Introduction
Breast cancer is the most prevalent type of cancer
diagnosed in the worldwide female population (1). In patients with breast cancer, ~60%
of pre-menopausal and ~75% of post-menopausal females have
hormone-dependent (estrogen receptor positive [ER+]) carcinomas
(2), and are therefore suitable
for endocrine therapy, which is a therapeutic strategy that aims to
suppress the mitogenic effects of estrogen on breast cancer cells
(3). There are various types of
hormonal therapies that may be used to treat ER+ breast cancers.
Recently, aromatase inhibitors (AIs) have been considered the
primary choice for hormonal treatment of ERα+ breast cancer in
postmenopausal females (4).
Third-generation AIs include the non-steroidal triazole
derivatives, anastrozole and letrozole, which act as competitive
inhibitors. Furthermore, a steroidal derivate of androstenedione,
exemestane, has been shown to be an effective alternative to
tamoxifen. Previous studies have demonstrated that exemestane is
superior to tamoxifen, with regards to its effects on disease
progression, incidences of locoregional and distant relapses, and
contralateral breast cancers (2,5).
However, despite advances in breast cancer treatment, ~25–40% of
patients will eventually develop metastatic disease, which is
largely incurable (6). Systemic
chemotherapy is currently considered the standard treatment for
patients with metastatic breast cancer (7). In addition, despite the success of
the most recent generation of AIs, the eventual occurrence of
adverse effects, including bone loss, fractures (8) and acquired resistance (9), reinforce the importance of searching
for novel potent and specific agents with lower side effects, which
may reverse the acquired resistance and extend the benefits of
AIs.
Statins are one of the most frequently prescribed
medications, which are used to decrease the risk of cardiovascular
events and overall mortality (10). Statins are known to decrease high
blood cholesterol levels through suppression of hepatic cholesterol
biosynthesis (11). Statins have a
similar structure to that of 3-hydroxy-3-methylglutaryl-coenzyme A
(HMG-CoA) and competitively inhibit HMG-CoA reductase, an enzyme
that catalyzes the rate-limiting step in cholesterol biosynthesis,
the conversion of HMG-CoA to mevalonate (12). As well as producing cholesterol,
the mevalonate pathway produces numerous non-sterol products,
including ubiquinone, dolichol, isopentenyladenine and prenyl
groups, which are essential for the isoprenylation of intracellular
second messenger mitogenic signaling proteins, such as Ras and
other small G proteins (12).
These non-sterol isoprenoid byproducts are important regulators of
numerous oncogenic properties, including angiogenesis,
proliferation and migration (13,14).
Since mevalonate is synthesized from HMG-CoA, HMG-CoA reductase
inhibitors, also known as statins, reduce the entry of mevalonate
into the pathway. Previous studies have demonstrated the
anti-neoplastic effects of statins in vitro (15–18).
Nielsen et al (19)
reported that statin use in Danish patients with cancer was
associated with reduced cancer-associated mortality in a large
observational study that included >295,000 patients with
cancer.
Simvastatin is the most commonly used lipid-lowering
statin drug, which is derived from lovastatin. Simvastatin has been
shown to exhibit anti-proliferative and apoptotic activity against
numerous types of cancer cell lines, including colon, prostate and
breast (16–18). The anti-tumor mechanisms of
simvastatin have been investigated and numerous potential
underlying mechanisms have been identified, including suppression
of downstream signaling of epidermal growth factor receptors, and
attenuation of extracellular signal-regulated kinases 1/2, nuclear
factor-κB, c-Jun N-terminal kinases, phosphoinositide 3-kinase/Akt
(17), generation of reactive
oxygen species (20), activation
of inducible nitric oxygen species resulting in increased levels of
nitric oxide (21), as well as
down regulation of B-cell lymphoma 2 (Bcl-2) and activation of
Bcl-2-associated X protein (Bax) (15). Furthermore, a large Danish
nationwide prospective cohort study demonstrated that use of
simvastatin, a highly lipophilic statin, reduced the recurrence
risk by 10 fewer cases per 100 females over 10 years among Danish
females with Stage I–III breast cancer (22).
Therapeutic strategies using a combination of drugs
in order to enhance the efficacy of cancer treatment have recently
garnered attention. The drugs may act together synergistically to
inhibit tumor progression through the regulation of various
signaling pathways, or reverse the cancer-specific upregulated cell
proliferation or evasion of apoptosis (23). The present study chose to evaluate
combinations of statin drugs, based upon previously reported
cytotoxic experience in breast cancer cell lines (24,25).
The present study tested the hypothesis that the combination of
simvastatin and exemestane may suppress the growth of ER+ breast
cancer, and investigated the effects of combined exemestane and
simvastatin treatment on breast cancer cell function, including
cell survival, cell cycle, cell apoptosis and alterations in
signaling pathways.
Materials and methods
Cell culture
The MCF-7 human breast cancer cell line was kindly
provided by the Laboratory of Molecular Biology of Anhui Medical
University (Anhui, China). The cells were purchased from American
Type Culture Collection (Manassas, VA, USA) and were cultured in
Dulbecco’s modified Eagle’s medium (DMEM; HyClone Laboratories,
Inc., Logan, UT, USA) supplemented with 10% heat-inactivated fetal
bovine serum, 100 U/ml penicillin, 100 μg/ml streptomycin
and 2 mM L-glutamine, which were all purchased from Sijiqing
Biological Engineering Materials (Hangzhou, China), and cells were
maintained at 37°C in a humidified incubator containing 5%
CO2 and 95% air. The cells were harvested with
trypsin-EDTA once they had reached the exponential growth
phase.
Reagents and antibodies
Exemestane was provided by Pfizer Inc. (New York,
NY, USA) and was dissolved in dimethyl sulfoxide (DMSO;
Sigma-Aldrich, St. Louis, MO, USA) at 100 mM, in order to produce a
stock solution. Simvastatin was purchased from Sigma-Aldrich and
was dissolved in DMSO to a stock concentration of 100 mM. The drugs
were stored at −20°C and diluted with culture medium prior to use.
Final concentrations of exemestane were 3.125, 6.25, 12.5, 25, 50
and 100 μM, and simvastatin were 1.5625, 3.125, 6.25, 12.5,
25 and 50 μM. The final concentration of DMSO in the DMEM
was kept at <0.1%, and equal amounts of the solvent were added
to the control cells. The following primary antibodies were used:
Phosphorylated (p)-mitogen-activated protein kinase (MAPK),
p-mammalian target of rapamycin (mTOR), mTOR, P70S6 kinase (K),
p-P70S6K (Cell Signaling Technology, Inc., Danvers, MA, USA);
Bcl-2, Bax and β-actin (Santa Cruz Biotechnology, Inc., Dallas, TX,
USA).
Growth inhibition assay
The anti-proliferative effects of exemestane and
simvastatin on the cells were evaluated using an MTT
(Sigma-Aldrich) assay. Exponentially growing cells were seeded in
96-well plates (1×104 cells/well). The cells were
incubated overnight for cell attachment and recovery. Following
treatment with various concentrations of exemestane or simvastatin
for 72 h, 20 μl MTT solution (5 mg/ml) was added to each
well, and the plates were incubated for a further 4 h at 37°C. The
colored formazan product was dissolved in 150 μl DMSO. The
96-well plates were then agitated for 10 min at room temperature in
order to thoroughly dissolve the MTT product. The optical density
(OD) of each well was measured at a wavelength of 490 nm on an
ELISA plate reader (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
The percentage of inhibited cell growth resulting from each drug
was calculated as follows: [(OD490control
cells-OD490tre ated cells)/OD490control
cells]×100%. The half maximal inhibitory concentration
(IC50) of the drugs was determined as the drug
concentration that resulted in 50% cell growth inhibition, as
compared with the growth of the control cells, following 72 h
exposure to the drugs. Six replicate wells were used for each drug
concentration. Experiments were repeated at least three times and
performed in triplicate.
Measurement of synergy
The anti-proliferative effects of the interaction
between exemestane and simvastatin were assessed by measuring the
combination-index (CI), a quantitative representation of the
pharmacological interaction between two drugs. The combined effect
of exemestane and simvastatin was assessed using the median effect
analysis method, as previously described by Chou and Talalay
(26). The two drugs were combined
in a fixed ratio of doses, which typically corresponded to 0.125,
0.25, 0.5, 1, 2, and 4 times that of the individual IC50
values. The CI values of interactions between exemestane and
simvastatin were assessed using CompuSyn 1.01 software (ComboSyn,
Inc., Paramus, NJ, USA): CI<1, CI=1, and CI>1 were considered
to indicate synergistic, additive and antagonistic effects,
respectively (26).
Cell cycle analysis by flow
cytometry
Equal numbers of MCF-7 cells (1×106/well)
were seeded in six-well dishes and were incubated for 24 h prior to
treatment with exemestane, simvastatin or a combination of the two
drugs for 72 h. The adhered cells were harvested by trypsinization
(Sijiqing Biological Engineering Materials), washed twice with
phosphate-buffered saline (PBS; Sigma-Aldrich) and fixed overnight
in 70% ethanol (Sijiqing Biological Engineering Materials) at 4°C.
The ethanol was removed and the cells were washed a further two
times with PBS, prior to resuspension in 1 ml propidium
iodide/Triton X-100 staining solution [PBS containing 0.1% Triton
X-100 (Sigma-Aldrich), 200 μg/ml RNAse A (Sigma-Aldrich) and
50 μg/ml propidium iodide (Sigma-Aldrich)] in the dark for
30 min. The cell cycle distribution was measured by flow cytometry
using a FACScan system equipped with Cell Quest software (BD
Biosciences, San Jose, CA, USA). The percentage of cells in the
G0/G1, S, and G2/M phases was
calculated using ModFit LT™ 4.0 software (Verity Software House,
Topsham, ME, USA) in order to determine the cell cycle
distribution.
Annexin V assay for the assessment of
apoptosis
The cells in the exponential growth phase were
plated (1×106 cells/well) in six-well plates, allowed to
attach overnight and treated with IC50 values of
exemestane and simvastatin, either alone or in combination, for 72
h. Following 72 h of treatment, the adherent and floating cells
were collected, washed twice with precooled (4°C) PBS and
resuspended in 400 μl binding buffer. The cells were then
incubated with 5 μl Annexin V-fluorescein isothiocyanate
(BestBio, Shanghai, China) at room temperature in the dark for 15
min, followed by an incubation with 10 μl prop-idium iodide
(40 μg/ml; Sigma-Aldrich) at room temperature in the dark
for 5 min. Following incubation, the stained cells were analyzed
using a FACScan system equipped with Cell Quest software. Untreated
cells were used as controls.
Western blot analysis
The MCF-7 cells treated with or without the drugs
were washed with ice-cold PBS and scraped into lysis buffer
(HyClone Laboratories, Inc.). The lysates were centrifuged at
16,853 × g for 30 min at 4°C, and the supernatants were collected.
Briefly, the protein concentration of each sample was determined
using a Bicinchoninic Acid Protein Assay kit (Beyotime Institute of
Biotechnology, Shanghai, China). Equal amounts of protein (5
μl; 0.62 mg/ml) from each sample were separated by 10%
SDS-PAGE (HyClone Laboratories, Inc.) and were transblotted to
polyvinylidene difluoride (PVDF) membranes (Millipore, Billerica,
MA, USA). The membranes were blocked with a solution of PBS
containing 5% milk and 0.1% Tween 20 (HyClone Laboratories, Inc.)
for 2 h. The PVDF membranes were then probed with the following
specific primary antibodies: p-MAPK, MAPK, p-mTOR (9208P; rabbit
monoclonal), mTOR (2983P; rabbit monoclonal), p70S6K (2708p; rabbit
monoclonal) and p-p70S6K (9234P; rabbit monoclonal), which were
used at a dilution of 1:1,000 and were purchased from Cell
Signaling Technologies, Inc. (Danvers, MA, USA), as well as Bcl-2
(ab32124; rabbit monoclonal), Bax (ab32503; rabbit monoclonal) and
β-actin (ab133626; mouse monoclonal) were used at a dilution of
1:500 in Tris-buffered saline with 0.1% Tween 20 (TBST) and were
purchased from Abcam, (Cambridge, MA, USA) at 4°C overnight.
Following rinsing with TBST three times, the PVDF membranes were
incu bated with a horseradish peroxidase-conjugated secondary
antibody (1:5,000) at room temperature for 1 h. Positive bands were
detected using Enhanced Chemiluminescence reagents (EMD Millipore,
Billerica, MA, USA). β-actin was used as a loading control.
Statistical analysis
Values are expressed as the mean ± standard
deviation, obtained from at least three independent experiments.
Student’s t-test and one-way analysis of variance were used to
determine the significant differences between the control and
treatment groups. Data processing was performed using the SPSS
version 16.0 software package (SPSS Inc., Chicago, IL, USA).
P<0.05 was considered to indicate a statistically signifi-cant
difference.
Results
Exemestane or simvastatin alone inhibit
the growth of MCF-7 cells
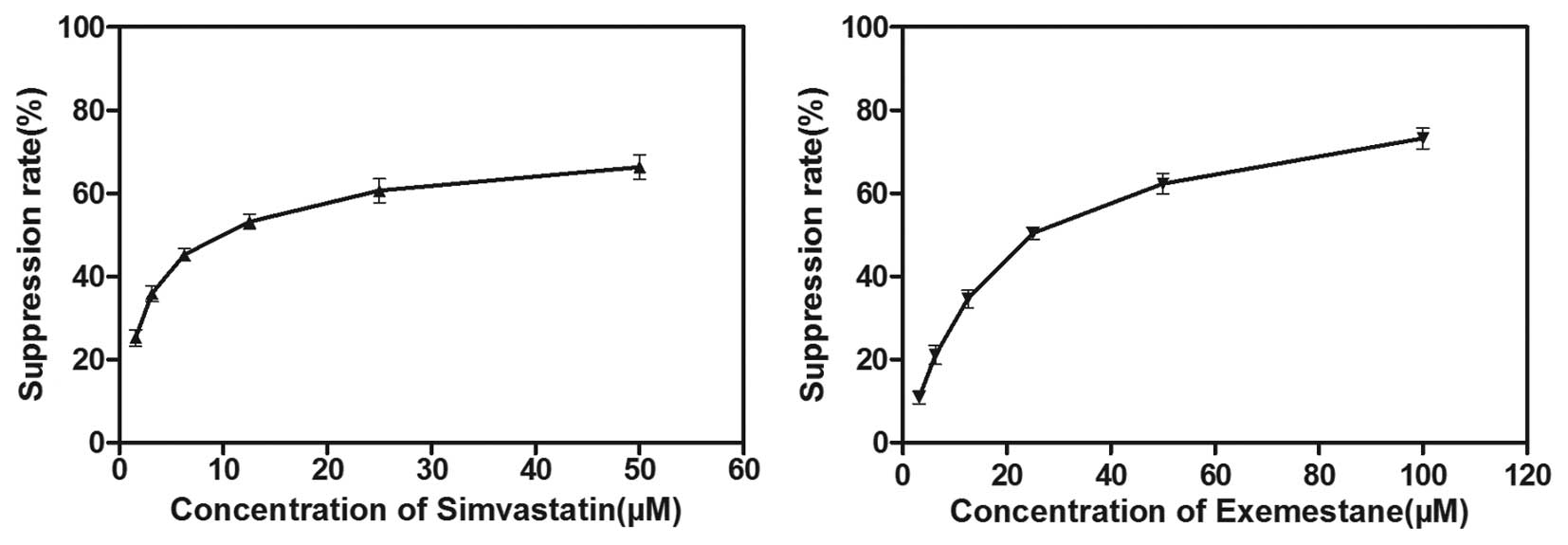
The anti-proliferative effects of exemestane and
simvastatin as single agents on MCF-7 cells were determined using
an MTT assay. The MCF-7 cells were treated with various
concentrations of exemestane (3.125–100 μM) or simvastatin
(1.5625–50 μM) for 72 h. A dose-dependent decrease in cell
viability was observed following treatment with either exemestane
or simvastatin. The IC50 values were 28.02±2.806
μM and 10.93±1.615 μM for exemestane and simvastatin
respectively, following 72 h exposure (Fig. 1). Therefore, 28 μM
exemestane and 11 μM simvastatin were used for all of the
subsequent experiments.
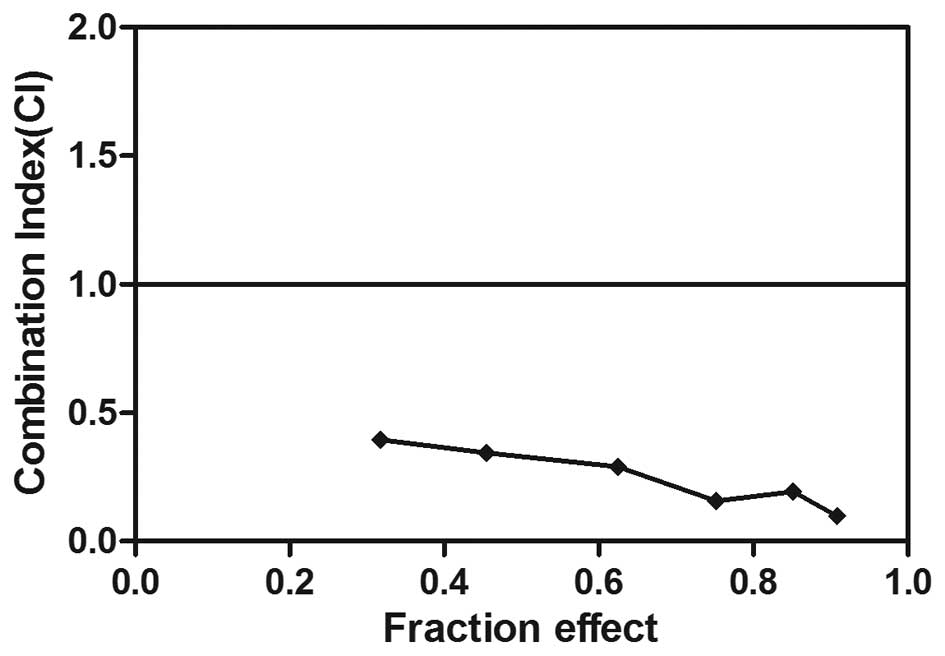
Synergistic interaction between
exemestane and simvastatin in MCF-7 cells
To investigate the effects of exemestane combined
with simvastatin on MCF-7 cells, the cells were exposed to various
concentrations of exemestane and simvastatin for 72 h. Combined
treatment with the two agents induced increased levels of cell
death, as compared with treatment with either exemestane or
simvastatin alone. In the cells treated with exemestane and
simvastatin concurrently, the CI values were all <1, with mean
CI values of 0.25. These results indicated a synergistic
interaction between exemestane and simvastatin on the growth
inhibition of MCF-7 cells (Fig.
2). In support of this result, photomicrographs demonstrated
that treatment with exemestane or simvastatin alone had only a
minor effect on the number of MCF-7 cells and their morphology,
whereas combined treatment resulted in a marked reduction of cell
proliferation after 72 h of treatment.
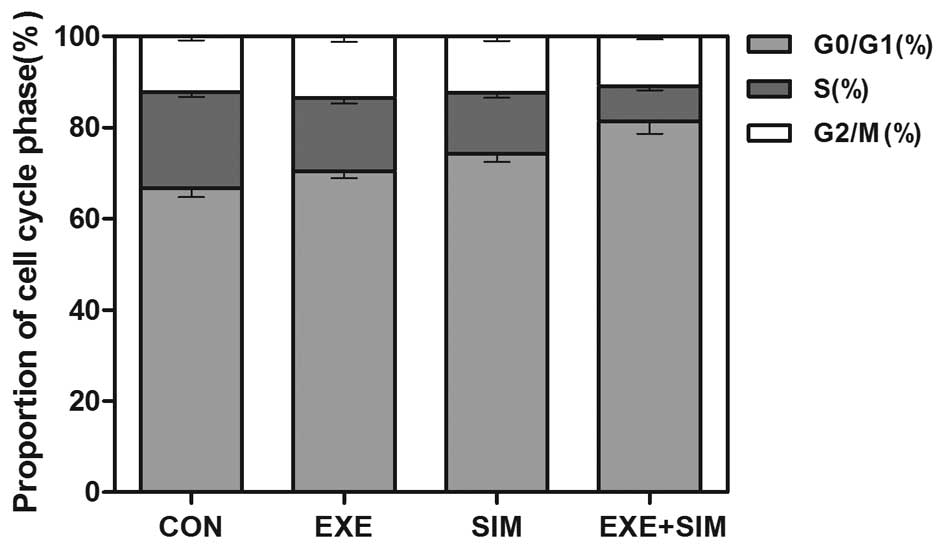
Detection of cell cycle distribution
using flow cytometry
To elucidate the mechanisms by which exemestane and
simvastatin inhibit the proliferation of MCF-7 cells, the cell
cycle distribution was analyzed by flow cytometry. Treatment with
either exemestane or simvastatin increased the population of cells
in G0/G1 phase, with a concomitant decrease
of cells in S phase (P<0.05) (Fig.
3). In addition, combined treatment with exemestane and
simvastatin further increased the percentage of MCF-7 cells in
G0/G1 phase, as compared with the cells
treated with either exemestane or simvastatin alone (P<0.01).
These results indicated that these two drugs may exert synergistic
growth-inhibitory effects, resulting in a cell cycle arrest in
G0/G1 phase.
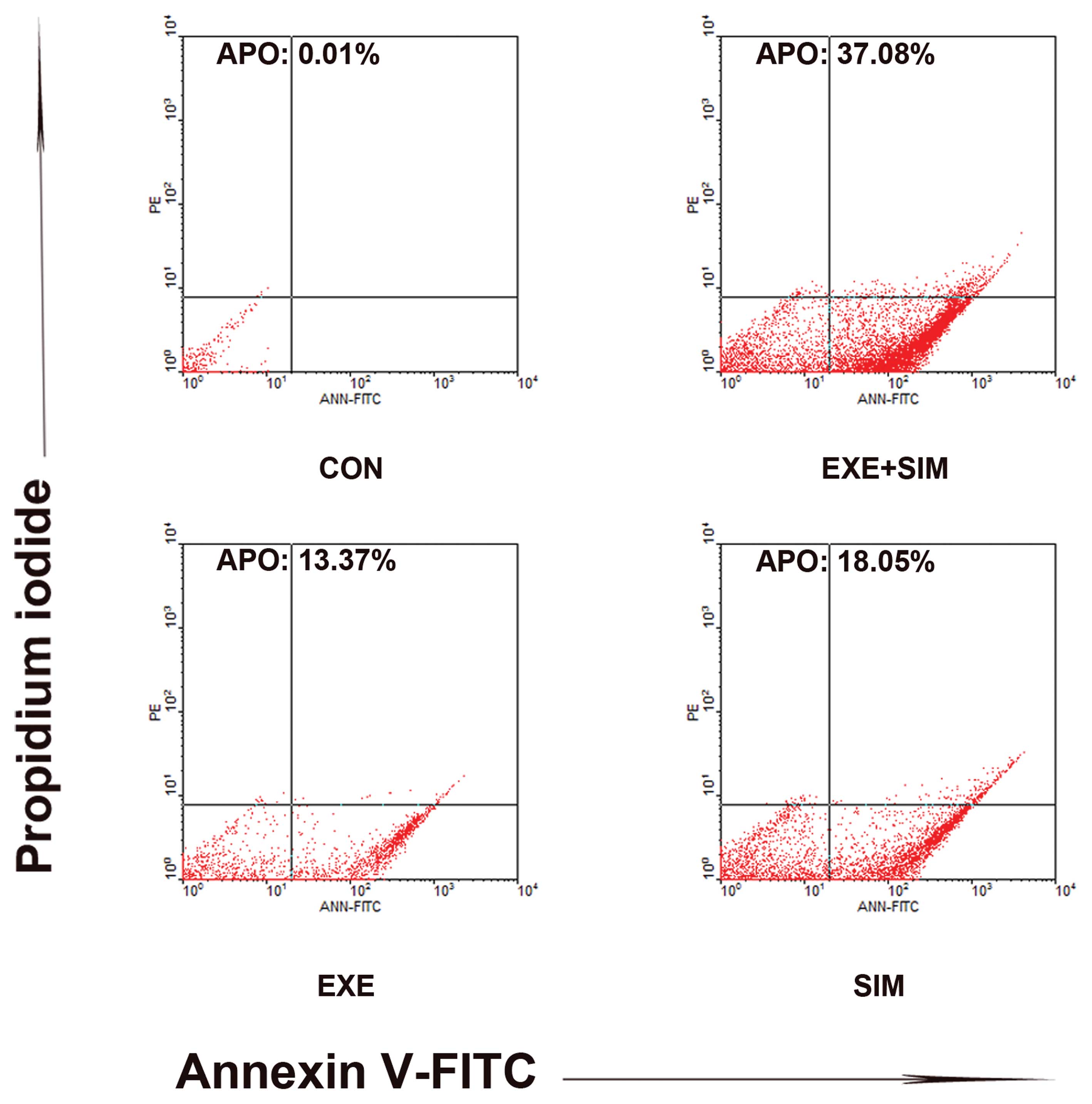
Effects of exemestane or simvastatin,
either alone or in combination, on cell apoptosis
To examine whether the observed suppression of
growth was due to an enhanced rate of apoptosis, the apoptotic
rates of the cells treated with exemestane and simvastatin, either
alone or in combination, were determined using Annexin V-propidium
iodide staining. Annexin V staining is markedly more sensitive for
detecting apoptosis, as compared with the methods based on
hypodiploid DNA content. Treatment with exemestane combined with
simvastatin significantly enhanced apoptosis of the cells, as
compared with the treatment with either drug alone. Individual
treatment with exemestane and simvastatin resulted in 13.37 and
18.05% apoptotic cells, respectively, whereas 37.08% Annexin
V-positive cells were observed following combined treatment with
the two drugs (Fig. 4). These data
indicated that concurrent exposure to exemestane and simvastatin
resulted in synergistic interaction in MCF-7 cells.
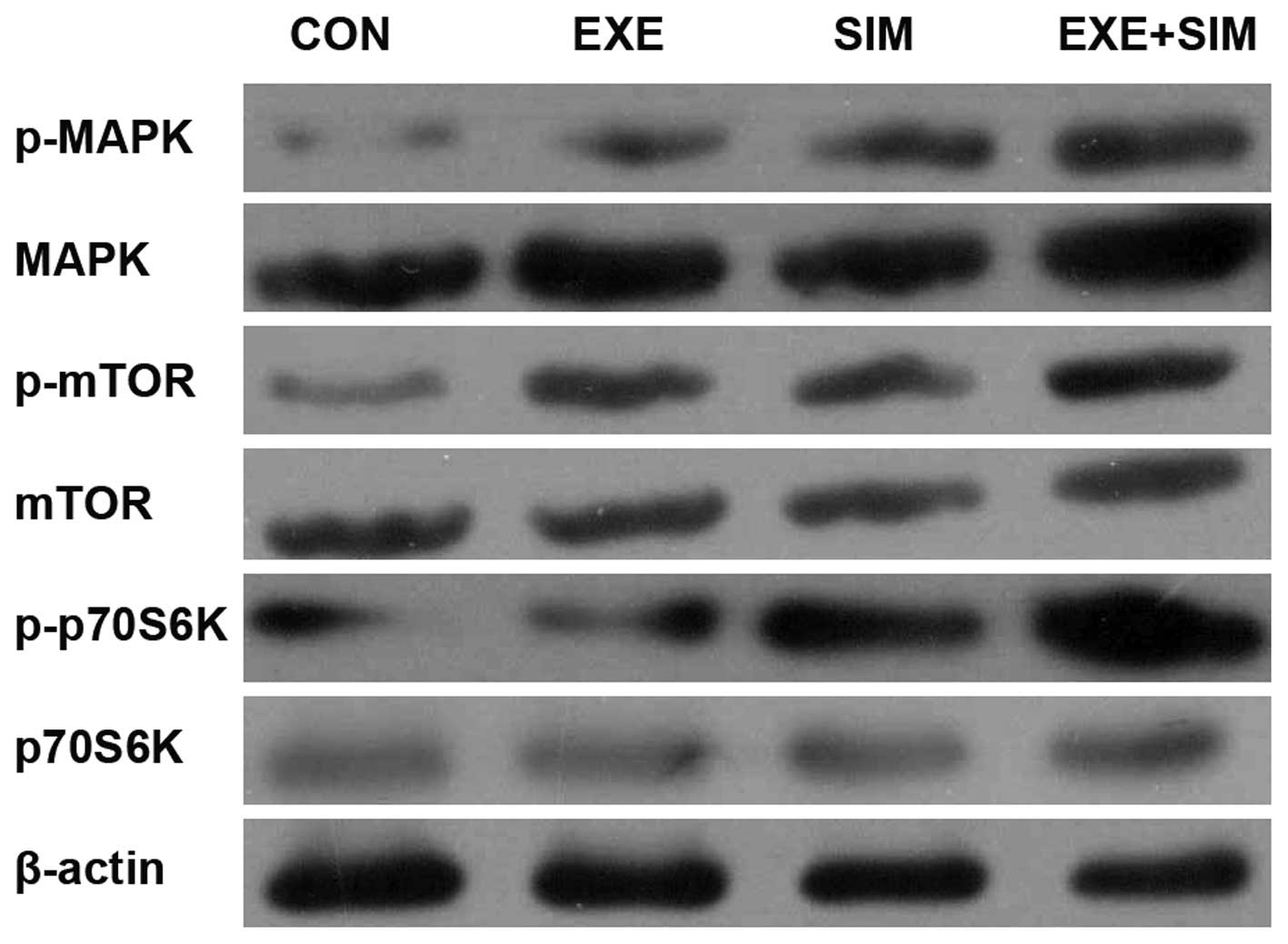
Exemestane and simvastatin alone or in
combination modify the expression levels of mitogen-activated
protein kinase (MAPK) and mTOR/p70S6K signaling-associated proteins
in MCF-7 cells
The main downstream effect of MAPK activation is
inhibition of the mTOR signaling pathways, which have been causally
associated with breast cancer cell proliferation, motility and
invasiveness. Therefore, the present study analyzed the expression
levels of MAPK and mTOR/p70S6 signaling-associated proteins in
MCF-7 cells. The combined treatment of simvastatin with exemestane
resulted in increased expression levels of p-MAPK and reduced
expression levels of p-mTOR and p-p70S6K, whereas the total MAPK,
mTOR and p70S6K expression levels were unchanged in response to
treatment with simvastatin, exemestane or a combination of the two
(Fig. 5).
Effects of simvastatin and exemestane
treatment on the expression levels of Bcl-2 and Bax
To further evaluate the potential synergistic
mechanisms of exemestane and simvas-tatin, the protein expression
levels of Bcl-2 and Bax in MCF-7 cells were detected by western
blotting. Combined treatment of exemestane with simvastatin
resulted in a marked reduction in the protein expression levels of
Bcl-2, and an increase in the expression levels of Bax, as compared
with those in the control cells and those treated with simvastatin
or exemestane alone (Fig. 6).
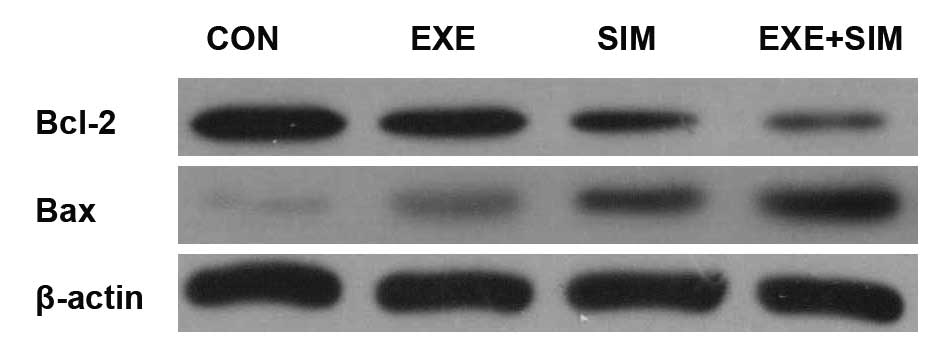 | Figure 6Effects of exemestane combined with
simvastatin on the protein expression levels of Bcl-2 and Bax in
MCF-7 human breast cancer cells. The Bcl-2 and Bax family have an
important role in the regulation of apoptosis, proliferation and
the invasion of tumor cells. MCF-7 cells were treated with
exemestane, simvastatin or a combination of both for 72 h, using
their IC50 concentrations. After treatment, the cells
were harvested and lysed, and equal amounts of extracted protein
were analyzed for Bcl-2 and Bax expression levels by western
blotting. β-actin was used as a loading control. CON, control; EXE,
exemestane; SIM, simvastatin; EXE+SIM, concurrent administration;
Bcl-2, B-cell lymphoma 2; Bax, Bcl-2-associated X protein. |
Discussion
Breast cancer is a hormone-dependent disease that
relies on the mitogenic effects of estrogen to drive
carcinogenesis. AIs are currently used as the standard first-line
treatment to significantly reduce the risk of recurrence for
postmenopausal females with ER+ metastatic breast cancer, as they
have been proven to be more effective than tamoxifen (5). An open-label, randomized, phase III
study conducted by the European Organisation for the Research and
Treatment of Cancer reported a significant improvement in median
progression-free survival and overall response rate for exemestane
treatment, as compared with tamoxifen (27). However, despite the proven clinical
efficacy of AIs in the treatment of breast cancer, de novo and
acquired drug resistance often occurs and presents a major obstacle
to successful therapy. In addition, patients with breast cancer
treated with AIs have a higher incidence of AI-associated
musculoskeletal symptoms. An International Standard Randomised
Controlled Trial reported that females receiving exemestane
experienced significantly higher rates of arthralgia (28). A previous study demonstrated that
Aromasin® (exemestane) combined with simvastatin was
able to significantly increase bone mineral density, thus
suggesting that simvastatin may have potential therapeutic
application in the treatment of osteoporosis, to counterbalance the
adverse effects of exemestane (29). Furthermore, numerous preclinical
studies have shown that statins possess anti-proliferative,
anti-angiogenic, anti-metastatic and pro-apoptotic properties in
various types of cancer cell (17,18).
In addition, some epidemiological studies have also demonstrated
that statins are associated with a lower incidence of invasive
breast cancer, these findings suggest that statins may contribute
to the primary prevention of breast cancer (19,22).
However, to the best of our knowledge, the effects of statins on
endocrine therapies for patients with ER+ breast cancer have
remained to be elucidated. Therefore, the present study
investigated the efficacy of simvastatin, either alone or in
combination with exemestane, on the MCF-7 ER+ breast cancer cell
line. The results of the present study demonstrated that
simvastatin enhanced the inhibitory effects of exemestane on the
growth of ER+ breast cancer cells. When simvastatin was combined
with exemestane, the concentration of exemestane required to
inhibit cancer cell growth was significantly reduced. These results
indicated the potential importance of combined treatment approaches
for increasing the efficacy of exemestane, and lessening its
associated side-effects.
Simvastatin is a widely used cholesterol-adjusting
drug, which selectively inhibits HMG-CoA reductase, leading to
decreased cholesterol biosynthesis. Population-based studies have
demonstrated that treatment with simvastatin is associated with a
substantial reduction in the risk of breast cancer (19,22).
Furthermore, the ex vivo tumor cell inhibition of
simvastatin and its additive effects upon combination with
cisplatin or docetaxel, provide the basis for epidemiological and
clinical studies on statins, potentially directed toward
co-medication in future treatment regimens (30). Cholesterol is a steroid hormone
precursor, and the majority of cases of breast cancer are
considered hormone responsive (31). Previous studies in genetic- or
diet-induced hypercholesterolemic murine models have demonstrated
an obvious association between high lipid levels and breast cancer
progression (32). Furthermore, a
primary metabolite of cholesterol, oxysterol
27-hydroxycho-lesterol, has been shown to promote the growth of ER+
breast cancer in in vivo models (33). Therefore, the reversal of these
processes by the oral lipid-lowering drug simvastatin is an
attractive anti-cancer strategy.
The results of the present study indicated that
simvastatin and exemestane inhibited the proliferation of MCF-7
cells in a concentration-dependent manner. Combined treatment of
simvastatin with exemestane for 72 h resulted in a marked increase
in the inhibition of cell growth, as compared with treatment with
exemestane or simvastatin alone. The present study also
demonstrated an enhanced effect on cell cycle progression and
apoptosis. MAPK is a key kinase that has an essential role in
energy homeostasis and regulates processes associated with the
development of cancer, including cell proliferation and survival
(34,35), cell cycle arrest (34) and protein synthesis (35). Despite uncontrolled cellular
proliferation in breast cancer, which is theoretically expected to
create a large demand for cellular energy, there is histological
evidence that phosphorylation of MAPK at Thr-172 is down-regulated,
particularly in tumors of high histological grade that are
associated with axillary node metastasis (36). The MAPK signaling pathway not only
promotes cell proliferation, but also induces cell apoptosis and is
known to be upregulated in cancer cells (37). The main downstream effect of MAPK
activation is the inhibition of the mTOR signaling pathways.
Misirkic et al (38)
reported that in simvastatin-treated glioma cells, inhibition of
mTOR, and its substrate S6K1, resulted in the activation of the
mTOR negative regulator MAPK. This result is concordant with the
previously reported ability of statins to activate MAPK in hepatic
and colorectal cancer cells in vitro (39), as well as to inhibit the Akt/mTOR
signaling pathway in renal cancer cells (40). In the present study, combined
treatment of exemestane with simvastatin caused a large decrease in
the relative protein expression levels of p-MAPK and decreased the
expression levels of p-mTOR, as compared with those in the control
or single-agent treatment groups. p70S6K is one of the main
downstream effectors of the mTOR signaling pathway, and its
activated form p-p70S6K was also shown to be inhibited in the
present study. To the best of our knowledge, the present study was
the first to examine the individual and combined effects of
simvastatin and exemestane on MCF-7 ER+ breast cancer cells, and to
investigate the underlying apoptotic and growth pathways
involved.
The results of the present study also demonstrated
that simvastatin and exemestane increased the expression levels of
Bax and decreased the expression levels of Bcl-2. The progression
of cancer depends on the balance between pro-apoptotic proteins,
such as Bax and anti-apoptotic proteins, such as Bcl-2 (15,41).
Bcl-2 and Bax are key apoptosis regulators in numerous types of
cells, which in response to treatment may lead to the activation of
apoptosis (15). The results of
the present study indicated that regulation of Bax and Bcl-2
protein expression may be involved in combination treatment-induced
cell death.
In conclusion, the results of the present study
indicated that simvastatin combined with exemestane may have
synergistic effects on cell proliferation and induce cell cycle
arrest and apoptosis of MCF-7 human breast cancer cells in
vitro. The present study further confirmed that the synergistic
effects of these two agents may involve the Bax/Bcl-2 apoptotic
pathway and the MAPK/mTOR/p70S6K growth pathway. The anti-tumor
effects of simvastatin are complex and remain to be fully
elucidated; however, these findings provided direct evidence of its
efficacy on ER+ breast cancer cells when used in combination with
exemestane. Futhermore, the results of the present study suggested
that the combination of simvastatin and exemestane may be a
potential therapeutic strategy used to treat breast cancer;
however, the synergistic effects of these two drugs require a
large-scale clinical trial for further validation.
Acknowledgments
The present study was supported by grants from the
Anhui Provincial Science and Technology Agency Foundation of China
(grant nos. 1301042214, 12070403072 and KJ2012A157). The authors
would also like to thank Mrs. YuanYuan, The Central Laboratory of
Binhu Hospital, The Third Affiliated Hospital of Anhui Medical
University (Anhui, China), for experimental instruction. The
authors declare that they have no conflict of interest.
References
|
1
|
Ferlay J, Parkin DM and Steliarova-Foucher
E: Estimates of cancer incidence and mortality in Europe in 2008.
Eur J Cancer. 46:765–781. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen S: An “omics” approach to determine
the mechanisms of acquired aromatase inhibitor resistance. OMICS.
15:347–352. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jankowitz RC and Davidson NE: Adjuvant
endocrine therapy for breast cancer: how long is long enough?
Oncology (Williston Park). 27:1210–1216. 2013.
|
|
4
|
Campagnoli C, Pasanisi P, Castellano I,
Abba C, Brucato T and Berrino F: Postmenopausal breast cancer,
androgens, and aromatase inhibitors. Breast Cancer Res Treat.
139:1–11. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Riemsma R, Forbes CA, Kessels A, et al:
Systematic review of aromatase inhibitors in the first-line
treatment for hormone sensitive advanced or metastatic breast
cancer. Breast Cancer Res Treat. 123:9–24. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li Z, Gu X, Tong J, et al: A meta-analysis
of internal mammary lymph node metastasis in breast cancer
patients. Onkologie. 36:747–752. 2013.PubMed/NCBI
|
|
7
|
Morris PG, McArthur HL and Hudis CA:
Therapeutic options for metastatic breast cancer. Expert Opin
Pharmacother. 10:967–981. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rizzoli R, Body JJ, De Censi A, Reginster
JY, Piscitelli P and Brandi ML; European Society for Clinical and
Economical aspects of Osteoporosis and Osteoarthritis (ESCEO):
Guidance for the prevention of bone loss and fractures in
postmenopausal women treated with aromatase inhibitors for breast
cancer: an ESCEO position paper. Osteoporos Int. 23:2567–2576.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Brodie A and Sabnis G: Adaptive changes
result in activation of alternate signaling pathways and
acquisition of resistance to aromatase inhibitors. Clin Cancer Res.
17:4208–4213. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wong WW, Dimitroulakos J, Minden MD and
Penn LZ: HMG-CoA reductase inhibitors and the malignant cell: the
statin family of drugs as triggers of tumor-specific apoptosis.
Leukemia. 16:508–519. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Superko HR, Momary KM and Li Y: Statins
personalized. Med Clin North Am. 96:123–139. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Goldstein JL and Brown MS: Regulation of
the mevalonate pathway. Nature. 343:425–430. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liao JK: Isoprenoids as mediators of the
biological effects of statins. J Clin Invest. 110:285–288. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wejde J, Blegen H and Larsson O:
Requirement for mevalonate in the control of proliferation of human
breast cancer cells. Anticancer Res. 12:317–324. 1992.PubMed/NCBI
|
|
15
|
Spampanato C, De Maria S, Sarnataro M, et
al: Simvastatin inhibits cancer cell growth by inducing apoptosis
correlated to activation of Bax and down-regulation of BCL-2 gene
expression. Int J Oncol. 40:935–941. 2012.
|
|
16
|
Kochuparambil ST, Al-Husein B, Goc A,
Soliman S and Somanath PR: Anticancer efficacy of simvastatin on
prostate cancer cells and tumor xenografts is associated with
inhibition of Akt and reduced prostate-specific antigen expression.
J Pharmacol Exp Ther. 336:496–505. 2011. View Article : Google Scholar
|
|
17
|
Campbell MJ, Esserman LJ, Zhou Y, et al:
Breast cancer growth prevention by statins. Cancer Res.
66:8707–8714. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cho SJ, Kim JS, Kim JM, Lee JY, Jung HC
and Song IS: Simvastatin induces apoptosis in human colon cancer
cells and in tumor xenografts, and attenuates colitis-associated
colon cancer in mice. Int J Cancer. 123:951–957. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nielsen SF, Nordestgaard BG and Bojesen
SE: Statin use and reduced cancer-related mortality. N Engl J Med.
367:1792–1802. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Guterres FA, Martinez GR, Rocha ME and
Winnischofer SM: Simvastatin rises reactive oxygen species levels
and induces senescence in human melanoma cells by activation of
p53/p21 pathway. Exp Cell Res. 319:2977–2988. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cheng WH, Ho WY, Chang CF, et al:
Simvastatin induces a central hypotensive effect via Ras-mediated
signalling to cause eNOS up-regulation. Br J Pharmacol.
170:847–858. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ahern TP, Pedersen L, Tarp M, et al:
Statin prescriptions and breast cancer recurrence risk: a Danish
nationwide prospective cohort study. J Natl Cancer Inst.
103:1461–1468. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hu LX, Du YY, Zhang Y and Pan YY:
Synergistic effects of exemestane and aspirin on MCF-7 human breast
cancer cells. Asian Pac J Cancer Prev. 13:5903–5908. 2012.
View Article : Google Scholar
|
|
24
|
Budman DR, Calabro A, Wang LG, et al:
Synergism of cytotoxic effects of vinorelbine and paclitaxel in
vitro. Cancer Invest. 18:695–701. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Budman DR and Calabro A: In vitro search
for synergy and antagonism: evaluation of docetaxel combinations in
breast cancer cell lines. Breast Cancer Res Treat. 74:41–46. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chou TC and Talalay P: Quantitative
analysis of dose-effect relationships: the combined effects of
multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 22:27–55.
1984. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Paridaens RJ, Dirix LY, Beex LV, et al:
Phase III study comparing exemestane with tamoxifen as first-line
hormonal treatment of metastatic breast cancer in postmenopausal
women: the European Organisation for Research and Treatment of
Cancer Breast Cancer Cooperative Group. J Clin Oncol. 26:4883–4890.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sestak I, Cuzick J, Sapunar F, et al ATAC
Trialists’ Group: Risk factors for joint symptoms in patients
enrolled in the ATAC trial: a retrospective, exploratory analysis.
Lancet Oncol. 9:866–872. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen SH, Chou FF and Ko JY: The use of
simvastatin with aromasin in an ovariectomized rat model: effects
on the skeletal system. Chang Gung Med J. 33:509–514.
2010.PubMed/NCBI
|
|
30
|
Stoehr M, Mozet C, Boehm A, Aigner A,
Dietz A and Wichmann G: Simvastatin suppresses head and neck
squamous cell carcinoma ex vivo and enhances the cytostatic effects
of chemotherapeutics. Cancer Chemother Pharmacol. 73:827–837. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Danilo C and Frank PG: Cholesterol and
breast cancer development. Curr Opin Pharmacol. 12:677–682. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Llaverias G, Danilo C, Mercier I, et al:
Role of cholesterol in the development and progression of breast
cancer. Am J Pathol. 178:402–412. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Nelson ER, Wardell SE, Jasper JS, et al:
27-Hydroxycholesterol links hypercholesterolemia and breast cancer
pathophysiology. Science. 342:1094–1098. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Imamura K, Ogura T, Kishimoto A, Kaminishi
M and Esumi H: Cell cycle regulation via p53 phosphory-lation by a
5′-AMP activated protein kinase activator,
5-aminoimidazole-4-carboxamide-1-beta-D-ribofuranoside, in a human
hepatocellular carcinoma cell line. Biochem Biophys Res Commun.
287:562–567. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Jones RG, Plas DR, Kubek S, et al:
AMP-activated protein kinase induces a p53-dependent metabolic
checkpoint. Mol Cell. 18:283–293. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hadad SM, Baker L, Quinlan PR, et al:
Histological evaluation of AMPK signalling in primary breast
cancer. BMC Cancer. 9:3072009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Bühler S and Laufer SA: p38 MAPK
inhibitors: a patent review (2012 – 2013). Expert Opin Ther Pat.
24:535–554. 2014. View Article : Google Scholar
|
|
38
|
Misirkic M, Janjetovic K, Vucicevic L, et
al: Inhibition of AMPK-dependent autophagy enhances in vitro
antiglioma effect of simvastatin. Pharmacol Res. 65:111–119. 2012.
View Article : Google Scholar
|
|
39
|
Yang PM, Liu YL, Lin YC, Shun CT, Wu MS
and Chen CC: Inhibition of autophagy enhances anticancer effects of
atorvastatin in digestive malignancies. Cancer Res. 70:7699–7709.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Woodard J, Sassano A, Hay N and Platanias
LC: Statin-dependent suppression of the Akt/mammalian target of
rapamycin signaling cascade and programmed cell death 4
up-regulation in renal cell carcinoma. Clin Cancer Res.
14:4640–4649. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Jaafar H, Abdullah S, Murtey MD and Idris
FM: Expression of Bax and Bcl-2 in tumour cells and blood vessels
of breast cancer and their association with angiogenesis and
hormonal receptors. Asian Pac J Cancer Prev. 13:3857–3862. 2012.
View Article : Google Scholar : PubMed/NCBI
|