Introduction
Hepatocellular carcinoma (HCC) is the fifth most
common type of cancer and has the third highest rate of
tumor-associated mortality worldwide (1). Surgical resection is one of the most
effective treatment options for patients with HCC (2). However, the majority of patients are
unable to undergo curative resection due to the advanced cancer
staging when first diagnosed. This is considered to be associated
with the distinctive rapid progression observed in HCC. Therefore,
there is an urgent requirement to understand the mechanism
underlying the rapid progression of HCC.
Metastasis-associated in colon cancer-1 (MACC1) is a
novel oncogenic factor involved in tumor growth and metastasis, and
is aberrantly overexpressed in various types of tumor, including
lung cancer (3), gastric cancer
(4), colorectal cancer (5,6) and
breast cancer (7). Our preliminary
investigation revealed that MACC1 was aberrantly upregulated in HCC
tissues compared with adjacent liver tissues, and in vitro
experiments revealed that the overexpression of MACC1 promoted the
migration and invasive ability of HCC cells, potentially via
upregulating matrix metalloproteinase (MMP)2 and MMP9 (8). Another study demonstrated that
overexpression of MACC1 induces the increasing expression of
downstream factors, including hepatocyte growth factor (HGF) and
C-met, and promotes tumor growth and metastasis in colorectal
cancer (9,10). However, the molecular mechanism
whereby MACC1 leads to HCC progression remains to be
elucidated.
In the 1930s, Warburg et al described the
phenomenon that glycolysis was increased despite a adequate supply
of oxygen, which is now well-known as the Warburg effect in cancer
cells (11). Hexokinase-2 (HK2),
identified as a key rate-limiting enzyme in glucose metabolism,
catalyzes the reaction of the first step of glycolysis and has an
irreplaceable role in cancer glucose metabolism (12). Furthermore, with the unlimited
proliferation of tumor cells, the demand for energy and the
expression of basic glycolytic enzymes, particularly HK2, increases
markedly in the majority of tumors (13,14).
In addition, the overexpression of HK2 enhances the affinity of
tumor cells for adenosine triphosphate (ATP), promotes tumor cells
to uptake ATP and, in turn, improves the level of oxidative
phosphorylation in an environment of low ATP (15).
The MACC1-HGF/C-met pathway is important in cellular
growth, epithelial-mesenchymal transition, angiogenesis, cell
motility, invasiveness and metastasis through the activation of the
mitogen-activated protein kinase (MAPK) and phosphoinositide
3-kinase (PI3K)-Akt signaling pathways (9,16,17).
To assess the correlation between MACC1 and cancer glucose
metabolism in HCC, the present study used in vitro assays to
investigate the expression of HK2, an essential glycolytic enzyme
and downstream factor of the PI3K/AKT signaling pathway (18,19),
and analyzed the correlation between the two proteins and their
role in postsurgical survival.
Materials and methods
Patients and specimens
A total of 80 patients with HCC [Child-Pugh A to B,
scored as previously described (20)] were registered in the present study
between February 2006 and January 2008, including 58 males and 22
females (mean age 51 years; range 24–76), who had not received
preoperative chemotherapy or embolization. Following necessary
preoperative examinations, all the patients underwent liver
resection. Tumor tissue and matched normal tumor-adjacent tissue
specimens (≥2 cm distance to the resection margin) were collected
and immediately stored in paraformaldehyde for immunohistochemical
analysis or at −80°C for western blot analysis, respectively.
Clinical data were obtained from the patient’s medical records.
Written informed consent was obtained from all
patients. The Xi’an Jiaotong University Ethics committee approved
all procedures, according to the The Declaration of Helsinki, 1975
(21).
Cell culture and transfection
Liver cell lines (HepG2, Hep3B, SMMC-7721, Bel-7402,
Huh7, MHCC-97H and LO2) were obtained from the Institute of
Biochemistry and Cell Biology, Chinese Academy of Sciences
(Shanghai, China). All the cells were maintained in Dulbecco’s
modified Eagle’s medium (DMEM; Hyclone, Logan, UT, USA) containing
10% fetal bovine serum (FBS; Gibco BRL, Gaithersburg, MD, USA) with
100 U/ml penicillin and 100 μg/ml streptomycin
(Sigma-Aldrich, St. Louis, MO, USA) and cultured in a humidified 5%
CO2 incubator at 37°C for 48–72 h.
The MACC1-expressing plasmid and control plasmid
(pCMV6-Entry; GenePharma, Shanghai, China) were trans fected into
HepG2 cells using Roche FuGENE® 6 Transfection reagent
(Roche Diagnostics, Indianapolis, IN, USA), and stably expressing
clones were selected by G418 at a dose of 300 μg/ml for 2
weeks. Western blot analysis confirmed the overexpression of MACC1
in the selected stably expressing clones (Fig. 5A). MACC1 small interfering (si)RNA
and control siRNA were transfected into the MHCC-97H cells using
the siPORT™ NeoFX™ transfection agent purchased from Applied
Biosystems (Carlsbad, CA, USA).
Immunohistochemistry
Tumor samples were fixed in 10% buffered formalin
solution (Shaanxi Xianfeng Biotechnology Co., Ltd, Xi’an, China)
and embedded in paraffin (Shaanxi Xianfeng Biotechnology Co., Ltd).
Rabbit polyclonal MACC1 (cat. no. ab106579; Abcam, Hong Kong,
China;1:200) and rabbit monoclonal HK2 (cat. no. C64G5; Cell
Signaling Technology, Danvers, MA, USA; 1:100) antibodies were used
in immunohistochemical analysis (IHC) using the
streptavidin-peroxidase conjugated method. In brief, following
antigen retrieval in a citrate buffer (Shaanxi Xianfeng
Biotechnology Co., Ltd) using a microwave (350W; Midea Group Co.,
Ltd, Guangdong, China) for 15 min; the sections were then incubated
with normal serum (Shaanxi Xianfeng Biotechnology Co., Ltd) at 37°C
for 30 min and then with the primary antibody against MACC1 or HK2
at 4°C overnight. Sections were washed with PBS (Shaanxi Xianfeng
Biotechnology Co., Ltd) and incubated with biotinylated goat
anti-rabbit monoclonal secondary antibodies (cat no. SP-9000-D;
Zhongshan Goldenbridge Biotechnology Co., Ltd, Beijing, China) and
avidin-biotin-peroxidase complex (Zhongshan Goldenbridge
Biotechnology Co., Ltd) according to the manufacturer’s
instructions. The positive signal was visualized by incubating the
sections with a diaminobenzidine solution (Shaanxi Xianfeng
Biotechnology Co., Ltd) and counterstaining with hematoxylin
(Shaanxi Xianfeng Biotechnology Co., Ltd). The sections were then
observed under an inverted microscope (Nikon China Co., Ltd, Tokyo,
Japan). The staining intensity was expressed as four grades: 0,
none; 1, weak; 2, moderate; and 3, strong under 10 randomly
selected independent high magnification (magnification, ×400)
fields. The percentages of positive carcinoma cells were expressed
as the following grades: 0, <10%; 1, 10–25%; 2, 26–50%; 3,
51–75%; and 4, >75%. The total score was calculated by summating
the staining intensity and the percentage of positive tumor cells.
Sections with a total score >2 were defined as exhibiting
positive staining for the above two proteins.
Western blot analysis
Rabbit polyclonal MACC1 (cat. no. ab106579; Abcam;
1:1,000), rabbit monoclonal HK2 (1:1,000) and mouse monoclonal
β-actin (cat. no. sc-47778, Santa Cruz Biotechnology, Inc., Santa
Cruz, CA, USA; 1:1,000) antibodies were used for western blot
analysis. Secondary horseradish peroxidase-conjugated goat
anti-mouse or rabbit antibodies (Bio-Rad, Laboratories, Inc.,
Hercules, CA, USA) were used at a 1:5,000 dilution.
Briefly, equal quantities of the protein samples
were separated by denaturing gel electrophoresis (Bio-Rad
Laboratories, Inc.). Following transfer onto a polyvinylidene
difluoride membrane (Millipore, Billerica, MA, USA) blots were
probed overnight with the primary antibodies (MACC1, HK2 and
β-actin) respectively. Following washing 3 times in Tris-buffered
saline with Tween 20 (Sino-American Biotechnology, He’nan, China),
blots were then incubated with the relevant goat anti-rabbit and
goat anti-mouse immunoglobulin G monoclonal secondary antibodies
(cat nos. sc-2004 and sc-2005; Santa Cruz Biotechnology, Inc.;
1:15,000) conjugated with horseradish peroxidase (HRP) and signals
were visualized using the HyGLO HRP detection kit from Denville
Scientific (Metuchen, NJ, USA).
Reverse transcription quantitative
polymerase chain reaction (RT-qPCR)
Total cellular RNA was extracted from the cultured
cells using a Fastgen200 RNA isolation System (Fastgen, Shanghai,
China). cDNA synthesis was achieved using the High Capacity cDNA
Reverse Transcription kit (Fermentas, Burlington, Canada). RT-qPCR
was performed using SYBR Green PCR master mix (Takara Bio, Inc.,
Dalian, China) using an IQ5 system (Bio-Rad Laboratories, Inc.).
The primers to detect mRNA were as follows: MACC1, forward
5′-CTTGCGGAGGTCACCATAGC-3′ and reverse 5′-GATTTCCAACAACGGGCTCA-3′.
All the samples were normalized to internal controls and
fold-changes were calculated through relative quantification. Each
measurement was performed in triplicate.
Metabolic assay of glycogen
Glycogen measurements were performed using a
glycogen concentration kit (Biovision, Milpitas, CA, USA) according
to the KOH-anthrone method, described by Liu et al (22). Lactate quantifications in the
media, following transfection or planting, were performed using a
Lactate assay kit II (Biovision). Experiments were performed in
triplicate.
MTT assay
Proliferation was determined using an MTT assay
(Roche Diagnostics). Following transfection of the MACC1 siRNA or
MACC1 expressing plasmids, the cells were cultured further for
10–20% area of a culture dish, in a humidified 5% CO2
incubator at 37°C for 0 to 72 h and the absorbance of the samples
was measured using a model 550 microplate reader (Bio-Rad
Laboratories, Inc.), at a wavelength of 570 nm corrected to 655 nm.
The experiments were performed in triplicate.
Colony formation assay
A total of 100 HepG2-vector and HepG2-MACC1 cells
were placed in a fresh six-well plate in triplicate and maintained
in DMEM containing 10% FBS in a humidified 5% CO2
incubator at 37°C for 2 weeks. The cell colonies were fixed with
20% methanol and stained with 0.1% coomassie brilliant blue R250 at
room temperature for 15 min. The colonies were counted using a
ELIspot Bioreader 5000 (Bio-Sys, Karben, Germany).
Statistical analysis
Differences between groups were compared using the
χ2 test, Fisher’s exact test, the Mann-Whitney test and
an analysis of variance. P<0.05 was considered to indicate a
statistically significant difference. The SPSS 13.0 statistical
package (SPSS, Inc., Chicago, IL, USA) was used for all
calculations.
Results
MACC1 is overexpressed and accompanied by
the expression of HK2 in HCC tissue
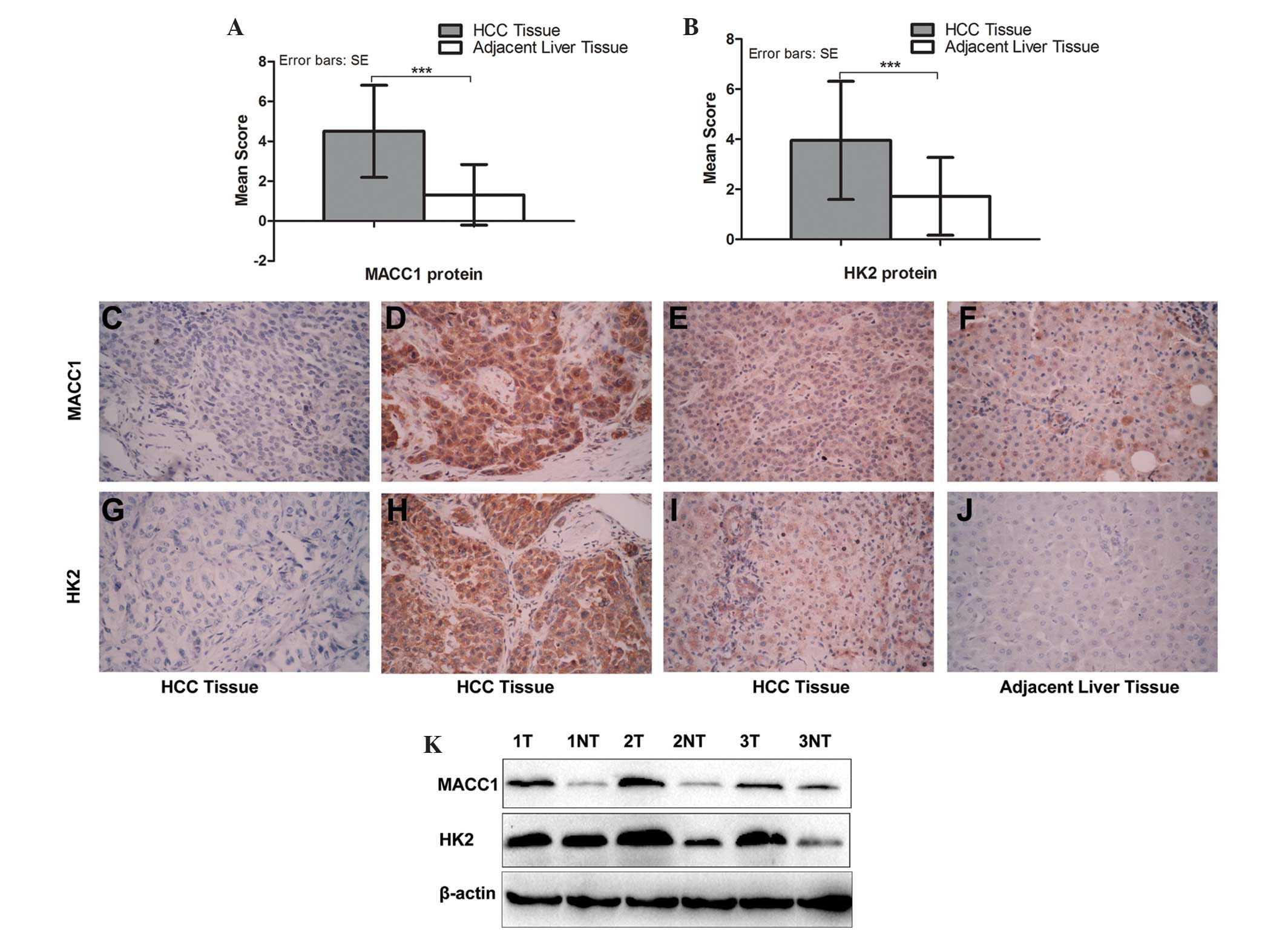
The IHC staining of the protein expression of MACC1
was positive in 59/80 (73.8%) of the HCC tissues compared with
23/80 (28.8%) of the adjacent liver tissues, while the protein
expression of HK2 was positive in 56/80 (70%) and 25/80 (31.3%;
P<0.001), respectively. In addition, the IHC scores also
demonstrated that the protein expression of MACC1 was significantly
higher in the HCC tissues compared with the adjacent liver tissues
(4.50±2.31, vs. 1.32±1.52; P<0.001; Fig. 1A), as was the protein expression of
HK2 (3.95±2.36 vs. 1.72±1.55; P<0.001; Fig. 1B). The results of the IHC assay
demonstrated that MACC1 protein was located predominantly in the
nucleus and cytoplasm, while it was located in the cytoplasm in
almost all the HK2-positive tumor tissues (Fig. 1).
In addition, western blot analysis revealed that
MACC1 was positive in 20/24 (83.3%) of the HCC tissues compared
with 11/24 (54.2%) in the adjacent liver tissues, while HK2 was
positive in 21/24 (87.5%) and 13/24 (65.0%; P<0.05),
respectively (Fig. 1K). The
expression levels of the two proteins were significantly higher in
the HCC tissues compared with the adjacent liver tissues.
Notably, the IHC data demonstrated that the
expression of MACC1 was significantly associated with tumor
staining for HK2 (odds ratio 3.89; 95% confidence interval
(CI)=1.36–11.18; Spearman’s correlation=0.569; P=0.009), as shown
in Fig. 1). This suggested that
overexpression of the MACC1 protein in HCC tissues occurred with an
increase in HK2 protein.
Correlation between the protein
expression levels of MACC1 and HK2 and the clinical characteristics
of HCC
Following analysis of the association between the
expression levels of MACC1 and HK2 in HCC tissues and
clinicopathological characteristics (Table I), it was revealed that positive
MACC1 expression was significantly associated with large tumor size
(r=0.332; P=0.004), a high Edmondson-Steiner classification
(r=0.269; P=0.016) and an advanced tumor-mode-metastasis (TNM)
stage (r=0.254; P=0.023). In addition, positive HK2 expression was
significantly correlated with high Edmonson-Steiner classification
(r=0.382; P=0.001) and advanced TNM stage (r=0.373; P=0.001),
whereas no correlation was identified between other
characteristics. These clinical data suggested that MACC1 and HK2
were closely associated with the process of HCC, and implied
advanced HCC progression.
 | Table IDistribution of MACC1 and HK2 positive
tumors in groups, defined by clinicopathological variables. |
Table I
Distribution of MACC1 and HK2 positive
tumors in groups, defined by clinicopathological variables.
| Variable | Group | MACC1
| P-value | HK2
| P-value |
|---|
| + | − | + | − |
|---|
| Age | >50 | 32 | 12 | 0.818 | 33 | 11 | 0.281 |
| ≤50 | 27 | 9 | | 23 | 13 | |
| Gender | Male | 41 | 17 | 0.312 | 40 | 18 | 0.743 |
| Female | 18 | 4 | | 16 | 6 | |
| HBV infection | Yes | 47 | 15 | 0.438 | 44 | 18 | 0.726 |
| No | 12 | 6 | | 12 | 6 | |
| Liver
cirrhosis | Yes | 43 | 14 | 0.589 | 40 | 17 | 0.957 |
| No | 16 | 7 | | 16 | 7 | |
| AFP (ng/ml) | >20 | 42 | 12 | 0.445 | 37 | 17 | 0.913 |
| ≤20 | 14 | 8 | | 16 | 6 | |
| Unknown | 3 | 1 | | 3 | 1 | |
| Tumor size
(cm) | ≥5 | 36 | 4 | 0.004a | 27 | 12 | 0.738 |
| <5 | 21 | 16 | | 28 | 10 | |
| Unknown | 2 | 1 | | 1 | 2 | |
| Edmondson | I+II | 30 | 17 | 0.016a | 26 | 21 | 0.001a |
| III+IV | 29 | 4 | | 30 | 3 | |
| TNM stage | T1+T2 | 28 | 16 | 0.023a | 24 | 20 | 0.001a |
| T3+T4 | 31 | 5 | | 32 | 4 | |
| Intrahepatic | Yes | 12 | 1 | 0.097 | 11 | 2 | 0.209 |
| metastases | No | 47 | 20 | | 45 | 22 | |
| Portal vein | Yes | 11 | 1 | 0.126 | 9 | 3 | 0.682 |
| Invasion | No | 48 | 20 | | 47 | 21 | |
Association between survival rates and
the tumor expression of MACC1 and HK2
Follow-up information was obtained from the 80 HCC
cases. The median survival rate of 80 patients was 30 months (range
0–60 months). As compared by a Kaplan-Meier survival curve,
MACC1-positive expression was significantly associated with a lower
overall survival (OS) rate, with the median survival rate of 29
months in the 59 HCC patients compared with 60 months in the 21 HCC
patients with MACC1-negative expression (Log-rank P=0.032; hazard
ratio (HR)=1.99; 95% CI=1.06–3.72), as shown in Fig. 2A. In addition, HK2-positive
expression was also significantly associated with a lower OS rate,
with a median survival rate of 26 months in the 56 HCC patients
compared with 56 months in the 24 HCC patients with HK2-negative
expression (Log-rank P=0.015; HR=2.11; 95% CI=1.55–3.84), as shown
in Fig. 2B.
In addition, among the 56 HCC patients with
HK2-positive expression, a lower OS rate was identified in HCC
patients whose samples were also MACC1-positive compared with
MACC1-negative tumor samples (Log-rank P=0.039, HR=2.31, 95%
CI=1.04–5.14; Fig. 2C). However,
among the 24 HCC patients with HK2-negative expression, no
significant change in mortality rate was observed if the patients
had MACC1-positive expression (Log-rank P=0.798; Fig. 2E). By contrast, among the 59 HCC
patients with MACC1-positive expression, a significantly decreased
survival rate was observed in the HCC patients whose samples were
also HK2-positive compared with HK2-negative tumor samples
(Log-rank P=0.016; HR=2.37; 95%CI=1.17–4.78; Fig. 2D). However, among the 21 HCC
patients with MACC1-negative expression, no significant change in
mortality rate was observed if the patients had HK2 positive
expression (Log-rank P=0.308; Fig.
2F). These data demonstrated that upregulation of the protein
expression levels of MACC1 and HK2 led to a poor prognosis in
HCC.
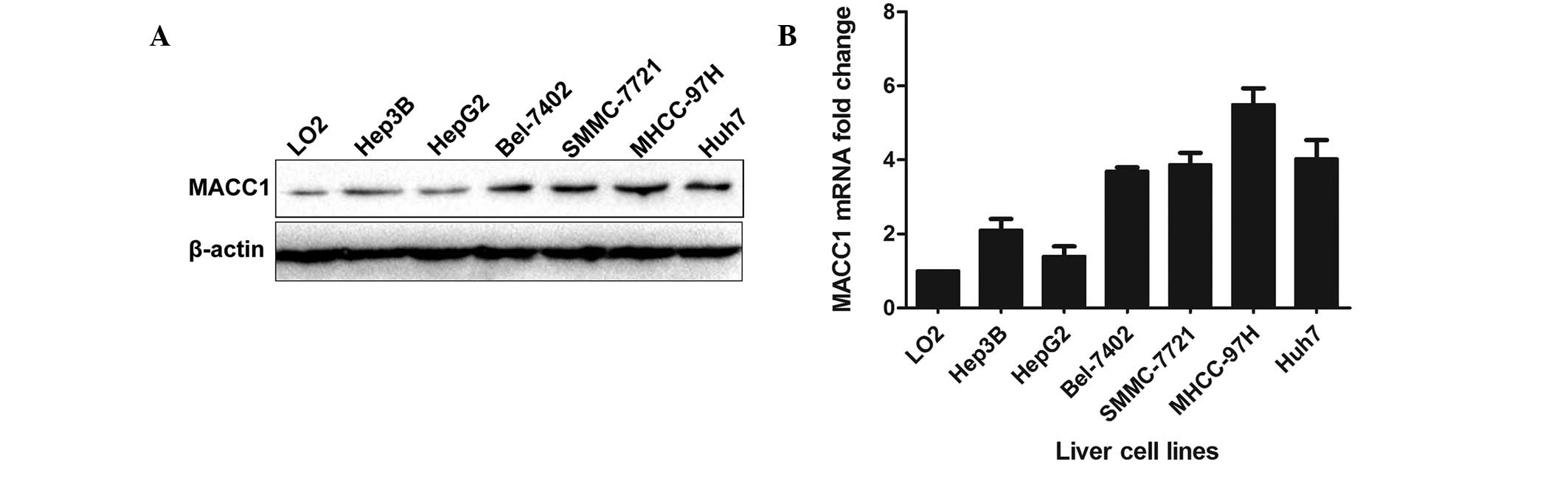
Differential protein and mRNA expression
levels of MACC1 in HCC cell lines
Subsequently, western blot analysis and RT-qPCR were
performed in six HCC cell lines (HepG2, Hep3B, SMMC-7721, Bel-7402,
Huh7 and MHCC-97H) and a non-transformed human liver cell line
(LO2), each of which had different biological characteristics.
Among these HCC cell lines, HepG2 expressed the lowest level of
MACC1 protein and mRNA, while MHCC-97H expressed a relatively high
level of MACC1 protein and mRNA (Fig.
3).
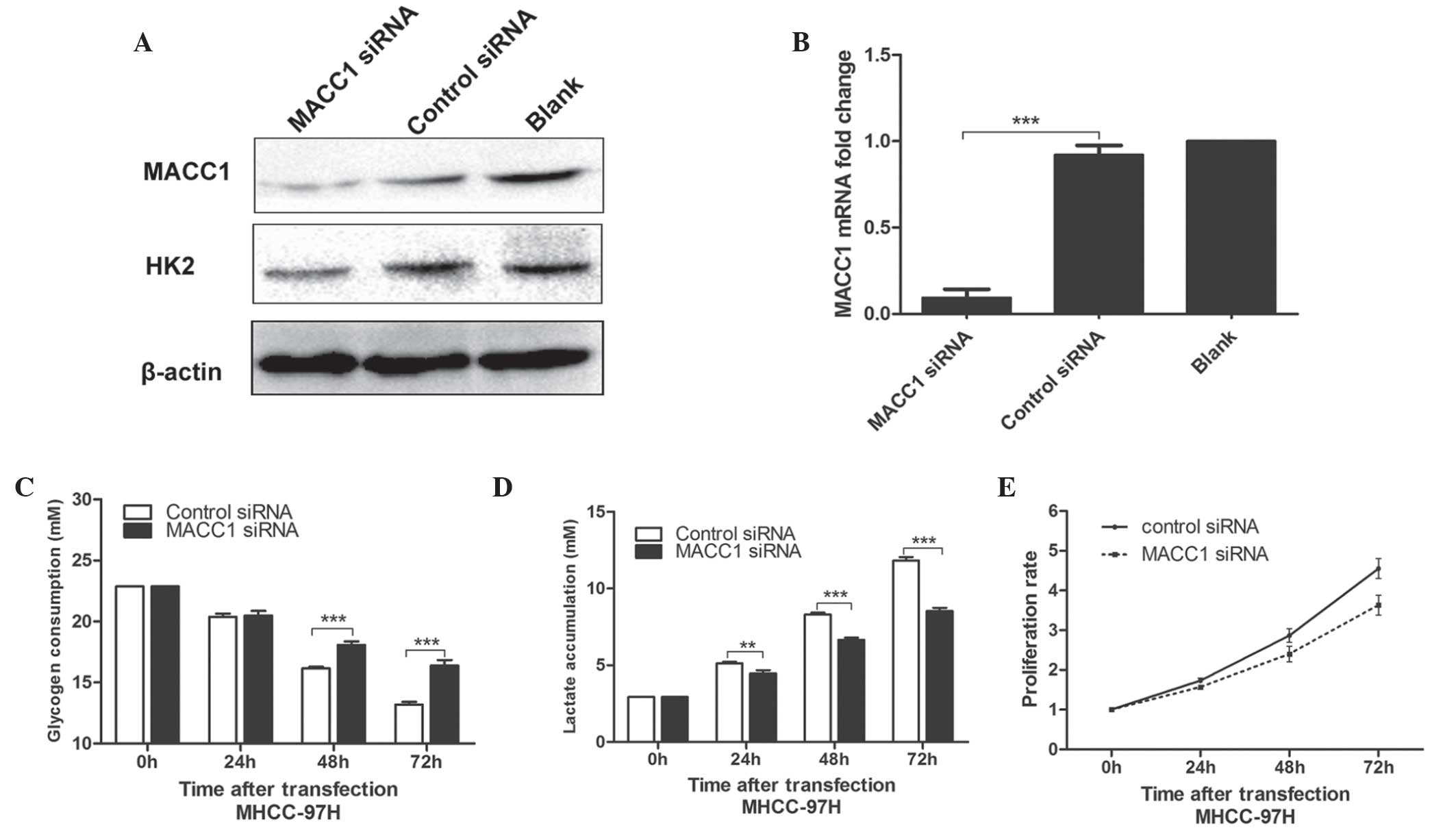
Knockdown of MACC1 in MHCC-97H cells
suppresses cell glucose metabolism and reduces proliferation
To further investigate the function of MACC1 in
glucose metabolism in cancer, the expression of MACC1 was silenced
in MHCC-97H cells by transfection with MACC1 siRNA. As shown in
Fig. 4A and B, the protein
expression levels of MACC1 and HK2 were significantly decreased
following transfection with MACC1 siRNA. The determination of the
glycogen concentration of the culture medium revealed that the
knockdown of MACC1 markedly suppressed the glucose catabolism of
MHCC-97H cells (48 and 72 h after transfection; P<0.001,
respectively; Fig. 4C).
Simultaneously, decreased lactate production also confirmed the
inhibition of glucose catabolism (48 and 72 h after transfection;
P<0.001, respectively; Fig.
4D). The proliferation of MHCC-97H cells was evaluated with or
without MACC1 siRNA transfection using an MTT assay. The MTT
activity of the MHCC-97H cells transfected with MACC1 siRNA was
markedly decreased compared with the control cells (48 and 72 h
after transfection; P<0.001, respectively), implying decreased
proliferation rates (Fig. 4E).
These data suggested that MACC1 promoted glucose metabolism and
proliferation.
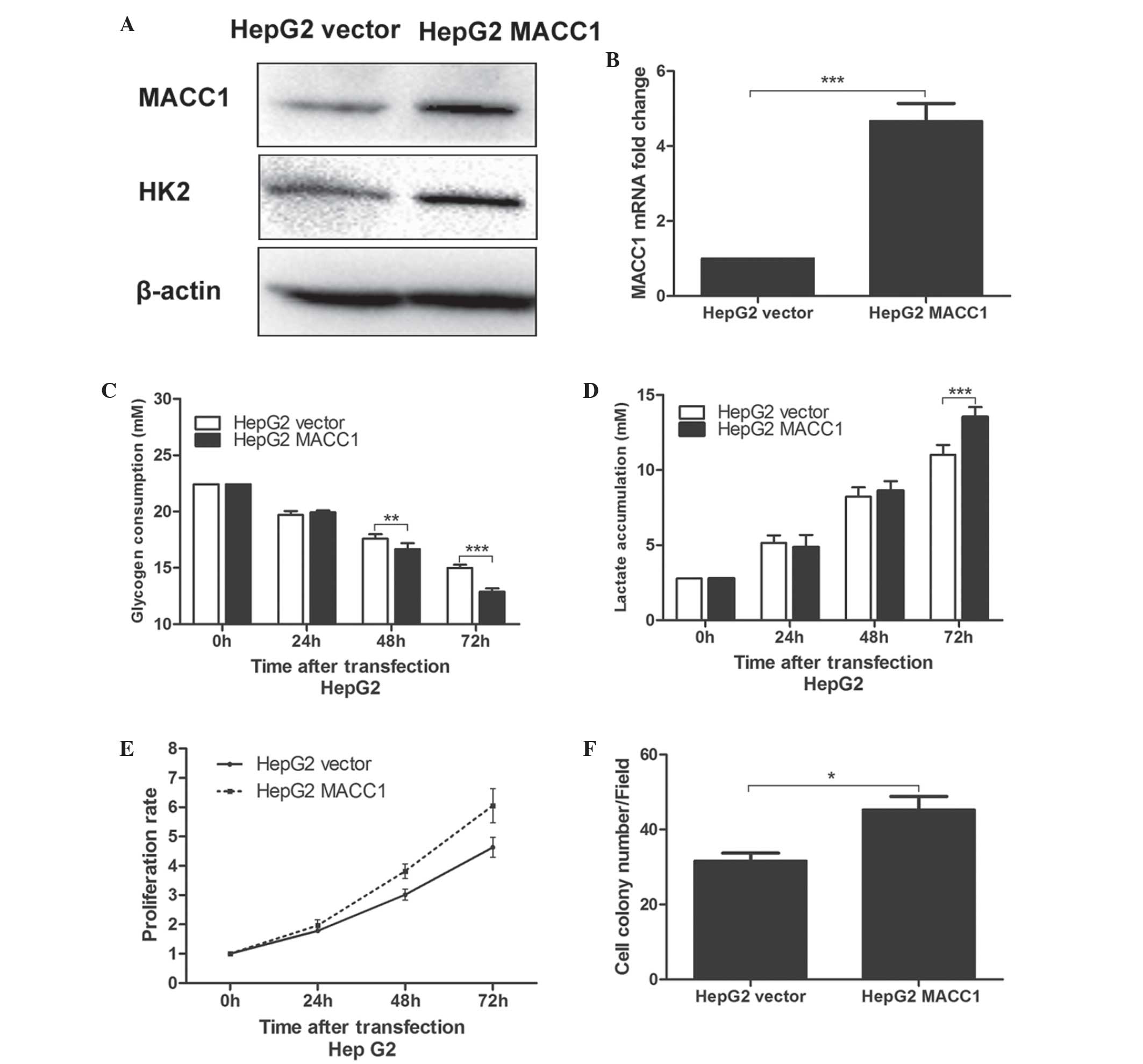
Overexpression of MACC1 in HepG2 cells
promotes cell glucose metabolism and increases replication
To confirm the observed findings, the protein and
mRNA expression of MACC1 in HepG2 cells was increased through
stable transfection with a MACC1-expressing plasmid, and the
protein expression of HK2 increased accordingly (Fig. 5A and B). Subsequently, it was
identified that the glycogen consumption of the HepG2 cells stably
transfected with the MACC1 expressing plasmid (HepG2 MACC1 cells)
was significantly higher compared with that of HepG2 cells
transfected with the vector plasmid (HepG2 vector cells) 48 and 72
h after planting (P<0.01; Fig.
5C), with a similar trend in lactate accumulation (72 h after
planting; P<0.01; Fig. 5D). The
proliferation of the transfected HepG2 was subsequently evaluated
using MTT and colony formation assays. The proliferation rate of
the HepG2 MACC1 cells was significantly increased compared with the
HepG2 vector cells via the MTT assay 48 and 72 h after planting
(P<0.01; Fig. 5E). Furthermore,
the colony forming ability of the HepG2 MACC1 cells was
significantly enhanced compared with the HepG2 vector cell, as
expected (P<0.05; Fig. 5F).
These results further clarified that the overexpression of MACC1
promoted proliferation, partly through enhanced glucose
metabolism.
Discussion
The present study introduced a noteworthy finding,
confirmed by a number of in vitro assays, that the
expression of MACC1 was significantly upregulated in HCC tissues
and was accompanied by the increased protein expression of HK2.
Clinical data and survival curve analysis revealed that increased
protein expression levels of MACC1 and HK2 indicated a poor
prognosis in HCC. In addition, knockdown of MACC1 in the MHCC-97H
cells led to a reduced rate of proliferation via a reduction in
glucose metabolism by the decreased protein expression of HK2.
Furthermore, overexpression of MACC1 in the HepG2 cells induced the
opposite result, providing confirmation. It was hypothesized that
MACC1 leads to a poor prognosis in HCC, partly through promoting
proliferation via enhanced glucose metabolism.
HCC is one of the most common types of cancer
worldwide and has a poor prognosis. Tumor resection at an early
stage remains the most effective treatment for HCC, however, the
lack of early diagnosis has resulted in numerous patients with HCC
being unable to undergo resection. In the present study, the role
of MACC1 and HK2, from clinical data, and the prognosis of HCC,
from immunohistochemical, immunoblotting and survival curve
analysis, were investigated and the protein expression levels of
MACC1 and HK2 were markedly upregulated in HCC tissue and
associated with high Edmondson-Steiner classification, advanced TNM
stage and a poor prognosis in patients with HCC. These data
indicated the potential development of MACC1 and HK2 as clinical
prognostic biomarkers in the future.
As an oncogene, MACC1 contributes to neoplastic
growth, invasion and metastasis through the activation of HGF/MET
signaling in several types of tumor (5,9).
Previous studies have revealed that increased protein expression
levels of MACC1 and HK2 are significantly associated with a poor
prognosis in HCC patients (17,23,24).
However, the mechanism by which MACC1 and HK2 lead to a poor
prognosis remains to be elucidated. The PI3K/Akt signaling pathway,
downstream of HGF/MET, is known as one of the basic elements in the
development of several types of tumor, involved in tumor cell
growth, differentiation and apoptosis (25,26),
and previous studies have demonstrated that the PI3K/Akt signaling
pathway is also involved in glucose metabolism in tumors, through
increased HK2 protein expression and phosphorylation (19,27–29).
Therefore, it was hypothesized that MACC1 induced the tumor growth
via promoting tumor glucose metabolism and led to a poor prognosis
in patients with HCC. In the present study, the correlation between
the protein expression levels of MACC1 and HK2 was initially
confirmed using immunohistochemical and western blot analysis in
HCC tissue. Subsequently, it was identified that knockdown of MACC1
in MHCC-97H cells decreased the protein expression of HK2, which
also inhibited tumor glucose metabolism and reduced proliferation,
and may improve prognosis. Subsequent overexpression investigations
confirmed these findings. These data suggested that MACC1 induced
tumor glucose metabolism by increasing the protein expression of
HK2 and caused rapid proliferation, which may lead to poor survival
rates in patients with HCC. Although MACC1 was found to enhance the
expression of HK2 in the MHCC-97H and HepG2 cells, whether MACC1
regulates the transcription of HK2 directly remains to be
elucidated. The present study aimed to examine the MACC1-binding
site of the HK2 promoter by performing out an electrophoretic
mobility shift assay, however no positive results were obtained,
which indicated that MACC1 may upregulate the expression of HK2
expression in an indirect manner.
In conclusion, the present study demonstrated that
aberrantly increased expression levels of MACC1 and HK2 in HCC
tissues were associated with a high Edmondson-Steiner
classification and advanced TNM stage, and led to a poor prognosis
in HCC patients. In addition, MACC1 induced tumor glucose
metabolism by upregulating HK2 indirectly and causing high levels
of proliferation, which may be, in part the reason for the poor
prognosis in HCC. MACC1 and HK2 may become potential clinical
prognosis factors and targets for the treatment of HCC, however,
further investigation is required to clarify the specific mechanism
by which MACC1 promotes the progression of HCC.
Acknowledgments
The present study was supported by grants from the
National Natural Scientific Foundation of China (grant no. 81071897
to Yingmin Yao) and the Research Fund for the Doctoral Program of
High Education of China from the Ministry of Education (grant no.
20120201120090 to Xin Zheng).
Abbreviations:
|
MACC1
|
metastasis-associated in colon
cancer-1
|
|
HK-2
|
hexokinase-2
|
|
HCC
|
hepatocellular carcinoma
|
|
OS
|
overall survival
|
|
CI
|
confidential intervals
|
|
HR
|
hazard ratio
|
References
|
1
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics, 2002. CA Cancer J Clin. 5:74–108. 2005.
View Article : Google Scholar
|
|
2
|
Blum HE: Treatment of hepatocellular
carcinoma. Best Pract Res Clin Gastroenterol. 19:129–145. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shimokawa H, Uramoto H, Onitsuka T, et al:
Overexpression of mACC1 mRNA in lung adenocarcinoma is associated
with postoperative recurrence. J Thorac Cardiovasc Surg. 4:895–898.
2011. View Article : Google Scholar
|
|
4
|
Shirahata A, Sakata M, Kitamura Y, et al:
MACC1 as a marker for peritoneal-disseminated gastric carcinoma.
Anticancer Res. 30:3441–3444. 2010.PubMed/NCBI
|
|
5
|
Shirahata A, Shinmura K, Kitamura Y, et
al: MACC1 as a marker for advanced colorectal carcinoma. Anticancer
Res. 30:2689–2692. 2010.PubMed/NCBI
|
|
6
|
Arlta F and Stein U: Colon cancer
metastasis: MACC1 and Met as metastatic pacemakers. Int J Biochem
Cell Biol. 41:2356–2359. 2009. View Article : Google Scholar
|
|
7
|
Huang Y, Zhang H, Cai J, et al:
Overexpression of MACC1 and Its significance in human breast cancer
progression. Cell Biosci. 1:162013. View Article : Google Scholar
|
|
8
|
Gao J, Ding FH, Liu QG and Yao YM:
Knockdown of MACC1 expression suppressed hepatocellular carcinoma
cell migration and invasion and inhibited expression of MMP2 and
MMP9. Mol Cell Biochem. 376:21–32. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Stein U, Walther W, Arlt F, et al: MACC1,
a newly identified key regulator of HGF-MET signaling, predicts
colon cancer metastasis. Nat Med. 15:59–67. 2009. View Article : Google Scholar
|
|
10
|
Qiu J, Huang P, Liu Q, et al:
Identification of MACC1 as a novel prognostic marker in
hepatocellular carcinoma. J Transl Med. 9:166–176. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wallace DC: Mitochondria and cancer:
Warburg addressed. Cold Spring Harb Symp on Quantit Biol.
70:363–374. 2005. View Article : Google Scholar
|
|
12
|
Mathupala SP, Rempel A and Pedersen PL:
Aberrant glycolytic metabolism of cancer cells: a remarkable
coordination of genetic, transcriptional, post-translational, and
mutational events that lead to a critical role for type II
hexokinase. J Bioenerg Biomembr. 29:339–343. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bustamante E and Pedersen PL: High aerobic
glycolysis of rat hepatoma cells in culture: role of mitochondrial
hexokinase. Proc Natl Acad Sci USA. 74:3735–3739. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bustamante E, Morris HP and Pedersen PL:
Energy metabolism of tumor cells: requirement for a form of
hexokinase with a propensity for mitochondrial binding. J Biol
Chem. 256:8699–8704. 1981.PubMed/NCBI
|
|
15
|
Pastorino JG and Hoek JB: Hexokinase II:
the integration of energy metabolism and control of apoptosis. Curr
Med Chem. 10:1535–1551. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sattler M and Salgia R: c-Met and
hepatocyte growth factor: potential as novel targets in cancer
therapy. Curr Oncol Rep. 9:102–108. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lang AH, Geller-Rhomberg S, Winder T, et
al: A common variant of the MACC1 gene is significantly associated
with overall survival in colorectal cancer patients. BMC Cancer.
12:202012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ahn KJ, Hwang HS, Park JH, et al:
Evaluation of the role of hexokinase type II in cellular
proliferation and apoptosis using human hepatocellular carcinoma
cell lines. J Nucl Med. 50:1525–1532. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Roberts DJ, Tan-Sah VP, Smith JM and
Miyamoto S: Akt phosphorylates HK-II at Thr473 and increases
mitochondrial HK-II association to protect cardiomyocytes. J Biol
Chem. 288:23798–23806. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Albers I, Hartmann H, Bircher J and
Creutzfeldt W: Superiority of the Child-Pugh classification to
quantitative liver function tests for assessing prognosis of liver
cirrhosis. Scand J Gastroenterol. 24:269–276. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shephard DA: The 1975 Declaration of
Helsinki and consent. Can Med Assoc J. 115:1191–1192.
1976.PubMed/NCBI
|
|
22
|
Liu F, Yang T, Wang B, et al: Resistin
induces insulin resistance, but does not affect glucose output in
rat-derived hepatocytes. Acta Pharmacol Sin. 29:98–104. 2008.
View Article : Google Scholar
|
|
23
|
Xie C, Wu J, Yun J, et al: MACC1 as a
prognostic biomarker for early-stage and AFP-normal hepatocellular
carcinoma. PLoS One. 8:e642352013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ge SH, Wu XJ, Wang XH, et al:
Over-expression of metastasis-associated in colon cancer-1 (MACC1)
associated with better prognosis of gastric cancer patients. Chin J
Cancer Res. 23:153–159. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gottlob K, Majewski N, Kennedy S, Kandel
E, Robey RB and Hay N: Inhibition of early apoptotic events by
Akt/PKB is dependent on the first committed step of glycolysis and
mitochondrial hexokinase. Genes Dev. 15:1406–1418. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bijur GN and Jope RS: Rapid accumulation
of Akt in mitochondria following phosphatidylinositol 3-kinase
activation. J Neurochem. 87:1427–1435. 2003. View Article : Google Scholar
|
|
27
|
Neary CL and Pastorino JG: Akt inhibition
promotes hexokinase 2 redistribution and glucose uptake in cancer
cells. J Cell Physiol. 228:1943–1948. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhu Y, Pereira RO, O’Neill BT, et al:
Cardiac PI3K-Akt impairs insulin-stimulated glucose uptake
independent of mTORC1 and GLUT4 translocation. Mol Endocrinol.
27:172–184. 2013. View Article : Google Scholar :
|
|
29
|
Gwak GY, Yoon JH, Kim Km, Lee HS, Chung JW
and Gores GJ: Gores. Hypoxia stimulates proliferation of human
hepatoma cells through the induction of hexokinase II expression. J
Hepatol. 42:358–364. 2005. View Article : Google Scholar : PubMed/NCBI
|