Introduction
Oxidative stress contributes to a cascade of
secondary damage following spinal cord injury (SCI), which results
in inflammatory cell infiltration, neuronal and glial cell
destruction, neuronal dysfunction and cell death (1). It has been demonstrated that
mitochondrial dysfunction is a principle source of increased
oxidative stress following SCI (2–4).
Pharmacological treatments have demonstrated that mitochondria are
important as a therapeutic target to promote neuronal recovery and
regeneration against oxidative SCI (3,5).
Thus, preventing mitochondrial dysfunction and stabilizing
mitochondrial integrity may be considered as a potential approach
for antioxidant based interventions following traumatic SCI.
The pathophysiological antioxidative defense
mechanisms against mitochondrial dysfunction and reactive oxygen
species (ROS) production in the injured spinal cord are complicated
(3–7). N-acetylcysteine (NAC), a derivative
of cysteine, acts as a mitochondrial enhancer, which has a broad
range of activities, including anti-inflammatory and antioxidant
effects in neuronal disorders associated with excessive free
radical production and oxidative tissue damage (8,9).
Previous studies have demonstrated that acute NAC administration
reduced neuronal degeneration and attenuated the microglia and
inflammatory responses following SCI in rodent models (9,10).
However, whether NAC has neuroprotective effects on long-term
neurological recovery in the injured spinal cord following
traumatic SCI remains to be elucidated. The aim of the present
study was to evaluate the effects of NAC on spinal cord trauma.
Materials and methods
SCI
Adult female C57BL/6 mice weighing 18–22 g were
anesthetized intraperitoneally with sodium pentobarbital (40 mg/kg)
and subjected to a moderate spinal cord contusion trauma. A
laminectomy was performed at the T9 level and the exposed dorsal
surface of the spinal cord was subjected to moderate contusion
injury as described previously (11). Following SCI, the skin was closed
with wound clips. Animal body temperature was maintained at 37°C
with a warming blanket throughout the surgery and during the
recovery from anesthesia. For the sham-surgery controls (SHAM), the
animals underwent a T9 laminectomy without contusion injury.
Surgical interventions and postoperative animal care were conducted
in accordance with the National Institutes of Health Guide for the
Care and Use of Laboratory Animals (National Institutes of Health,
Bethesda, MD, USA) and with approval from the Animal Subjects
Committee at Zhejiang University (Hangzhou, China). The study was
approved by the ethics committee of Zhejiang University (Hangzhou,
China).
Drug treatment
NAC dissolved in phosphate-buffered saline (PBS;
Sigma-Aldrich, St. Louis, MO, USA) was administered to
corresponding SHAM and SCI mice (SHAM+NAC and SCI+NAC) via
intraperitoneal injection (100 mg/kg) (12–15)
1 day prior to SCI and then once a day for 28 days for behavioral
assessment or for the time-points indicated on the corresponding
figures for other experiments following SCI. The current dose of
NAC administration was applied on the basis of mitochondrial
function restoration in our experiments. Sterile saline for vehicle
control (VEH) was administered into corresponding SHAM and SCI mice
(SHAM+VEH and SCI+VEH). Significant side effects resulting from NAC
treatment, including alterations in body weight or an increase in
the mortality rate, were not observed during the present
experiments.
Mitochondrial integrity and function
Mitochondrial isolation was performed as described
previously with the following modifications (3–7). The
final mitochondria was stored on ice during assessments of
mitochondrial function. Mitochondrial respiration and enzyme
activity was assessed using a miniature Clark-type electrode
following procedures described previously (3–7). The
oxidative substrates and inhibitors of different enzyme complexes
of the mitochondrial electron transport system were added to assess
mitochondrial respiration. A total of three independent oxymetric
traces for each sample were obtained and the respiration rates
averaged.
Histology and immunohistochemistry
Histological and immunohistochemistry procedures
were performed as previously described with certain modifications
(16). Spinal cord tissues were
transferred to a 25% sucrose solution prior to processing for
histological analysis. The spinal cords were subsequently frozen,
sectioned at 7 μm thickness and stained with hematoxylin and
eosin for detection of spinal cord injuries and leukocyte
infiltration. A scale of 0–4 represented the severity of spinal
cord injury: 0, none or minor; 1, modest or limited; 2,
intermediate; 3, widespread or prominent and 4, widespread and most
prominent. These tasks were conducted in a blinded manner.
To assess white matter sparing and neuronal
survival, the serial 7 μm transverse sections at 250
μm intervals surrounding the epicenter of the lesion 28 days
after SCI were stained with Nissl for neurons and Luxol fast blue
for myelin. A total of 13 sequential sections spanning 3,000
μm of spinal cord length, which included six sections,
rostral and caudal to the section at the epicenter, were assessed
in SHAM and SCI mice. The images of Nissl and Luxol fast blue
staining were analyzed using Image J software version 1.48
(National Institutes of Health).
Non-heme iron was analyzed using Perls’ prussian
blue to assess iron deposition in the spinal cord. Quantitative
measurement of non-heme iron was performed on spinal cord tissue as
previously described with a number of modifications (17). Integrated density of non-heme iron
accumulation was determined using Image J software (National
Institutes of Health).
For immunofluorescence staining, the transverse
sections 250 μm caudal and rostral to the epicenter of the
lesion (T10) 28 days after SCI were used. The spinal sections were
washed in PBS for 10 min, followed by washing with PBS containing
0.3% Tween 20 (Sigma-Aldrich) for 10 min and blocked with blocking
serum for 2 h. The sections were incubated with mouse anti-neuronal
nuclei (NeuN; 1:100; Santa Cruz Biotechnology, Inc., Santa Cruz,
CA, USA) diluted in PBS overnight at 4°C. Nuclei were stained for
10 min at room temperature with 300 ng/ml DAPI (Sigma-Aldrich).
Images were acquired using a Leica DM LA upright microscope (Leica,
Mannheim, Germany) and were analyzed using Image J software by
three investigators.
Myeloperoxidase (MPO) activity assay
MPO activity, as an indicator of leukocyte
infiltration was performed in spinal cord tissue as previously
described (18). The MPO activity
was defined as the quantity of enzyme required to degrade 1 mmol
H2O2 per minute at 37°C, expressed as units
of MPO/g wet tissue.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was prepared with TRIzol reagent
(Invitrogen Life Technologies, Carlsbad, CA, USA). Complementary
DNA synthesis and RT-qPCR were performed using a previously
described method (11). The primer
sequences used were as follows: Tumor necrosis factor-α (TNF-α),
forward 5′-CCCAGACCCTCACACTCAGAT-3′ and reverse
5′-TTGTCCCTTGAAGAGAACCTG-3′; interleukin (IL)-1β, forward
5′-GCAGCTACCTATGTCTTGCCCGTG-3′ and reverse
5′-GTCGTTGCTTGTCTCTCCTTGTA-3′; IL-6, forward
5′-AAGTTTCTCTCCGCAAGATACTTCCAGCCA-3′ and reverse
5′-AGGCAAATTTCCTGGTTATATCCAGTT-3′; inducible nitric oxide synthase
(iNOS), forward 5′-CTCCATGACTCTCAGCACAGAG-3′ and reverse
5′-GCACCGAAGATATCCTCATGAT-3′.
ELISA
To determine the cytokine and ROS levels, the lesion
site was rapidly dissected and homogenized in PBS 24 h after SCI.
Following centrifugation at 4°C for 15 min at 900 × g, the
supernatants were used to measure the concentrations of IL-6,
IL-1β, TNF-α (R&D Systems, Minneapolis, MN, USA), iNOS,
glutathione peroxidase (GSH-Px) and superoxide dismutase (SOD;
Cusabio Biotech Co., Ltd., Wuhan, China) using corresponding ELISA
kits.
Western blot analysis
A 0.5 cm length of cord, centered over the site of
impact and representing the epicenter, was lysed on ice for 30 min
with 50 mM Tris-HCl (pH 7.5), 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 25
mM NaF, 5 mM sodium pyrophosphate, 1 mM
Na3VO4 and protease inhibitors (Roche
Diagnostics GmbH, Mannheim, Germany). Cell lysates were clarified
by centrifugation at 10,400 × g for 15 min at 4°C and the
supernatants were collected and assayed for protein concentration
using a bicinchoninic acid protein assay kit (Pierce Biotechnology,
Inc., Rockford, IL, USA). Immunoblot analysis was performed as
described previously (11). The
following antibodies were used: Rabbit polyclonal anti-heme
oxygenase-1 (HO-1; 1:200; cat no. sc-10789; Santa Cruz
Biotechnology, Inc.) and rabbit polyclonal anti-α/β-Tubulin
(1:1,000; cat no. 2148; Cell Signaling Technology, Inc., Danvers,
MA, USA). Tubulin served as a loading control. Subsequently, the
membrane was incubated with goat anti-rabbit Alexa Fluor 488
(Jackson Immunoresearch, West Grove, PA, USA). at a concentration
of 1:200. The immunoblot analysis was repeated three times and the
band density of immunoblots obtained using Image J software were
subjected to statistical analysis.
Behavioral analysis
The behavioral test was scored in accordance with
the rules of Basso, Beattie and Bresnahan (BBB), which comprise 21
criteria for the movement of lower limbs, from complete paralysis
to complete mobility (19). These
criteria are based on the accurate observation of the lower limbs,
including movement, step and coordinated motor action. This rating
scale takes into account limb movement, stepping, coordination and
trunk stability. Mice were assessed at 1 and 3 days and weekly
thereafter until euthanasia 28 days after SCI. Motor performance on
a rotarod was also evaluated as well as the ability to traverse a
wire grid (17), in sequence, at
26, 27 and 28 days after SCI. There were three trials daily, with a
total of nine trials for each test. In each of these tests, the
mean score from each mouse was used.
Statistical analysis
Statistical analysis was performed using GraphPad
Prism software version 6 (GraphPad Software, Inc., La Jolla, CA,
USA). P<0.05 was considered to indicate a statistically
significant difference. Data are presented as the mean ± standard
error of the mean of three independent experiments.
Results
Effects of NAC on mitochondrial
dysfunction-induced oxidative stress
Mitochondrial dysfunction is critical in the
progression of secondary injury in SCI (4,7,20).
To assess whether mitochondrial integrity or function were directly
affected by SCI, alterations in the mitochondrial respiratory
control ratio (RCR) and respiration rates were analyzed in the
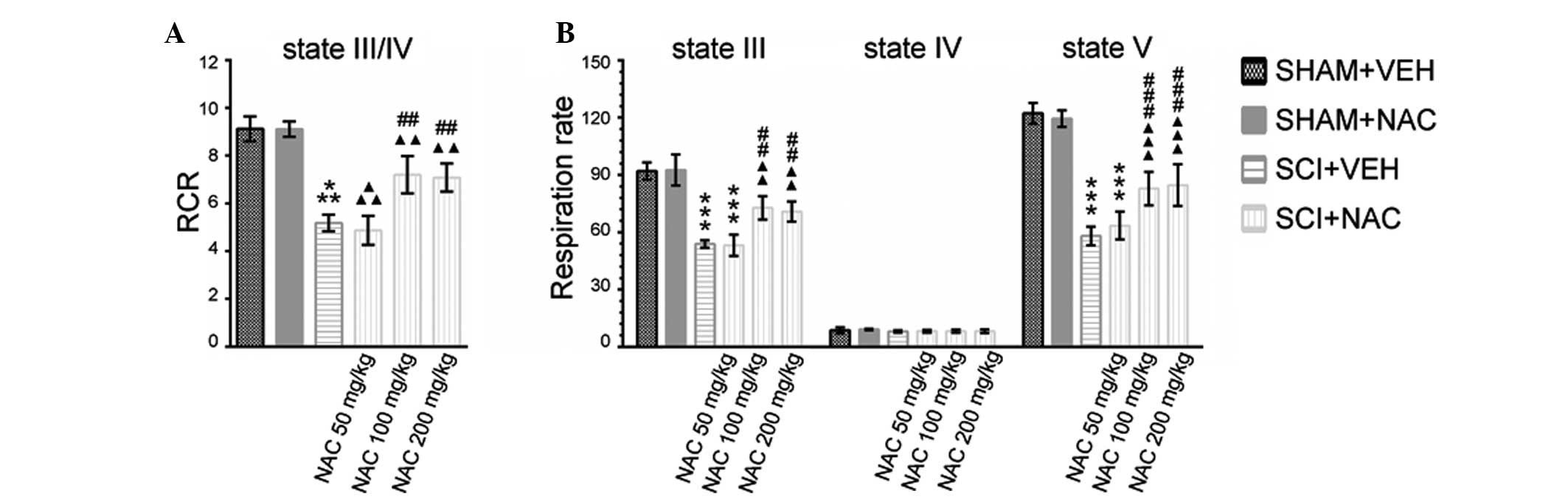
injured spinal cord (Fig. 1A and
B). A significant decrease in RCR and state III and state
V-complex I respiration in SCI+VEH mice compared with SHAM mice was
observed 24 h after SCI. NAC treatment at 50 mg/kg/day had no
effect on a lower mitochondrial respiratory capacity caused by SCI.
When NAC treatment in SCI mice was at dosages of 100 or 200 mg/kg
per day, a significant restoration of mitochondrial RCR and
respiration rates was observed compared with VEH treatment in SCI
mice, although the RCR and respiration rates in SCI+NAC mice
remained significantly lower than SHAM mice. No significant
differences were identified in improved mitochondrial respiratory
function between SCI+NAC 100 mg/kg/day mice and SCI+NAC 200
mg/kg/day mice.
The effects of NAC on the oxidant and antioxidant
status induced by mitochondrial dysfunction in SCI were
subsequently evaluated. Indicators of oxidative stress, including
GSH-Px, HO-1, non-heme iron and SOD were measured in all SHAM and
SCI mice. Analysis of immunoblots revealed an elevation of HO-1 in
SCI mice treated with VEH or with NAC (100 mg/kg/day) at 24 h
compared with SHAM mice (Fig. 2A).
However, the expression of HO-1 was significantly decreased at 3
days in SCI mice treated with NAC, but not in those treated with
VEH. No significant difference was identified in HO-1 protein
expression between SCI and SHAM mice at 7 days. In addition,
accumulation of non-heme iron was reduced in the injured spinal
cord at 14 days (Fig. 2B) in SCI
mice treated with NAC (100 mg/kg/day). The GSH-Px and SOD activity
in SCI+VEH mice was significantly lower than SHAM mice, whereas NAC
(100 mg/kg/day) significantly rescued the GSH-Px and SOD activities
in mice at 24 h after SCI (Fig. 2C and
D). Furthermore, significant positive correlations were
observed between the higher GSH-Px/SOD activities and increased
mitochondrial RCR (Fig. 2E). By
contrast, negative correlations were identified between HO-1
expression/non-heme iron accumulation and mitochondrial RCR
(Fig. 2E), indicating that
mitochondrial respiratory dysfunction is directly linked to
oxidative stress-induced redox imbalance in SCI.
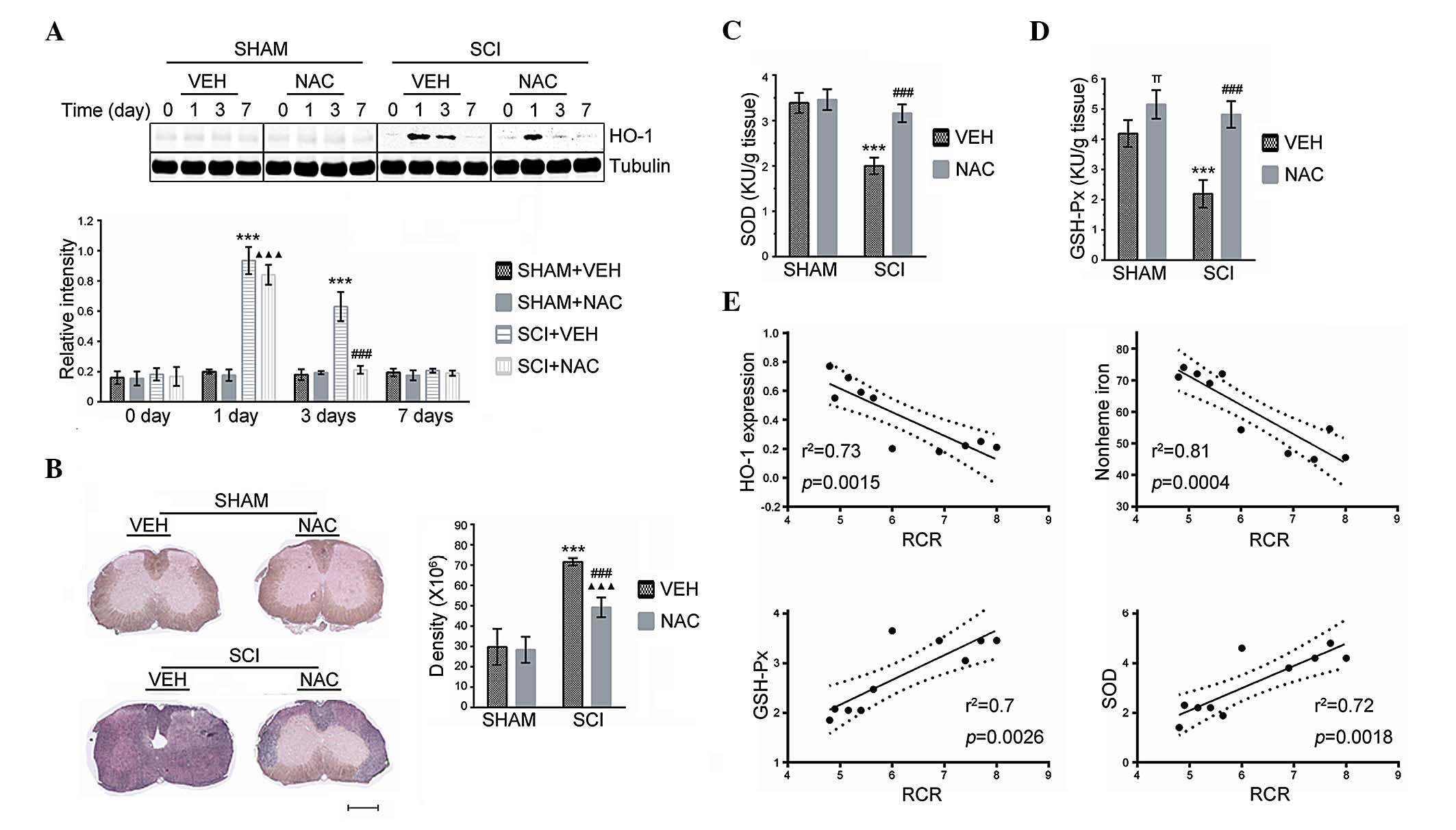 | Figure 2Effects of NAC on oxidative stress.
Data are presented as the mean ± standard error of the mean of
three independent experiments (n=3 per group)
***P<0.001, SCI+VEH mice versus SHAM+VEH mice;
###P<0.001, SCI+NAC mice versus SCI+VEH mice;
ΔΔΔP<0.001, SCI+NAC mice versus SHAM+NAC mice;
πP<0.05, SHAM+NAC mice versus SHAM+VEH mice. (A) HO-1
expression by quantitative immunoblot analysis was performed at the
indicated time points in SHAM and SCI mice treated with NAC or VEH.
(B) Modified Perl’s staining was performed to detect non-heme iron
in SHAM and SCI mice treated with NAC or VEH at 14 days. Scale bar
indicates 500 μm. Accumulation of non-heme iron detected by
Perl’s staining was analyzed using NIH Image J and expressed as
integrated density measurements. (C) ELISA of SOD was performed at
24 h in SHAM and SCI mice treated with NAC or VEH. (D) ELISA of
GSH-Px was performed at 24 h in SHAM and SCI mice treated with NAC
or VEH. (E) Correlation between mitochondrial RCR and oxidative
stress in injured spinal cord. NAC, N-Acetyl-Cysteine; VEH,
vehicle; SCI, spinal cord injury; RCR, respiratory control ratio;
SOD, superoxide dismutase; GSH-Px, glutathione peroxidase; HO-1,
heme oxygenase-1. |
Effects of NAC on histological
alterations and neuronal survival
Subsequently, the anti-oxidative protection of NAC
on spinal cord histological and neurological alterations following
SCI was examined. There were homogenous and intact tissue
structures in uninjured spinal cord in all SHAM mice, whereas SCI
caused mechanical insults, including edema, hemorrhage, leukocyte
recruitment and loosened tissue structure in all SCI mice on day 1
(Fig. 3A). Compared with SCI+VEH
mice, morphological changes and inflammatory cell infiltration were
significantly alleviated in SCI mice treated with NAC (100
mg/kg/day) at 28 days. Subsequently, it was investigated whether
NAC repressed demyelination following SCI. White matter sparing was
compared between SHAM and SCI mice using Luxol fast blue staining
(Fig. 3B). Similarly, white matter
sparing at the epicenter (T10) was significantly decreased in
SCI+VEH mice compared with SCI+NAC mice at 28 days. Statistically
significant differences were identified at the epicenter and at
rostral (250 μm) and caudal (250, 500 and 750 μm)
sites between SCI+VEH and SCI+NAC mice.
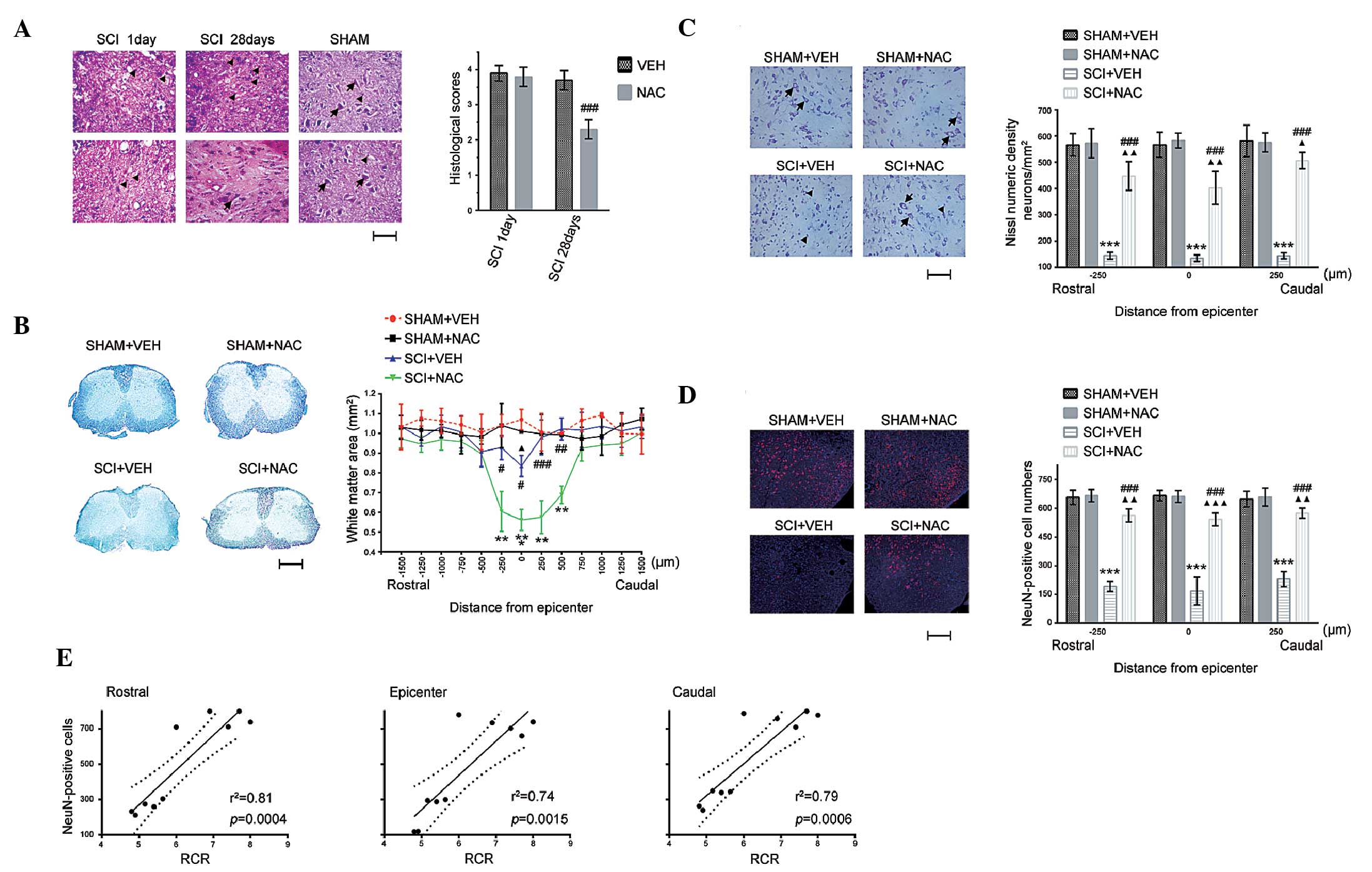 | Figure 3Effects of NAC on histological
changes. Data are presented as the mean ± standard error of the
mean of three independent experiments (n=3 per group).
**P<0.01, ***P<0.001, SCI+VEH mice
versus SHAM+VEH mice; #P<0.05,
##P<0.01, ###P<0.001, SCI+NAC mice
versus SCI+VEH mice; ΔP<0.05, ΔΔP<0.01,
ΔΔΔP<0.001, SCI+NAC mice versus SHAM+VEH mice. (A)
SCI and leukocyte infiltration by hematoxylin and eosin staining at
1 day and 28 days in SHAM and SCI mice treated with NAC or VEH
(scale bar=100 μm). Arrows indicate normal neurons;
triangles indicate glia. Histological scores were measured for the
SCI severity at 1 and 28 days in SCI mice treated with NAC or VEH.
(B) White matter sparing by Luxol fast blue staining at 28 days in
SHAM and SCI mice treated with NAC or VEH (scale bar=500
μm). Data are expressed as the proportional area. (C)
Quantification of Nissl numeric density (neurons/mm2) at
28 days in SHAM and SCI mice treated with NAC or VEH (scale bar=100
μm). Arrows indicate normal neurons; triangles indicate
neuron death. (D) Quantification of immunofluorescence staining of
NeuN (red) and 4′,6-diamidino-2-phenylindole (blue) at 28 days in
SHAM and SCI mice treated with NAC or VEH (scale bar=100
μm). (E) Correlation between mitochondrial RCR and neuronal
survival in SCI. NAC, N-acetylcysteine; VEH, vehicle; SCI, spinal
cord injury; RCR, respiratory control ratio; NeuN, neuronal
nuclei. |
To identify whether NAC treatment (100 mg/kg/day)
suppressed neuronal death in SCI, the protective effects of NAC on
neuronal survival was examined using Nissl and NeuN staining in
SHAM and SCI mice (Fig. 3C and D).
No significant difference was identified in Nissl-stained neurons
and NeuN-positive cell numbers in the spinal cord between SHAM+VEH
mice and SHAM+NAC mice, whereas the number of Nissl-stained neurons
and NeuN-positive cells in SCI+NAC mice were higher than that in
SCI+VEH mice at the epicenter and at rostral (250 μm) and
caudal (250 μm) sites at 28 days. In addition, mitochondrial
RCR is positively correlated with neuronal survival (NeuN-positive
cell numbers) in the injured spinal cord. These results suggest
that recovery of mitochondrial function using NAC may promote
histological improvement and neuronal survival following SCI.
Effects of NAC on leukocyte inflammatory
responses
The histological changes of SCI were associated with
leukocyte and inflammatory mediator accumulation in the injured
spinal cord of mice (16).
Therefore, the effects of NAC (100 mg/kg/day) on neutrophil
infiltration were investigated using an MPO activity assay in SHAM
and SCI mice at 1 day. The MPO activity was higher in SCI mice
treated with VEH than those treated with NAC (Fig. 4A). The effects of NAC treatment on
the expression of inflammatory mediators were also examined,
including IL-1β, IL-6, iNOS and TNF-α by RT-qPCR and ELISA assays
in SHAM and SCI mice at the indicated time points. It was
identified that the mRNA expression levels of IL-1β, TNF-α (at 2
h), IL-6 and iNOS (at 6 h) were increased in SCI mice treated with
VEH compared with mice treated with NAC, or than all SHAM mice and
SCI mice (Fig. 4B). ELISA assays
also revealed similar expression levels of inflammatory mediators
at 24 h in SHAM and SCI mice (Fig.
4C). This suggests that NAC may inhibit leukocyte infiltration
and inflammatory responses in SCI mice.
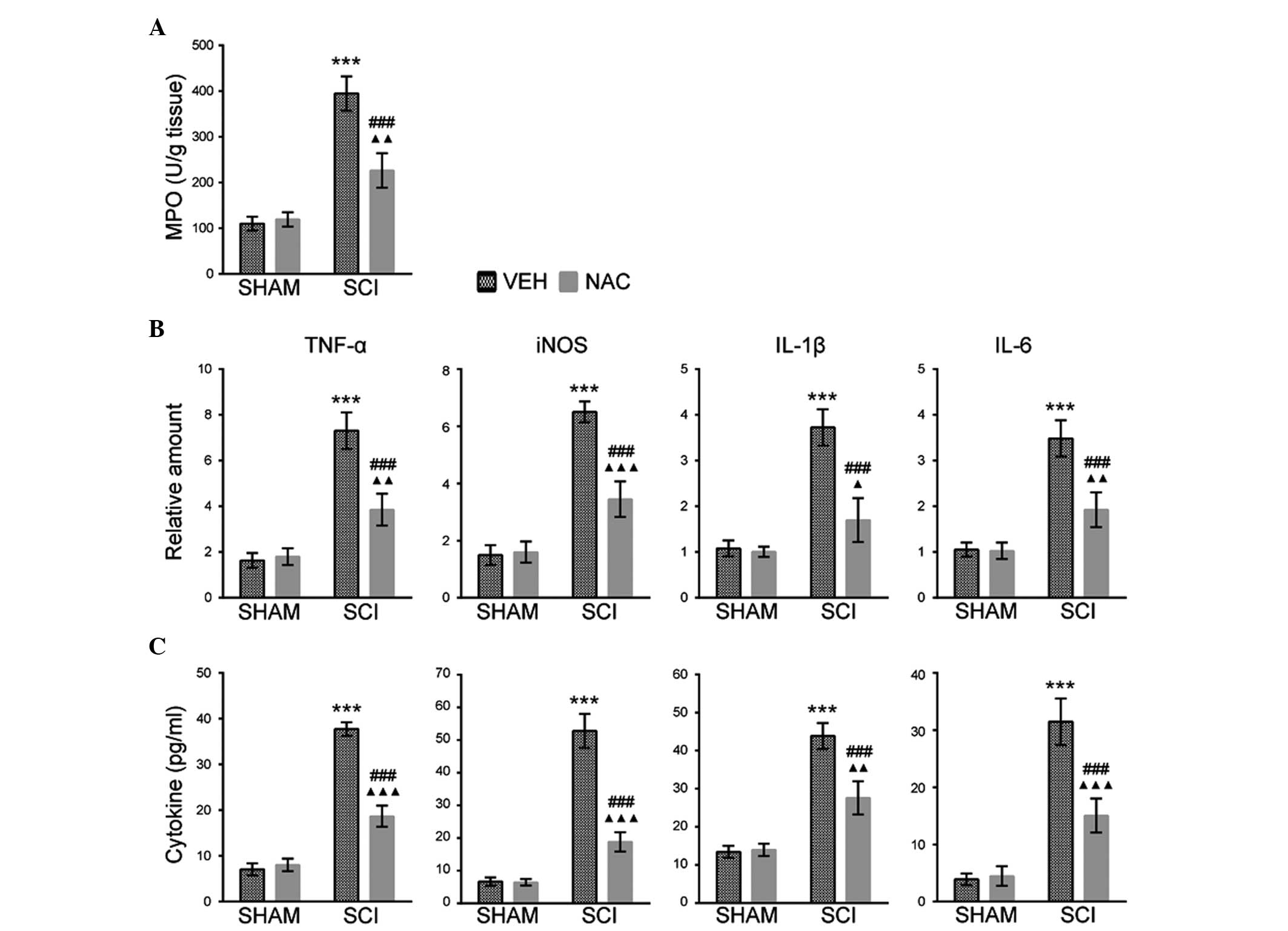 | Figure 4Effects of NAC on MPO activity and
inflammatory mediator expression. Data are presented as the mean ±
standard error of the mean of three independent experiments (n=3
per group). ***P<0.001, SCI+VEH mice versus SHAM+VEH
mice; ###P<0.001, SCI+NAC mice versus SCI+VEH mice;
ΔP<0.05, ΔΔP<0.01,
ΔΔΔP<0.001, SCI+NAC mice versus SHAM+NAC mice. (A)
MPO activity as an indicator of leukocyte infiltration was analyzed
at 24 h in SHAM and SCI mice treated with NAC or VEH. (B) Reverse
transcription-quantitative polymerase chain reaction of TNF-α,
IL-1β (at 2 h), IL-6 and iNOS (at 6 h) was performed in SHAM and
SCI mice treated with NAC or VEH. (C) ELISA of TNF-α, IL-1β, IL-6
and iNOS was performed at 24 h in SHAM and SCI mice treated with
NAC or VEH. NAC, N-acetylcysteine; VEH, vehicle; SCI, spinal cord
injury; RCR, respiratory control ratio; TNF-α, tumor necrosis
factor-α; IL, interleukin; MPO, myeloperoxidase; iNOS, inducible
nitric oxide synthase. |
Effects of NAC on long-term functional
recovery
In order to evaluate whether the neuroprotective
effects of NAC (100 mg/kg/day) were associated with long-term
functional recovery, the BBB, rotarod and grid-walk test scores
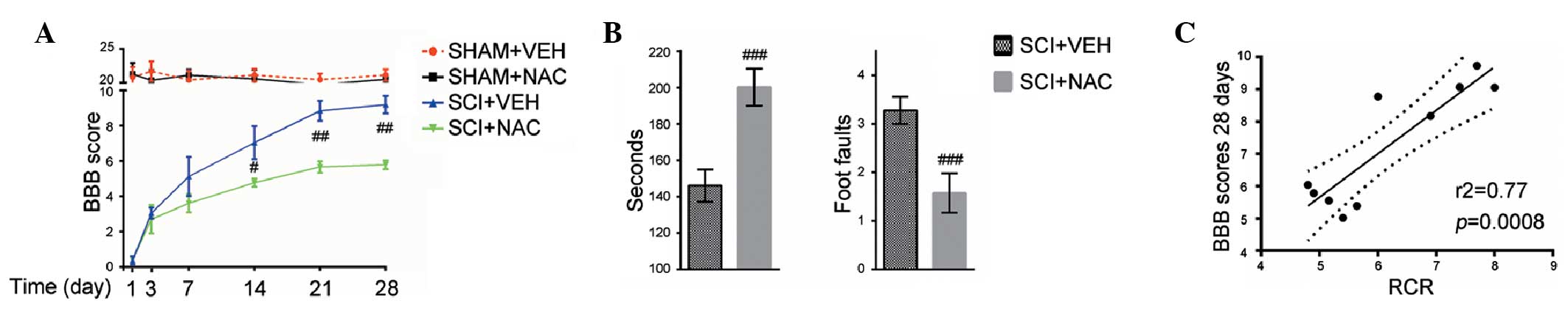
were measured for 28 days. It was observed that hind-limb movements
were eradicated immediately following SCI compared with SHAM mice
as assessed using the BBB scale. The BBB scores in all SCI mice
increased after 1 day. The averages of the BBB scores were higher
in SCI mice treated with NAC than in SCI+VEH mice from days 14–28
(Fig. 5A). Subsequent assessment
of rotarod and grid-walk performance (Fig. 5B) revealed similar locomotor
functional improvements in SCI mice treated with NAC, relative to
their respective controls, thereby confirming that NAC treatment
may enhance long-term recovery of locomotor function in SCI mice. A
significant positive correlation was observed between the higher
BBB scores (28 days) and increased mitochondrial RCR in SCI mice
treated with NAC (Fig. 5C). These
results indicate that mitochondrial function improved by NAC is
beneficial for long-term locomotor recovery in SCI mice.
Discussion
Oxidative stress contributes to several pathological
responses following SCI, including leukocyte infiltration,
demyelination and neuronal death that exacerbate spinal cord damage
and impair neurological recovery (4,7,20,21).
Suppression of oxidative damage in the spinal cord has been
verified to ameliorate the neurological dysfunction following
spinal cord trauma (16,17,21).
In the present study, it was demonstrated that the mitochondrial
enhancer NAC exerted antioxidant and neuroprotective effects in SCI
mice. The administration of NAC in SCI mice suppressed oxidative
stress, attenuated histopathological alterations, the degeneration
of injured spinal cord and the reduction of neuronal loss,
inhibited the expression of proinflammatory factors, preserved
white matter area and promoted locomotor functional recovery. These
results clearly suggest that NAC is an effective therapy for the
treatment of traumatic SCI.
Spinal cord trauma initially causes immediate,
primarily mechanical neuronal and vascular tissue damage followed
by secondary neural dysfunction and neurodegeneration in the
injured spinal cord (1). There
were multiple independent, however, interrelated, biochemical and
pathophysiological pathways underlying the secondary damage
following SCI (7,10). It is apparent that oxidative stress
is one of the principle pathogenic pathways involved in SCI
(7,10). Previous studies have demonstrated
that increased oxidative damage was primarily associated with
mitochondrial dysfunction in the injured spinal cord (2–4).
Thus, the regulation of oxidative stress through pharmacological
targeting of mitochondrial dysfunction may provide critical
neuroprotection for traumatic SCI. In the present study, it was
revealed that SCI severely impairs mitochondrial function and
integrity in the injured spinal cord of mice and acute NAC
treatment at 100 mg/kg concentration was able to significantly
preserve mitochondrial dynamics and integrity. Correlation analyses
between neurological recoveries and mitochondrial respiratory
activities also provided marked evidence that improved
mitochondrial oxidative function with NAC treatment following
spinal cord trauma was a direct result of greater neuronal survival
and locomotor response.
The present study assessed whether NAC conspicuously
decreased oxidative stress and restored redox state balance in SCI
mice. These results are consistent with previous findings that
excessive oxidative stress following SCI results in decreased
activities of key mitochondrial respiratory enzymes and NAC is also
reported to directly improve mitochondrial energy production and
stabilize mitochondrial membranes (3–7). It
was also observed that NAC treatment rapidly inhibited HO-1
expression, which acts as an antioxidant and neuroprotective
effector molecule in the injured spinal cord (21). These results may be explained by
the suppression of the nuclear erythroid 2-related factor 2
(NRF2)/antioxidant response elements pathway by NAC (22) and NRF2 may directly regulate HO-1
promoter activity (23). However,
the potential disadvantageous function of NAC may be
counterbalanced by its beneficial effects on maintaining
mitochondrial function and integrity following SCI (7). In addition, NAC may enhance neuronal
glutathione levels through the extracellular signal-regulated
kinase (ERK)/eukaryotic initiation factor 2/activating
transcription factor 4 pathway, which reduces oxidant-induced
neuronal injury and cell death and scavenges free radical species
in the injured spinal cord (24).
It is well established that traumatic SCI results in
neuronal loss and degeneration (7,10,21).
Spinal cord trauma also leads to the delayed retrograde reaction in
spinal motor and sensory neurons (25,26).
The present study confirmed previous observations that traumatic
SCI induces spinal neuron degeneration, spinal cord dysfunction and
associated locomotor deficits, whereas NAC treatment exhibited
marked neuroprotective effects on lesioned spinal neurons,
including larger areas of white matter sparing and less neuronal
death in SCI mice. Consistent with previous studies (8,9), NAC
treatment may immediately suppress leukocyte infiltration and the
expression of several important neuroinflammatory mediators,
including IL-1β, IL-6, iNOS and TNF-α in SCI mice. Furthermore, NAC
significantly improved hind limb function within the first week of
administration and promoted long-term locomotor recovery following
SCI. The mechanisms underlying the effects of NAC on recovery of
the injured spinal cord neurons and locomotor function following
traumatic SCI is at least partially dependent upon the ability of
NAC to positively regulate the neurotrophic factor activated
Ras/ERK pathway. These neurotrophic factors, including
brain-derived neurotrophic factor, nerve growth factor and glial
cell line-derived neurotrophic factor are essential to maintain
neuronal survival and neuronal structure (27–29).
Accordingly, the current findings indicate that neuroprotection of
spinal cord neurons following NAC treatment after SCI has a major
impact on long-term biochemical, histological and neurological
recovery.
Taken together, the neuroprotective effects of NAC
on oxidative stress and mitochondrial integrity following traumatic
SCI have been demonstrated. The present study revealed that NAC
suppressed excessive oxidative stress, attenuated mitochondrial
dysfunction and inflammatory responses, promoted white matter
sparing and improved long-term neuronal survival and neurological
recovery in SCI mice. The current results suggest that NAC may
provide potential therapeutic applications for preventing
mitochondrial dysfunction-induced oxidative stress following spinal
cord trauma.
Acknowledgments
This study was supported by the Science and
Technology Bureau Foundation of Ningbo (grant no. 2012A610230).
References
|
1
|
Mautes AE, Weinzierl MR, Donovan F and
Noble LJ: Vascular events after spinal cord injury: contribution to
secondary pathogenesis. Phys Ther. 80:673–687. 2000.PubMed/NCBI
|
|
2
|
Luo J, Borgens R and Shi R: Polyethylene
glycol improves function and reduces oxidative stress in
synaptosomal preparations following spinal cord injury. J
Neurotrauma. 21:994–1007. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
McEwen ML, Sullivan PG and Springer JE:
Pretreatment with the cyclosporin derivative, NIM811, improves the
function of synaptic mitochondria following spinal cord contusion
in rats. J Neurotrauma. 24:613–624. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sullivan PG, Krishnamurthy S, Patel SP,
Pandya JD and Rabchevsky AG: Temporal characterization of
mitochondrial bioenergetics after spinal cord injury. J
Neurotrauma. 24:991–999. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Patel SP, Sullivan PG, Lyttle TS and
Rabchevsky AG: Acetyl-L-carnitine ameliorates mitochondrial
dysfunction following contusion spinal cord injury. J Neurochem.
114:291–301. 2010.PubMed/NCBI
|
|
6
|
Patel SP, Sullivan PG, Pandya JD and
Rabchevsky AG: Differential effects of the mitochondrial uncoupling
agent, 2,4-dinitrophenol, or the nitroxide antioxidant, Tempol, on
synaptic or nonsynaptic mitochondria after spinal cord injury. J
Neurosci Res. 87:130–140. 2009. View Article : Google Scholar
|
|
7
|
Patel SP, Sullivan PG, Lyttle TS, Magnuson
DS and Rabchevsky AG: Acetyl-L-carnitine treatment following spinal
cord injury improves mitochondrial function correlated with
remarkable tissue sparing and functional recovery. Neuroscience.
210:296–307. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Arakawa M and Ito Y: N-acetylcysteine and
neurodegenerative diseases: basic and clinical pharmacology.
Cerebellum. 6:308–314. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Berk M, Kapczinski F, Andreazza AC, et al:
Pathways underlying neuroprogression in bipolar disorder: focus on
inflammation, oxidative stress and neurotrophic factors. Neurosci
Biobehav Rev. 35:804–817. 2011. View Article : Google Scholar
|
|
10
|
Karalija A, Novikova LN, Kingham PJ,
Wiberg M and Novikov LN: Neuroprotective effects of
N-acetyl-cysteine and acetyl-L-carnitine after spinal cord injury
in adult rats. PLoS One. 7:e410862012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lee J, Kim H, Choi H, Oh T and Yune T:
Fluoxetine inhibits matrix metalloprotease activation and prevents
disruption of blood-spinal cord barrier after spinal cord injury.
Brain. 135:2375–2389. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Welin D, Novikova LN, Wiberg M, Kellerth
JO and Novikov LN: Effects of N-acetyl-cysteine on the survival and
regeneration of sural sensory neurons in adult rats. Brain Res.
1287:58–66. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fu L, Guo Z and Longhurst J: Endogenous
endothelin stimulates cardiac sympathetic afferents during
ischaemia. J Physiol. 588:2473–2486. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Douglas SA, Vickery-Clark LM, Louden C and
Ohlstein EH: Selective ETA receptor antagonism with BQ-123 is
insufficient to inhibit angioplasty induced neointima formation in
the rat. Cardiovasc Res. 29:641–646. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Okada M and Nishikibe M: BQ-788, a
selective endothelin ETB receptor antagonist. Cardiovasc Drug Rev.
20:53–66. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yin X, Yin Y, Cao FL, et al: Tanshinone
IIA attenuates the inflammatory response and apoptosis after
traumatic injury of the spinal cord in adult rats. PLoS One.
7:e383812012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lee S, Rosen S, Weinstein P, van Rooijen N
and Noble-Haeusslein L: Prevention of both neutrophil and monocyte
recruitment promotes recovery after spinal cord injury. J
Neurotrauma. 28:1893–1907. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yang L, Jones NR, Blumbergs PC, et al:
Severity-dependent expression of pro-inflammatory cytokines in
traumatic spinal cord injury in the rat. J Clin Neurosci.
12:276–284. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xu J, Kim GM, Chen S, et al: iNOS and
nitrotyrosine expression after spinal cord injury. J Neurotrauma.
18:523–532. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Vaishnav RA, Singh IN, Miller DM and Hall
ED: Lipid peroxidation-derived reactive aldehydes directly and
differentially impair spinal cord and brain mitochondrial function.
J Neurotrauma. 27:1311–1320. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kanno H, Ozawa H, Dohi Y, Sekiguchi A,
Igarashi K and Itoi E: Genetic ablation of transcription repressor
Bach1 reduces neural tissue damage and improves locomotor function
after spinal cord injury in mice. J Neurotrauma. 26:31–39. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li N, Alam J, Venkatesan MI, et al: Nrf2
is a key transcription factor that regulates antioxidant defense in
macrophages and epithelial cells: protecting against the
proinflammatory and oxidizing effects of diesel exhaust chemicals.
J Immunol. 173:3467–3481. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Goven D, Boutten A, Leçon-Malas V,
Boczkowski J and Bonay M: Prolonged cigarette smoke exposure
decreases heme oxygenase-1 and alters Nrf2 and Bach1 expression in
human macrophages: roles of the MAP kinases ERK(1/2) and JNK. FEBS
Lett. 583:3508–3518. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Avivar-Valderas A, Salas E,
Bobrovnikova-Marjon E, et al: PERK integrates autophagy and
oxidative stress responses to promote survival during extracellular
matrix detachment. Mol Cell Biol. 31:3616–3629. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wilson AD, Hart A, Brännström T, Wiberg M
and Terenghi G: Delayed acetyl-L-carnitine administration and its
effect on sensory neuronal rescue after peripheral nerve injury. J
Plast Reconstr Aesthet Surg. 60:114–118. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang CG, Welin D, Novikov L, Kellerth JO,
Wiberg M and Hart AM: Motorneuron protection by N-acetyl-cysteine
after ventral root avulsion and ventral rhizotomy. Br J Plast Surg.
58:765–773. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yan CY and Greene LA: Prevention of PC12
cell death by N-acetylcysteine requires activation of the Ras
pathway. J Neurosci. 18:4042–4049. 1998.PubMed/NCBI
|
|
28
|
Novikova LN, Novikov LN and Kellerth JO:
Survival effects of BDNF and NT-3 on axotomized rubrospinal neurons
depend on the temporal pattern of neurotrophin administration. Eur
J Neurosci. 12:776–780. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Manfridi A, Forloni GL, Arrigoni-Martelli
E and Mancia M: Culture of dorsal root ganglion neurons from aged
rats: effects of acetyl-L-carnitine and NGF. Int J Dev Neurosci.
10:321–329. 1992. View Article : Google Scholar : PubMed/NCBI
|