Introduction
Expression of heat shock proteins (HSPs) is induced
in response to various types of biological stress to protect cells
against different types of damage as molecular chaperones (1,2).
Human HSPs are recently classified into seven groups, HSPH
(HSP110), HSPC (HSP90), HSPA (HSP70), DNAJ (HSP40), HSPB (small
HSP), HSPD/E (HSP60/HSP10) and CCT (TRiC) (1). Among them, the small HSP family
(HSPB) contains 10 members, including αB-crystallin (HSPB5)
(3), HSP27 (HSPB1) (4) and HSP20 (HSPB6) (5) with molecular masses ranging from 15
to 30 kDa. A number of HSPB family members, including αB-crystallin
and HSP27, are ubiquitously expressed in cells and tissues, such as
skeletal and smooth muscle (1,2). The
HSPB family members have a highly homologous structure in their
amino acid sequence, termed the α-crystallin domain (2). It is currently recognized that HSPB
binds improperly folded proteins and transfers them to the
ATP-dependent chaper-ones, such as HSPA (HSP70), or to the protein
degradation machines (6).
Accumulating evidence indicates that small HSPs participate in the
regulation of numerous intracellular processes in a wide range of
cell types and are important in maintaining the function of
tissues, such as muscle and nerve tissue (1). However, the exact mechanism
underlying HSPB effects on cell function remains to be
clarified.
Platelets are important in primary hemostasis and
repairing vascular injury, and are initially activated via adhesive
receptors, such as glycoprotein Ib/IX/V receptors. Glycoprotein
Ib/IX/V receptors mediate rolling and tethering of the platelets to
von Willebrand factor at the sites of vascular injury, which is
followed by glycoprotein IIb/IIIa activation resulting in platelet
aggregation (7,8). In addition, it is generally
recognized that shear stress stimulates platelet activation in a
physiological or pathological mechanism in vivo (7). Under the condition of sheer stress,
the activation of platelets is dependent upon the interaction of
von Willebrand factor-glycoprotein Ib/IX/V (7,8).
Ristocetin, an activator of glycoprotein Ib/IX/V, potently induces
the interaction between von Willebrand factor and glycoprotein
Ib/IX/V in vitro (9). It
has been reported that glycoprotein Ib activation induced by
ristocetion leads to thromboxane (TX) A2 generation by
cytosolic phospholipase A2 in platelets (8). Our group recently reported that
ristocetin induces the release of soluble CD40 (sCD40) ligand from
human platelets via TXA2 generation (10).
Our group has also demonstrated that human platelets
possess specific binding sites for αB-crystallin and that
αB-crystallin functions extracellularly and suppresses the human
platelet aggregation induced by ristocetin, an activator of
glycoprotein Ib/IX/V and thrombin (11,12).
In addition, we have recently reported that aB-crystallin
suppresses the adenosine diphosphate (ADP)-induced platelet granule
secretion by inhibition of HSP27 phosphorylation via p44/p42 MAPK
and p38 MAPK (13). However, the
exact mechanism underlying the extracellular effect of
αB-crystallin on human platelets has not been clarified. The
present study aimed to investigate whether αB-crystallin
extracellularly affects glycoprotein Ib/IX/V-induced sCD40 ligand
release from human platelets.
Materials and methods
Materials
Ristocetin was purchased from Sigma-Aldrich (St.
Louis, MO, USA). U46619 was obtained from Enzo Life Sciences
International, Inc. (Plymouth Meeting, PA, USA). αB-crystallin, a
native protein purified from the bovine eye lens, was purchased
from Assay Designs Inc. (Ann Arbor, MI, USA). sCD40 ligand ELISA
kits were purchased from R&D Systems, Inc. (Minneapolis, MN,
USA). TXB2 enzyme-linked immunosorbent assay (ELISA)
kits were obtained from Cayman Chemical Company (Ann Arbor, MI,
USA). Rabbit anti-human monoclonal phospho-specific p38 MAP kinase
antibodies (cat. no. 4511) and rabbit anti-human polyclonal p38 MAP
kinase antibodies (cat. no. 9212) were obtained from Cell
Signaling, Inc. (Beverly, MA, USA). SZ2, a mouse anti-human
monoclonal antibody against the sulfated tyrosine/anionic
glycoprotein Iba residues Tyr-276-Glu-282 (14), was obtained from Beckman Coulter
(Krefeld, Germany; cat. no. IM0409) for immunoprecipitation and
from Santa Cruz Biotechnology Inc. (Santa Cruz, CA; cat. no.
sc-59052) for western blotting. All other materials and chemicals
were obtained from commercial sources.
Preparation of platelets
The present study used seven healthy volunteers (5
male and 2 female) between the ages of 27 and 50 years. All
participants were provided a medical checkup at Gifu University
Hospital. Human blood (15 ml) was donated from the median cubital
vein and and combined with 3.8% sodium citrate (1/10 volume).
Platelet-rich plasma (PRP) was obtained from blood samples by
centrifugation at 155 × g for 12 min at room temperature.
Platelet-poor plasma was prepared from residual blood by
centrifugation at 2,500 × g for 5 min. All participants signed an
informed consent agreement after receiving a detailed explanation
of the study. The study was approved by the Committee of Ethics in
Gifu University Graduate School of Medicine (Gifu, Japan).
Measurement of platelet aggregation
induced by ristocetin or U46619
Platelet aggregation using citrated PRP was observed
in a PA-200 aggregometer (Kowa Co. Ltd., Tokyo, Japan), which can
determine the size of platelet aggregates based upon particle
counting using laser scattering methods (small size, 9–25
μm; medium size, 25–50 μm; and large size, 50–70
μm) (15), at 37°C with a
stirring speed of 800 rpm. The platelets were pre-incubated for 1
min, and then platelet aggregation was monitored for 4 min. The
percentage of transmittance of the isolated platelets was recorded
as 0%, and that of the appropriate platelet-poor plasma (blank) was
recorded as 100%. When indicated, PRP was pretreated with
αB-crystallin (0.6, 1.8 and 6.0 μg/ml) for 15 min.
Protein preparation after stimulation by
ristocetin or U46619
Following stimulation with ristocetin or U46619,
platelet aggregation was terminated by the addition of 10 mM
ice-cold EDTA (Katayama Chemical Industries Co., Ltd., Osaka,
Japan) solution. The mixture was centrifuged at 10,000 × g at 4°C
for 2 min. To measure the levels of sCD40 ligand, PDGF-AB and
TXB2 as described below, the supernatant was isolated
and stored at −20°C for subsequent ELISA. For western blot
analysis, the pellet was washed twice with phosphate-buffered
saline and then lysed and immediately boiled in a lysis buffer
containing 62.5 mM Tris/Cl, pH 6.8; 2% SDS, 50 mM dithiothreitol
and 10% glycerol. For immunoprecipitation, the pellet was washed
twice with phosphate-buffered saline and lysed in 0.5 ml ice-cold
TNE lysis buffer [containing 10 mM Tris-HCl, pH 7.8; 1% Nonidet
P-40, 150 mM NaCl, 1 mM EDTA, 1 mM dithiothreitol, 1 mM sodium
fluoride, 1 mM sodium vanadate and protease inhibitor cocktail (all
from Roche Applied Science, Mannheim, Germany)]. The lysates were
then centrifuged at 10,000 × g at 4°C for 30 min and the
supernatant was collected as TNE-soluble protein.
Measurement of sCD40 ligand and
11-dehydro-TXB2 levels
The sCD40 ligand and 11-dehydro-TXB2 levels in
samples were determined using each ELISA kit according to the
manufacturer’s instructions.
Western blot analysis
A western blot analysis was performed as described
previously (16). Briefly, sodium
dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was
performed according to a previous method (17) on a 10% polyacrylamide gel. The
proteins in the gel were transferred onto polyvinylidine fluoride
(PVDF) membranes (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
The membranes were then blocked with 5% fat-free dry milk in
Tris-buffered saline with 0.1% Tween-20 (TBS-T; 20 mM Tris, pH 7.6;
137 mM NaCl and 0.1% Tween-20) for 2 h prior to incubation with the
indicated primary antibodies. The primary antibodies used in this
experiment were phospho-specific p38 MAP kinase antibodies or p38
MAP kinase antibodies, respectively. Peroxidase-labeled anti-mouse
IgG (cat. no. NA931; 1:1,000; GE Healthcare, Little Chalfont, UK)
or anti-rabbit IgG (cat. no. 074–1506; Kirkegaard & Perry
Laboratories, Inc., Gaithersburg, MD, USA) antibodies were used as
secondary antibodies. The primary and secondary antibodies were
diluted to obtain optimum concentrations with 5% fat-free dry milk
in TBS-T. Peroxidase activity on PVDF membranes was visualized on
X-ray films by means of an enhanced chemiluminescnece western
blotting detection system according to the manufacturer’s
instructions.
Immunoprecipitation
SZ2 (Beckman Coulter) was added to the TNE-soluble
proteins, and the mixture was shaken gently for overnight at 4°C,
followed by the addition of 50 ml protein G Dynabeads (Life
Technologies, Carlsbad, CA, USA) and a further incubation for 1 h
with continuous mixing. Protein immunocomplexes were isolated with
the use of a magnetic particle concentrator (6-tube magnetic
separation rack; New England BioLabs, Inc., Ipswich, MA, USA).
Immunoprecipitated proteins were resuspended in SDS-PAGE loading
buffer, heated at 95°C for 5 min, and analyzed by western blot
analysis using SZ2 (Santa Cruz Biotechnology, Inc.) as a primary
antibody.
Statistical analysis
The data were analyzed by Student’s t-test, and a
P<0.05 was considered to indicated a statistically significant
difference. All data are presented as the mean ± standard error of
the mean. All statistical analyses were performed using PASW
statistics version 18 (IBM SPSS, Tokyo, Japan).
Results
Effect of αB-crystallin on the
ristocetin-stimulated release of sCD40 ligand from human
platelets
Our group has recently shown that ristocetin
stimulates sCD40 ligand release from human platelets (10). The effect of αB-crystallin on the
ristocetin-stimulated sCD40 ligand release from human platelets was
examined. αB-crystallin significantly suppressed the
ristocetin-stimulated release of sCD40 ligand (Fig. 1). The inhibitory effect was dose
dependent and αB-crystallin at 6.0 μg/ml caused an ~80%
reduction in the ristocetin-effect (Fig. 1).
Effect of αB-crystallin on the
ristocetin-stimulated production of TXA2 in human
platelets
Our group previously demonstrated that ristocetin
induces TXA2 generation, which leads to the release of
sCD40 ligand from human platelets (10). In the present study, the ristocetin
(1.5 mg/ml)-stimulated TXB2 production was significantly
reduced by αB-crystallin, which was determined by measuring the
generation of 11-dehyro-TXB2, a stable TXA2
metabolite (18) (Fig. 2). The suppressive effect of
αB-crystallin on the TXB2 production was dose-dependent and 6.0
μg/ml αB-crystallin caused ~90% reduction in the
ristocetin-effect (Fig. 2).
Effect of αB-crystallin on platelet
aggregation by U46619
Our group previously demonstrated that ristocetin
stimulates the release of sCD40 ligand through TXA2
production as an autacoid (10).
However, αB-crystallin did not affect the platelet aggregation
induced by U46619, which is a selective TXA2 receptor
(TP) agonist (19). In the present
study, αB-crystallin had little effect on the distribution of
aggregated particle sizes (small size, medium size or large size)
even when the platelets were treated with 6.0 μg/ml
αB-crystallin (Fig. 3).
Effects of αB-crystallin on the
U46619-stimulated release of sCD40 ligand and phosphorylation of
p38 MAP kinase in human platelets
Our group have previously demonstrated that
TXA2 receptor activation induces the release of the
sCD40 ligand via MAP kinases, such as p38 MAP kinase in human
platelets (10). Thus, in the
present study, the effect of αB-crystallin on the release of sCD40
ligand stimulated by U46619 from human platelets was analyzed.
αB-crystallin was not observed to significantly reduce the
U46619-induced release of sCD40 ligand (Fig. 4). Furthermore, the U46619-induced
phosphorylation levels of p38 MAP kinase were not affected by
αB-crystallin (Fig. 5).
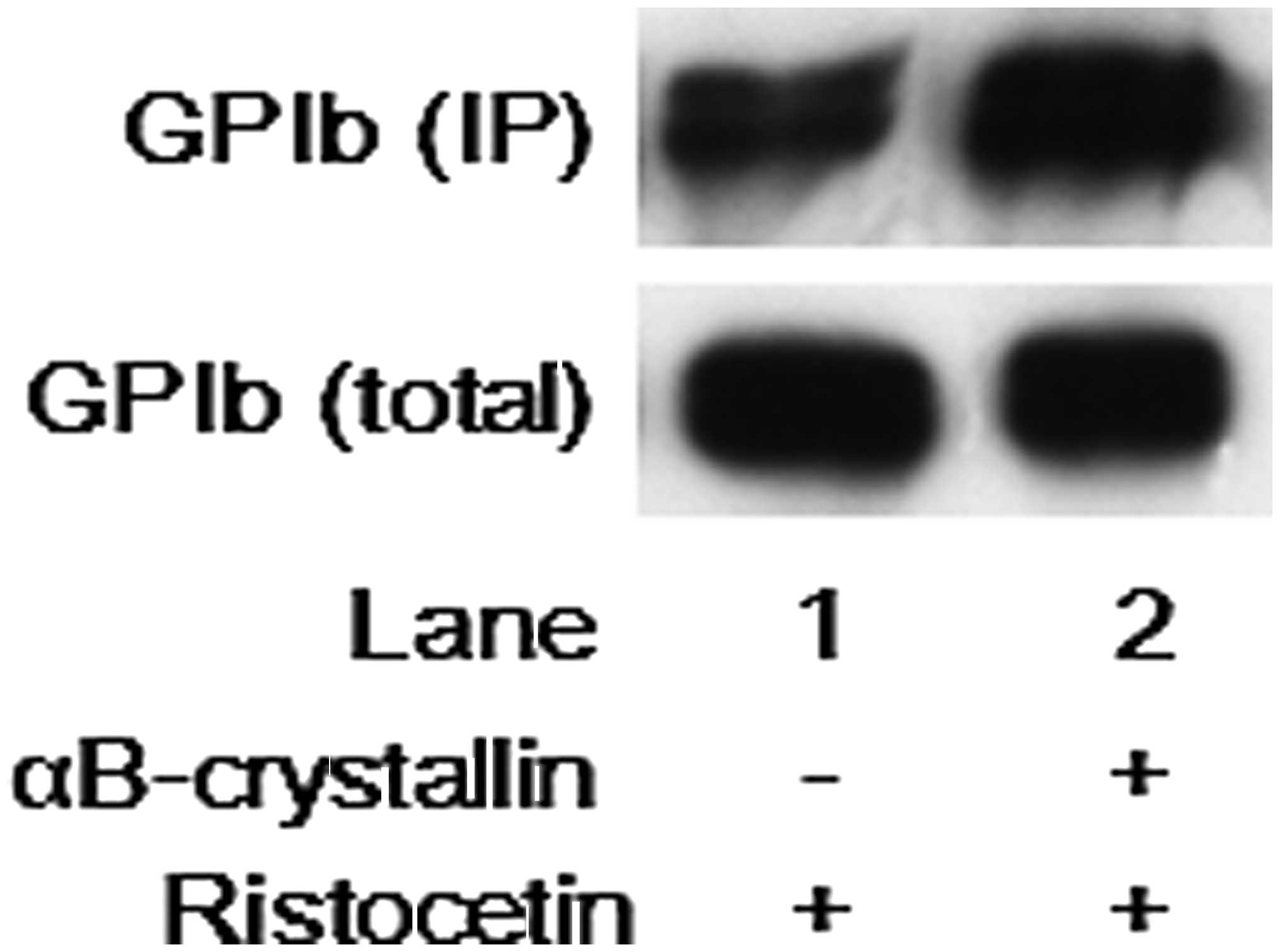
Effect of αB-crystallin on the binding of
SZ2 to ristocetin-stimulated human platelets
It is recognized that von Willebrand factor binds
glycoprotein Ib/IX/V on the platelet membrane and initiates signals
leading to platelet activation under shear stress or in the
presence of modulators, such as ristocetin. Thus, the effect of
αB-crystallin on the binding of SZ2, a monoclonal antibody to the
sulfated tyrosine/anionic glycoprotein Iba residues Tyr-276-Glu-282
(14), to the
ristocetin-stimulated human platelets was further examined.
However, αB-crystallin failed to suppress SZ2-binding to the
ristocetin-stimulated platelets (Fig.
6).
Discussion
αB-crystallin, a small HSP, is ubiquitously
expressed in a variety of types of tissues and cells, including
cardiac, smooth and skeletal muscle (1,2). It
is firmly established that HSPs act intracellularly as molecular
chaperones (1). In our previous
study (11), it was demonstrated
that the αB-crystallin levels in injured arteries are markedly
lower than those in non-injured arteries in vivo and that
αB-crystallin levels in the plasma of hamsters with cardiomyopathy
are markedly higher than those of control hamsters. Recently, it
has been shown that αB-crystallin is secreted from epithelial cells
(20). Our group has previously
reported that the specific binding sites of αB-crystallin exist on
human platelets and that αB-crystallin affects human platelets
extracellularly and inhibits the platelet aggregation induced by
ristocetin (11,12). In addition, it was demonstrated
that glycoprotein Ib/IX/V activation induces the release of sCD40
ligand, an inflammatory mediator, via TXA2 production in
human platelets (10). On the
basis of these findings, in the present study, the extracellular
effect of αB-crystallin on the ristocetin-induced release of sCD40
ligand in human platelets and the underlying mechanism were
investigated. It was observed that αB-crystallin significantly
suppressed the release of sCD40 ligand from platelets stimulated by
ristocetin. In addition, αB-crystallin failed to affect the
platelet aggregation induced by U46619, an agonist of TP
(TXA2 receptor). It is currently recognized that the MAP
kinases, such as p38 MAP kinase, are activated downstream of
TP-mediated responses (21). In
our previous study (10), it was
shown that TP-induced activation of MAP kinases is involved in the
ristocetin-stimulated sCD40 ligand release from human platelets.
Additionally, the present study demonstrated that the
U46619-induced phosphorylation of p38 MAP kinase or the release of
sCD40 ligand was not significantly affected by αB-crystallin in
human platelets. Based on these findings, it is unlikely that
αB-crystallin suppresses ristocetin-induced sCD40 ligand release at
a point downstream of TP.
In the present study, αB-crystallin significantly
inhibited the ristocetin-induced the production of TXB2,
a stable metabolite of TXA2. It has been reported that
ristocetin-activated glycoprotein Ib results in activation of
cytosolic phospholipase A2, which stimulates the release
of arachidonic acid and leads to the production of TXA2
in human platelets (8). In our
previous study (10), ristocetin
induced TXA2 generation via cyclooxygenase, which lead
to the release of sCD40 ligand from human platelets through TP.
Thus, it is most likely that αB-crystallin reduces
ristocetin-stimulated sCD40 ligand release via inhibiting
TXA2 generation in human platelets.
Furthermore, it was demonstrated that αB-crystallin
failed to reduce the SZ2-binding to the ristocetin-stimulated
platelets. SZ2 is known to be a monoclonal antibody against the
heparin-like, sulfated tyrosine/anionic glycoprotein Iba residues
Tyr-276-Glu-282, recognized as a binding site for von Willebrand
factor (14). Therefore, it is
unlikely that αB-crystallin suppresses the ristocetin-dependent
binding of von Willebrabd factor to glycoprotein Ib. Our group have
recently reported that antithrombin III inhibits ristocetin-induced
release of sCD40 ligand via inhibition of TXA2
production accompanied by the reduction of SZ2 binding in human
platelets (22). Thus, the
mechanism of αB-crystallin affecting GPIb/IX/V signaling may be
different from that of antithrombin III in human platelets.
Considering all findings, it is most likely that αB-crystallin
suppresses the TXA2 production induced by ristocetin,
resulting in the inhibition of sCD40 ligand release from human
platelets.
When exposed to various stimuli, human platelets
rapidly respond and release inflammatory mediators causing
atherosclerosis, such as sCD40 ligand in addition to granule
secretion of PDGF-AB and serotonin (5-HT) (23). CD40 ligand, which is a member of
the tumor necrosis factor-α family, exists in the cytoplasm of
resting platelets and is immediately translocated to the surface
following platelet activation (24). sCD40 ligand is subsequently
released from the platelet membrane as a functional soluble
fragment into the circulation. It is recognized that the
platelet-derived sCD40 ligand induces inflammatory responses via
CD40 expressed on vascular endothelial cells that produce
inflammatory mediators, such as reactive oxygen species and
chemokines (23,25). Platelet-derived sCD40 ligand
becomes mobilized in patients with acute coronary syndrome
(26). Reportedly, the elevation
of plasma sCD40 ligand is associated with an increased risk of
cardiovascular events in patients with acute coronary syndrome
(27). In the present study, it
was demonstrated that αB-crystallin obviously suppressed the
glycoprotein Ib/IX/V activation-induced release of sCD40 ligand
from human platelets. Therefore, our findings indicate that
αB-crystallin may be an anti-inflammatory agent for patients under
high shear stress conditions. Further investigation is required to
clarify the exact mechanism underlying the effects of αB-crystallin
on human platelets.
In conclusion, the results suggest that
αB-crystallin extra-cellularly suppresses the ristocetin-induced
release of sCD40 ligand by inhibiting TXA2 production in
human platelets.
Acknowledgments
The authors would like to thank Miss Yumiko Kurokawa
for her skillful technical assistance. This study was supported in
part by a Grant-in-Aid for Scientific Research (grant nos. 20590565
and 20591825) from the Ministry of Education, Science, Sports and
Culture of Japan and the Research Funding for Longevity Sciences
(25–4) from the National Center for Geriatrics and Gerontology,
Japan.
References
|
1
|
Mymrikov EV, Seit-Nebi AS and Gusev NB:
Large potentials of small heat shock proteins. Physiol Rev.
91:1123–1159. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Taylor RP and Benjamin IJ: Small heat
shock proteins: a new classification scheme in mammals. J Mol Cell
Cardiol. 38:433–444. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kato K, Shinohara H, Goto S, Inaguma Y,
Morishita R and Asano T: Copurification of small heat shock protein
with αB crystallin from human skeletal muscle. J Biol Chem.
267:7718–7725. 1992.PubMed/NCBI
|
|
4
|
Kato K, Hasegawa K, Goto S and Inaguma Y:
Dissociation as a result of phosphorylation of an aggregated form
of the small stress protein, hsp27. J Biol Chem. 269:11274–11278.
1994.PubMed/NCBI
|
|
5
|
Kato K, Goto S, Inaguma Y, Hasegawa K,
Morishita R and Asano T: Purification and characterization of a
20-kDa protein that is highly homologous to a B crystallin. J Biol
Chem. 269:15302–15309. 1994.PubMed/NCBI
|
|
6
|
Vos MJ, Hageman J, Carra S and Kampinga
HH: Structural and functional diversities between members of the
human HSPB, HSPH, HSPA and DNAJ chaperone families. Biochemistry.
47:7001–7011. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Berndt MC, Shen Y, Dopheide SM, Gardiner
EE and Andrews RK: The vascular biology of the glycoprotein Ib-IX-V
complex. Thromb Haemost. 86:178–188. 2001.PubMed/NCBI
|
|
8
|
Garcia A, Quinton TM, Dorsam RT and
Kunapuli SP: Src family kinase-mediated and Erk-mediated
thromboxane A2 generation are essential for vWF/GPIb-induced
fibrinogen receptor activation in human platelets. Blood.
106:3410–3414. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dong JF, Berndt MC, Schade A, McIntire LV,
Andrews RK and Lopez JA: Ristocetin-dependent, but not
botrocetin-dependent, binding of von Willebrand factor to the
platelet glycoprotein Ib-IX-V complex correlates with
shear-dependent interactions. Blood. 97:162–168. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Enomoto Y, Adachi S, Matsushima-Nishiwaki
R, Doi T, Niwa M, Akamatsu S, et al: Thromboxane A2 promotes
soluble CD40 ligand release from human platelets. Atherosclerosis.
209:415–421. 2010. View Article : Google Scholar
|
|
11
|
Kozawa O, Matsuno H, Niwa M, Hatakeyama D,
Kato K and Uematsu T: αB-crystallin, a low-molecular-weight heat
shock protein, acts as a regulator of platelet function. Cell
Stress Chaperones. 6:21–28. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Matsuno HI, Ishisaki A, Nakajima K, Kato K
and Kozawa O: A peptide isolated from αB-crystallin is a novel and
potent inhibitor of platelet aggregation via dual prevention of
PAR-1 and GPIb/V/IX. J Thromb Haemost. 12:2636–2642. 2003.
View Article : Google Scholar
|
|
13
|
Enomoto Y, Adachi S, Matsushima-Nishiwaki
R, Niwa M, Tokuda H, Akamatsu S, et al: Alpha B-crystallin
extracellularly suppresses ADP-induced granule secretion from human
platelets. FEBS Lett. 583:2464–2468. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ward CM, Andrews RK, Smith AI and Berndt
MC: Mocarhagin, a novel cobra venom metalloproteinase, cleaves the
platelet von Willebrand factor receptor glycoprotein Iba.
Identification of the sulfated tyrosine/anionic sequence
Tyr-276-Glu-282 of glycoprotein Iba as a binding site for von
Willebrand factor and a-thrombin. Biochemistry. 35:4929–4938. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fabre JE, Nguyen M, Latour A, Keifer JA,
Audoly LP, Coffman TM and Koller BH: Decreased platelet
aggregation, increased bleeding time and resistance to
thromboembolism in P2Y1-deficient mice. Nature Med. 5:1199–1202.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kato K, Ito H, Hasegawa K, Inaguma Y,
Kozawa O and Asano T: Modulation of the stress-induced synthesis of
hsp27 and α B-crystallin by cyclic AMP in C6 rat glioma cells. J
Neurochem. 66:946–950. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Laemmli UK: Cleavage of structural
proteins during the assembly of the head of bacteriophage T4.
Nature. 227:680–685. 1970. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Catella F, Healy D, Lawson JA and
FitzGerald GA: 11-Dehydrothromboxane B2: a quantitative index of
thromboxane A2 formation in the human circulation. Proc Natl Acad
Sci USA. 83:5861–5865. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bertele V, Di Minno G and de Gaetano G:
U-46619, a stable analogue of prostaglandin H2, induces retraction
of human platelet-rich plasma clots. Thromb Res. 18:543–545. 1980.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gangalum RK, Atanasov IC, Zhou ZH and Bhat
SP: αB-crystallin is found in detergent-resistant membrane
microdomains and is secreted via exosomes from human retinal
pigment epithelial cells. J Biol Chem. 286:3261–3269. 2011.
View Article : Google Scholar :
|
|
21
|
Nakahata N: Thromboxane A2:
physiology/pathophysiology, cellular signal transduction and
pharmacology. Pharmacol Ther. 118:18–35. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Doi T, Tokud H, Matsushima-Nishiwaki R,
Cuong NT, Kageyama Y, Iida Y, Kono A, Akamatsu S, Otsuka T, Iida H,
Kozawa O and Ogura S: Effect of antithrombin III on glycoprotein
Ib/IX/V activation in human platelets: suppression of thromboxane
A2 generation. Prosta Leukot Essent Fatty Acids. 87:57–62. 2012.
View Article : Google Scholar
|
|
23
|
Davi G and Patrono C: Platelet activation
and atherothrombosis. New Engl J Med. 357:2482–2494. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hermann A, Rauch BH, Braun M, Schror K and
Weber AA: Platelet CD40 ligand (CD40 L)-subcellular localization,
regulation of expression and inhibition by clopidogrel. Platelets.
12:74–82. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Henn V, Slupsky JR, Grafe M,
Anagnostopoulos I, Forster R, Muller-Berghaus G, et al: CD40 ligand
on activated platelets triggers an inflammatory reaction of
endothelial cells. Nature. 391:591–594. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Heeschen C, Dimmeler S, Hamm CW, van den
Brand MJ, Boersma E, Zeiher AM, et al: Soluble CD40 ligand in acute
coronary syndromes. New Engl J Med. 348:1104–1111. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Varo N, de Lemos JA, Libby P, Morrow DA,
Murphy SA, Nuzzo R, et al: Soluble CD40L: risk prediction after
acute coronary syndromes. Circulation. 108:1049–1052. 2003.
View Article : Google Scholar : PubMed/NCBI
|