Maurya SK, Khare P, Srivastava A
and Bandyopadhyay S: Characterization of developmental
neurotoxicity of As, Cd, and Pb mixture: synergistic action of
metal mixture in glial and neuronal functions. Toxicol Sci.
118:586–601. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Padilla S, Marshall RS, Hunter DL, et al:
Neurochemical effects of chronic dietary and repeated high-level
acute exposure to chlorpyrifos in rats. Toxicol Sci. 88:161–171.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen L, Qu G, Sun X, et al:
Characterization of the interaction between cadmium and
chlorpyrifos with integrative techniques in incurring synergistic
hepatoxicity. PloS One. 8:e595532013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mingrone G, Rosa G, Greco AV, et al:
Intramyocitic lipid accumulation and SREBP-1c expression are
related to insulin resistance and cardiovascular risk in morbid
obesity. Atherosclerosis. 170:155–161. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Xia C, Li R, Zhang S, et al: Lipid
accumulation product is a powerful index for recognizing insulin
resistance in non-diabetic individuals. Eur J Clin Nutr.
66:1035–1038. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Krssak M and Roden M: The role of lipid
accumulation in liver and muscle for insulin resistance and type 2
diabetes mellitus in humans. Rev Endocr Metab Disord. 5:127–134.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Moreau A, Téruel C, Beylot M, et al: A
novel pregnane X receptor and S14-mediated lipogenic pathway in
human hepatocyte. Hepatology. 49:2068–2079. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hao J, Zhu L, Zhao S, Liu S, Liu Q and
Duan H: PTEN ameliorates high glucose-induced lipid deposits
through regulating SREBP-1/FASN/ACC pathway in renal proximal
tubular cells. Exp Cell Res. 317:1629–1639. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Vazquez-Martin A, Colomer R, Brunet J,
Lupu R and Menendez JA: Overexpression of fatty acid synthase gene
activates HER1/HER2 tyrosine kinase receptors in human breast
epithelial cells. Cell Prolif. 41:59–85. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wu X, Qin L, Fako V and Zhang JT:
Molecular mechanisms of fatty acid synthase (FASN)-mediated
resistance to anti-cancer treatments. Adv Biol Regul. 54:214–221.
2014. View Article : Google Scholar
|
|
29
|
Jeon BN, Kim YS, Choi WI, et al: Kr-pok
increases FASN expression by modulating the DNA binding of SREBP-1c
and Sp1 at the proximal promoter. J Lipid Res. 53:755–766. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Choi WI, Jeon BN, Park H, et al:
Proto-oncogene FBI-1 (Pokemon) and SREBP-1 synergistically activate
transcription of fatty-acid synthase gene (FASN). J Biol Chem.
283:29341–29354. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Horton JD, Goldstein JL and Brown MS:
SREBPs: activators of the complete program of cholesterol and fatty
acid synthesis in the liver. J Clin Invest. 109:1125–1131. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yahagi N, Shimano H, Hasty AH, et al:
Absence of sterol regulatory element-binding protein-1 (SREBP-1)
ameliorates fatty livers but not obesity or insulin resistance in
Lep(ob)/Lep(ob) mice. J Biol Chem. 277:19353–19357. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Goda Y, Shimizu T, Kato Y, et al: Two
acylated anthocyanins from purple sweet potato. Phytochemistry.
44:183–186. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Qu G, Liu S, Zhang S, et al: Graphene
oxide induces toll-like receptor 4 (TLR4)-dependent necrosis in
macrophages. ACS Nano. 7:5732–5745. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hwang YP, Choi JH, Han EH, et al: Purple
sweet potato anthocyanins attenuate hepatic lipid accumulation
through activating adenosine monophosphate-activated protein kinase
in human HepG2 cells and obese mice. Nutr Res. 31:896–906. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Akhtar N, Srivastava MK and Raizada RB:
Assessment of chlorpyrifos toxicity on certain organs in rat,
Rattus norvegicus. J Environ Biol. 30:1047–1053. 2009.
|
|
37
|
Zhukalin M, Blanksma MK, Silva TD, et al:
Characterization and in vitro cytotoxicity testing of
ethanolamine-derived cadmium chelating agents. Biometals. 20:61–72.
2007. View Article : Google Scholar
|
|
38
|
Gottschalg E, Moore NE, Ryan AK, et al:
Phenotypic anchoring of arsenic and cadmium toxicity in three
hepatic-related cell systems reveals compound- and cell-specific
selective up-regulation of stress protein expression: implications
for fingerprint profiling of cytotoxicity. Chem Biol Interact.
161:251–261. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Goel A, Dani V and Dhawan DK: Protective
effects of zinc on lipid peroxidation, antioxidant enzymes and
hepatic histoarchitecture in chlorpyrifos-induced toxicity. Chem
Biol Interact. 156:131–140. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Dondero F, Piacentini L, Banni M, Rebelo
M, Burlando B and Viarengo A: Quantitative PCR analysis of two
molluscan metallothionein genes unveils differential expression and
regulation. Gene. 345:259–270. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ediz L, Hiz O, Ozkol H, Gulcu E, Toprak M
and Ceylan MF: Relationship between anti-CCP antibodies and oxidant
and anti-oxidant activity in patients with rheumatoid arthritis.
Int J Med Sci. 8:139–147. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Steevens JA and Benson WH: Interactions of
chlorpyrifos and methyl mercury: a mechanistic approach to assess
chemical mixtures. Mar Environ Res. 50:113–117. 2000. View Article : Google Scholar
|
|
43
|
Viollet B, Foretz M, Guigas B, et al:
Activation of AMP-activated protein kinase in the liver: a new
strategy for the management of metabolic hepatic disorders. J
Physiol. 574:41–53. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Yuan H, Shyy JY and Martins-Green M:
Second-hand smoke stimulates lipid accumulation in the liver by
modulating AMPK and SREBP-1. J Hepatol. 51:535–547. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Lee MS, Kim D, Jo K and Hwang JK:
Nordihydroguaiaretic acid protects against high-fat diet-induced
fatty liver by activating AMP-activated protein kinase in obese
mice. Biochem Biophys Res Commun. 401:92–97. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Rawson RB: Control of lipid metabolism by
regulated intramembrane proteolysis of sterol regulatory element
binding proteins (SREBPs). Biochem Soc Symp. 2003.221–231.
2003.PubMed/NCBI
|
|
47
|
Leach RM Jr, Wang KW and Baker DE: Cadmium
and the food chain: the effect of dietary cadmium on tissue
composition in chicks and laying hens. J Nutr. 109:437–443.
1979.PubMed/NCBI
|