Introduction
Colorectal cancer is one of the most commonly
diagnosed types of cancer and is a leading cause of
cancer-associated mortality worldwide, particularly among
populations in Eastern Asia, Eastern Europe and South America
(1,2). Risk factors for colorectal cancer
include infection, cigarette smoking, alcohol consumption, diet and
genetic variations (3). Although
certain progress has been made in the treatment of colorectal
cancer, the early diagnosis and long-term survival rates remain low
(4); in addition, colorectal
cancer was reported to have the second highest mortality rate of
all cancer types (5). Therefore,
it is essential to gain mechanistic insights into the oncogenesis
of colorectal cancer and to identify novel molecular targets for
the development of effective therapeutic agents for this highly
lethal disease.
The human short chain enoyl coenzyme A hydratase 1
(ECHS1) gene, located at chromosome 10q26.2–q26.3, is a 290 amino
acid enzyme which is known to have an essential role in the second
step of the mitochondrial fatty acid oxidation pathway (6). ECHS1 is highly conserved among
different species and is homologous to other hydrolases and
isomerases (7). ECHS1 expression
levels were reported to be downregulated in a non-alcoholic fatty
liver disease model as well as in patients with hepatic simple
steatosis (8). Previous studies
have implicated the involvement of ECHS1 in numerous types of
cancers, including breast, prostate, colon and liver cancer
(9,10). In addition, ECHS1 has been
identified as a novel interacting protein of signal transducer and
activator of transcription 3 (STAT3) (11). ECHS1 binding was found to
specifically inhibit STAT3 activity through repressing STAT3
phosphorylation, thus downregulating the expression of STAT3 target
genes (11). Proteomic analysis
and an in vitro study revealed that the phosphoinositide 3
kinase (PI3K)-Akt and STAT3 signaling pathways were functionally
linked in cancer (12). These
findings therefore suggested the possible function of ECHS1 in
colorectal cancer.
The present study aimed to investigate the roles of
ECHS1 in colorectal cancer development and progression in order to
elucidate a potential molecular biomarker or target for the
diagnosis or treatment of colorectal cancer. The expression levels
of the ECHS1 gene were determined in the HCT-8 cell line and a
stably transfected HCT-8 cell line was established, in which ECHS1
expression was knocked down using RNA interference technology.
These cells were then used to explore the effect of ECHS1
attenuation on cell proliferation, cell migration and the
activation of Akt and glycogen synthase kinase (GSK)3β
proteins.
Materials and methods
Cell lines and culture conditions
The human colorectal cancer cell line HCT-8 was
purchased from the Cell Bank of the Shanghai Institute of
Biochemistry and Cell Biology, Chinese Academy of Sciences
(Shanghai, China). HCT-8 cells were cultured in RPMI 1640 media
(Invitrogen Life Technologies, Carlsbad, CA, USA) supplemented with
10% fetal calf serum (Abcam, Cambridge, UK). ECHS1 small
interfering (si)RNA vector or empty vector (Santa Cruz
Biotechnology, Inc., Dallas, TX, USA) stably transfected cell lines
were grown in Dulbecco’s modified Eagle’s medium (DMEM; Invitrogen
Life Technologies) supplemented with 10% fetal bovine serum [FBS;
Shanghai Pharmaecuetical (Group) Co., Ltd., Shanghai, China] and 1
μg/ml puromycin (Invitrogen Life Technologies). All cells
were cultured in an incubator at 37°C in a 5% CO2
atmosphere and cell passage was performed using trypsinization with
0.02% EDTA/0.25% trypsin (Abcam) (1:1).
Construction and screening of
ECHS1-targeting siRNA vectors
The pU6 siRNA expression vector was provided by the
Anhui Medical Center laboratory (Anhui, China) and was used to
express siRNA targeting the ECHS1 gene (pU6-siECHS1). The pU6
vector expressed the sense- and antisense-strands of the siRNA in
tandem under the control of the U6 promoter.
A series of siRNA expression vectors were produced
by inserting antisense sequence expression cassettes into sense
sequence expression vectors. Sequences inserted immediately
downstream of U6 promoter were as follows: siRNA1 sense,
5′-GCUAUGAAACGAUAUGGGCUU-3′ and antisense,
5′-GCCCAUAUCGUUUCAUAGCUU-3′; siRNA2 sense,
5′-GUUCGUCACAUCUCAUCUACUU-3′ and antisense,
5′-GUAGAUGAGAUGUGACGAAUU-3′.
ECHS1 siRNA stably transfected cell
lines
In order to establish ECHS1 siRNA-expressing stably
transfected cell lines, HCT-8 cells were seeded in 60-mm culture
dishes and cultured with complete media without antibiotics at 37°C
in a 5% CO2 atmosphere. Plasmid transfections were
performed using the Lipofectamine 2000 transfection reagent (Life
Technologies, Shanghai, China) according to manufacturer’s
instructions when the cells reached 90% confluence. Cells were
transfected with the ECHS1 siRNA plasmid or the empty pU6 vector
and cultured for 24 h. At 24 h post transfection, 10 μg/ml
puromycin was added to the complete media without antibiotics in
order to select for the stably transfected cell clones. The
selection media was replaced every 2–4 days as necessary.
Puromycin-resistant colonies started to form at ~10 days post
transfection and single colonies were isolated at ~14 days post
transfection. The selected stably transfected cells were expanded
and the examined using western blot analyses in order to confirm
ECHS1 knockdown.
Western blot analysis
Cultured cells (~80% confluent) were washed three
times with pre-chilled phosphate-buffered saline (PBS; Abcam),
detached by trypsinization with 0.25% trypsin and then centrifuged
at 25,200 g for 30 min at 4°C. Cell pellets were first lysed with
protein lysis buffer with a protease inhibitor cocktail (1:100) and
sonicated on ice (5-sec sonication with intervals of 10 sec, at
100–120 W) until the lysate was clear. The cell lysates were
centrifuged at 25,200 × g at 4°C for 15 min and the supernatants
were stored at −80°C until further use. Protein concentrations were
determined using a bicinchoninic acid assay. The proteins (10
μg) were subjected to 10% SDS-PAGE gel electrophoresis and
transferred onto polyvinylidene difloride membranes (Millipore
Corp., Billerica, MA, USA). The membranes were incubated for 1 h at
room temperature in blocking buffer (Abcam), followed by an
incubation overnight at 4°C with the following antibodies:
Anti-ECHS1 (1:400; Abcam; cat. no. sc-1562) and anti-tubulin
(1:2,000; Santa Cruz Biotechnology, Inc.; cat. no. sc-47778). The
membranes were then washed with tris-buffered saline, Tween-20
(Cell Signaling Technology, Inc., Danvers, MA, USA) and Triton
X-100 (Bio-Rad Laboratories, Inc., Hercules, CA, USA), and
incubated with horseradish peroxidase-conjugated anti-rabbit
antibody (1:8,000; ZSGB-BIO, Beijing, China; cat. no. sc-81656) for
2 h at 37°C. The blots were visualized with enhanced
chemiluminescence solution (Applygen Technologies, Inc., Beijing,
China) and exposed to X-ray films (LAS-1000; Fujifilm, Tokyo,
Japan). The density of the protein bands were assessed using
TotalLab analysis software, version 2.01 (Nonlinear USA, Inc.,
Durham, NC, USA).
Cell proliferation assay
A Cell Counting Kit-8 (CCK-8; Bio-Rad Laboratories,
Inc.) assay was used to determine the rate of cell proliferation in
HCT-8 cells following ECHS1 siRNA transfection. ECHS1 siRNA and
empty pU6 vector stably transfected cells as well as the parental
HCT-8 cells growing in logarithmic phase were seeded into 96-well
plates at a density of 5,000 cells per well (in 200 μl
culture media) in quadruplicate. The CCK-8 reagent (10 μl)
was added to each well and cells were then incubated at 37°C for 90
min. Absorbance values were measured at 450 nm using a micro-plate
reader (DNM-9606; Nanjing Perlove Medical Equipment Co., Ltd.,
Nanjing, China). CCK-8 assays were performed following 12, 24, 36
and 48 h of culture post transfection.
Cell migration assay
The Transwell® method was used in order
to determine cell migration of colorectal cancer cells. Cell
suspensions were prepared by trypsinizing the cultured cells,
washing twice with serum-free media (Abcam), triturating the cells
into single cell suspensions and then adjusting the cell
concentration to 1×105 cells/ml. The upper chambers of
the Transwell® (Santa Cruz Biotechnology, Inc.) were
placed onto a 24-well plate containing 500 μl media with 20%
FBS or chemokine (Applygen Technologies, Inc.). Cell suspension
(200 μl) was added to the upper chamber of the
Transwell® assembly and incubated at 37°C in a 5%
CO2 atmosphere for 16 h. The Transwell®
assembly was then washed with PBS twice and fixed with pre-chilled
methanol (Invitrogen Life Technologies) at −20°C for 10 min. The
upper chamber of the Transwell® assembly was washed with
PBS twice. The cells which remained on the top surface of upper
chamber were removed using a wet cotton swab. The upper chamber was
then washed with PBS three times and air-dried in an upside-down
position. The chamber membrane was stained with 500 μl/well
0.1% crystal violet staining solution (GE Healthcare Life Sciences,
Little Chalfont, UK) at 37°C for 30 min, washed with PBS three
times and then air-dried. The crystal violet retained on the
Transwell® was eluted with 33% acetic acid
(Sigma-Aldrich, St. Louis, MO, USA) and the optical density
(OD)570nm value of the eluent was measured using a
microplate reader in order to indirectly estimate the number of
cells migrated through the Transwell® membrane.
Statistical analysis
GraphPad Prism 5 statistical analysis software was
used for data processing (GraphPad Software, Inc., La Jolla, CA,
USA). Values are presented as the mean ± standard deviation.
Differences between two sets of values were compared using the
Student’s t-test. Analyses for fitness to normal
distribution and homogeneity of variance of the various
experimental groups were performed. P<0.05 was considered to
indicate a statistically significant difference between values.
Results
Establishment of a stably transfected
ECHS1-silenced cell line
In order to study the functions of ECHS1 in
colorectal cancer cells, a stably transfected HCT-8 cell line
expressing siRNA targeting the ECHS1 gene was established using the
pU6-siECHS1 vector, which were named siECHS1 cells. In addition,
HCT-8 cell lines stably transfected with the empty pU6 vector were
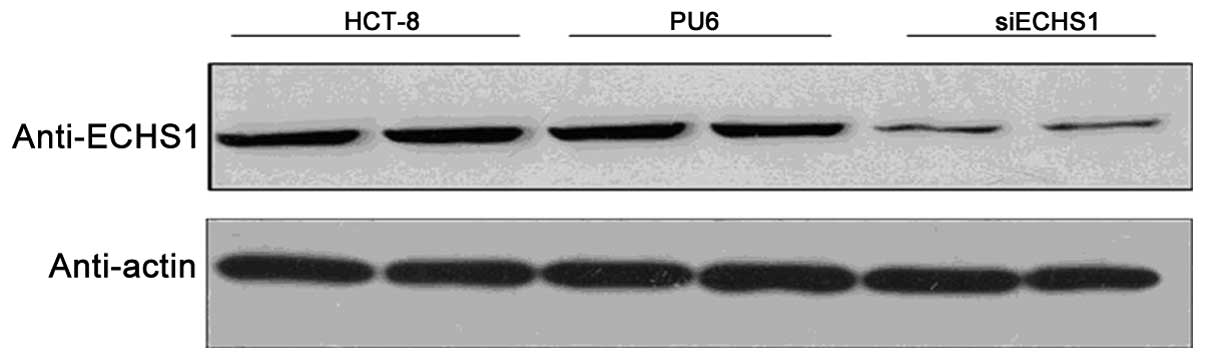
established as controls and were named PU6 cells. As shown in
Fig. 1, compared with that of the
parental HCT-8 cells, there was an >40% decrease in ECHS1
protein expression in the siECHS1 cells, whereas no obvious
decrease in ECHS1 protein levels was observed in the control PU6
cells. This therefore confirmed that stably transfected siECHS1
cells that expressed less ECHS1 protein than the parental cells
were successfully established.
Cell proliferation is inhibited in
siECHS1 cells
Cell proliferation profiles of siECHS1 cells were
compared to those of the PU6 and parental HCT-8 cells using CCK-8
assays. Cells were cultured for 24, 36 and 48 h prior to assaying
with CCK-8 and the number of living cells was proportional to the
OD450nm value. The results showed that the
OD450nm value of siECHS1 cell cultures was significantly
lower compared with those of PU6 and the parental HCT-8 cells at
each time-point (P<0.05) (Fig.
2). These results suggested that silencing of ECHS1 in HCT-8
cells inhibited cell proliferation.
Cell migration is suppressed in siECHS1
cells
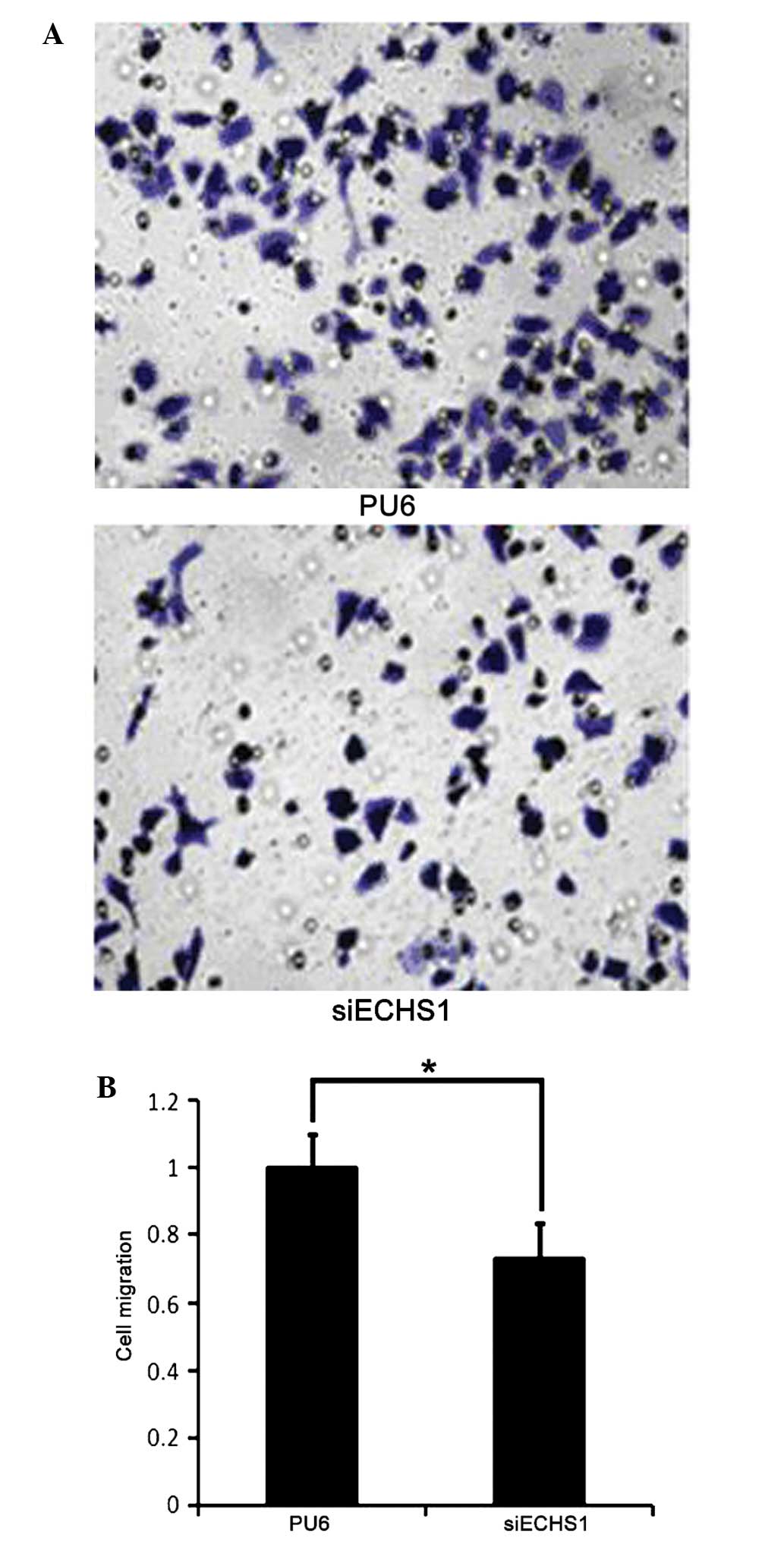
The effect of ECHS1 knockdown on HCT-8 cell
migration was investigated using a Transwell® assay. It
was observed that fewer siECHS1 cells migrated through the
transwell membrane compared with PU6 cells (Fig. 3A). Cell migration was quantified by
measuring the OD570nm value of the eluted crystal violet
stain retained on the Transwell® membrane. The results
showed that there was a significant (~30%) decrease in the number
of migrated cells in the siECHS1 group compared with that of the
PU6 group (P<0.05; Fig. 3B).
These results suggested that the decrease in ECHS1 expression
suppressed cell migration in HCT-8 cells.
ECHS1 knockdown decreases Akt and GSK3β
phosphorylation in HCT-8 cells
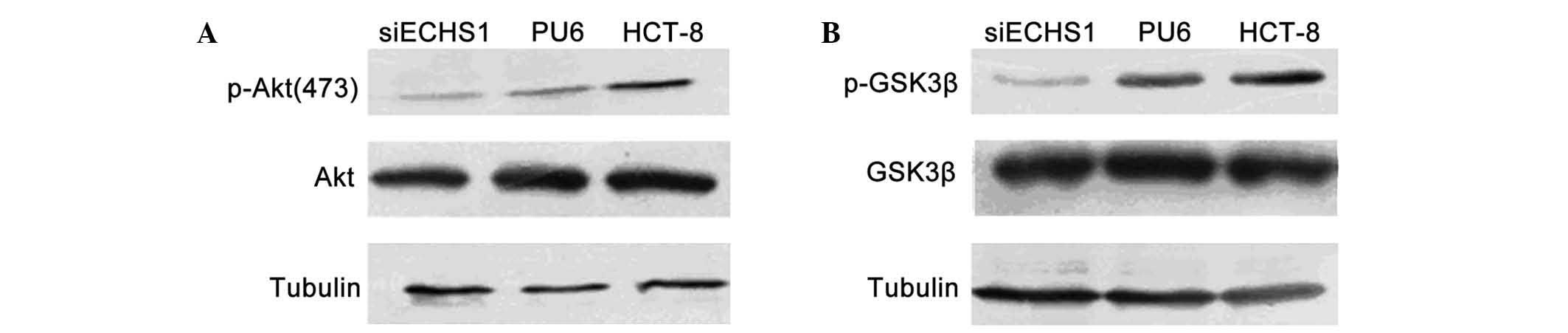
The phosphorylation of Akt and GSK3β proteins in
siECHS1 cells was assessed in order to explore the roles of ECHS1
in the cell cycle regulation of colorectal cancer cells. Compared
with that of the parental HCT-8 and PU6 cells, the level of Akt
phosphorylation at Serine 473 was decreased in siECHS1 cells
(Fig. 4A). In addition, the
phosphorylation level of the Akt downstream factor GSK3β was found
to be decreased in siECHS1 cells (Fig.
4B). These results indicated that the pro-proliferation and
pro-migration functions of ECSH1 may proceed via Akt- and
GSK3β-associated signaling pathways.
Discussion
ECHS1 has been identified by proteomic or gene
expression profiling in numerous types of cancer cells and patient
biopsies; however, there have been a limited number of functional
studies into ECHS1 (13,14). The results of the present study
demonstrated that proliferation and migration were inhibited in
HCT-8 cells with reduced ECHS1 protein levels due to the
constitutive expression of ECHS1-specific siRNA. In addition, ECHS1
knockdown suppressed Akt and GSKβ phosphorylation. These results
indicated that ECHS1 was involved in colorectal cancer cell
proliferation and migration, the mechanism of which may proceed via
PI3K-Akt- and GSKβ-associated signaling pathways. These findings
were consistent with those of previous reports on the role of ECHS1
in breast, prostate and liver cancers (9); in addition, the present study showed
that ECHS1 was highly expressed in colorectal cancer cells.
Furthermore, a previous study demonstrated that short hairpin
RNA-mediated knockdown of ECHS1 protein expression inhibited Akt
activation in hepatocellular carcinoma HepG2 cells (15), which was comparable to the
inhibition of Akt observed following ECHS1 knockdown in colorectal
cancer HCT-8 cells in the present study. Therefore, the activation
of Akt signaling may be a common mechanism for ECHS1-induced cell
proliferation in cancer cells. Future studies are therefore
required in order to investigate the detailed mechanism for
ECHS1-mediated Akt activation. As an essential mitochondrial enzyme
for fatty acid metabolism, ECHS1 expression is important for
mitochondrial integrity and function (16). Since ECHS1 regulates carbohydrate
synthesis and glycolysis, it may have a key role in cancer cell
survival during tumor growth. In the present study, ECHS1 knockdown
was found to suppress GSK3β phosphorylation. Notably, Akt-dependent
phosphorylation of GSK3β was reported to regulate the Wnt signaling
pathway (17); however, whether
ECHS1 acts in a similar manner in colorectal cancers cells requires
further exploration.
Cell migration is an important property of malignant
cancer. Previous studies have established the key roles of the
PI3K-Akt signaling and GSK3β in cell migration regulation (18,19).
In addition, GSK3β was reported to regulate the expression of
miRNAs miR-96, miR-182 and miR-183, which have been shown to affect
the proliferation and migration of colorectal cancer cells
(20). In line with these studies,
the results of the present study revealed that compared with that
of PU6 cells, GSK3β phosphorylation and cell migration was
decreased in siECHS1 cells. These results strongly indicated that
ECHS1 regulated colorectal cancer cell migration through the
GSK3β-associated pathways.
Numerous genes associated with metabolism also have
important regulatory roles in cell proliferation and migration, as
energy supply is a limiting factor for tumor growth and metastasis
(21). Therefore, modulation of
these metabolic genes may be a plausible therapeutic approach for
cancer treatment.
In conclusion, the results of the present study
showed that siRNA-mediated silencing of ECHS1 expression was able
to suppress colorectal cancer cell proliferation and migration.
However, it remains to be elucidated whether the decreased
proliferation and migration of siECHS1 cells was due to the direct
functional defects of ECHS1 or the indirect effects through other
molecules. Further studies are therefore required to decipher the
ECHS1-interacting factors in colorectal cancer cells in order to
gain mechanistic insight into ECHS1 over-expression and
oncogenesis. In addition, ECHS1 knockdown suppressed Akt and GSKβ
phosphorylation, which indicated that the mechanisms of
ECHS1-mediated decreased cell proliferation and migration in
colorectal cancer cells may proceed via PI3K-Akt- and
GSKβ-associated signaling pathways. Furthermore, the present study
suggested that ECHS1 may be a potential therapeutic target for
colorectal cancer treatment and since ECHS1 is highly expressed in
colorectal cancer cells, it may also be a candidate biomarker for
colorectal cancer diagnosis.
References
|
1
|
Orannapalai N, Attawettayanon W, Kanngern
S, Boonpipattanapong T and Sangkhathat S: Predicting the occurrence
of cancer-associated colorectal polyp using a metabolic risk score.
Mol Clin Oncol. 2:124–128. 2014.PubMed/NCBI
|
|
2
|
Zhang GJ, Zhou T, Liu ZL, Tian HP and Xia
SS: Plasma miR-200c and miR-18a as potential biomarkers for the
detection of colorectal carcinoma. Mol Clin Oncol. 1:379–384.
2013.
|
|
3
|
Chen G, Mao B, Pan Q, Liu Q, Xu X and Ning
Y: Prediction rule for estimating advanced colorectal neoplasm risk
in average-risk populations in southern Jiangsu Province. Chin J
Cancer Res. 26:4–11. 2014.PubMed/NCBI
|
|
4
|
Crncec I, Pathria P, Svinka J and Eferl R:
Induction of colorectal cancer in mice and hitomorphometric
evaluation of tumors. Methods Mol Biol. 1267:145–164. 2015.
|
|
5
|
Zhang J, Sun M, Li R, Liu S, Mao J, Huang
Y, Wang B, Hou L, Ibrahim MM and Tang J: Ech1 is a potent
suppressor of lymphatic metastasis in hepatocarcinoma. Biomed
Pharmacother. 67:557–560. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Janssen U, Davis EM, Le Beau MM and
Stoffel W: Human mitochondrial enoyl-CoA hydratase gene (ECHS1):
structural organization and assignment to chromosome 10q26.2–q26.3.
Genomics. 40:470–475. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sakai C, Yamaguchi S, Sasaki M, Miyamoto
Y, Matsushima Y and Goto Y: ECHS1 mutations cause combined
respiratory chain deficiency resulting in Leigh syndrome. Hum
Mutat. 36:232–239. 2015. View Article : Google Scholar
|
|
8
|
Zhang X, Yang J, Guo Y, Ye H, Yu C, Xu C,
Xu L, Wu S, Sun W, Wei H, Gao X, Zhu Y, Qian X, Jiang Y, Li Y and
He F: Functional proteomic analysis of nonalcoholic fatty liver
disease in rat models: enoylcoenzyme a hydratase down-regulation
exacerbates hepatic steatosis. Hepatology. 51:1190–1199. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu X, Feng R and Du L: The role of
enoyl-CoA hydratase short chain 1 and peroxiredoxin 3 in
PP2-induced apoptosis in human breast cancer MCF-7 cells. FEBS
Lett. 584:3185–3192. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gong X, Zhu Y, Dong J, Chen J, You J,
Zheng Q, Rao Z, Mao Q and Jiang J: Small hepatitis B surface
antigen interacts with and modulates enoyl-coenzyme A hydratase
expression in hepatoma cells. Arch Virol. 158:1065–1070. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chang Y, Wang SX, Wang YB, Zhou J, Li WH,
Wang N, Fang DF, Li HY, Li AL, Zhang XM and Zhang WN: ECHS1
interacts with STAT3 and negatively regulates STAT3 signaling. FEBS
Lett. 587:607–613. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yang G, Ma F, Zhong M, Fang L, Peng Y, Xin
X, Zhong J, Zhu W and Zhang Y: Interleukin-11 induces the
expression of matrix metalloproteinase 13 in gastric cancer SCH
cells partly via the PI3K-AKT and JAK-STAT3 pathways. Mol Med Rep.
9:1371–1375. 2014.PubMed/NCBI
|
|
13
|
Von Ohlen T, Luce-Fedrow A, Ortega MT,
Ganta RR and Chapes SK: Identification of critical host
mitochondrion-associated genes during Ehrlichia chaffeensis
infections. Infect Immun. 80:3576–3586. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhu XS, Gao P, Dai YC, Xie JP, Zeng W and
Lian QN: Attenuation of enoyl coenzyme A hydratase short chain 1
expression in gastric cancer cells inhibits cell proliferation and
migration in vitro. Cell Mol Biol Lett. 19:576–589. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhu XS, Dai YC, Chen ZX, Xie JP, Zeng W,
Lin YY and Tan QH: Knockdown of ECHS1 protein expression inhibits
hepatocellular carcinoma cell proliferation via suppression of Akt
activity. Crit Rev Eukaryot Gene Expr. 23:275–282. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xie JP, Zhu XS, Dai YC, Yu C, Xie T and
Chen ZX: Expression of enoyl coenzyme A hydratase, short chain, 1,
in colorectal cancer and its association with clinicopathological
characteristics. Mol Clin Oncol. 2:1081–1084. 2014.PubMed/NCBI
|
|
17
|
Zhang L, Zhang J, Dong Y, Swain CA, Zhang
Y and Xie Z: The potential dual effects of sevoflurane on
AKT/GSK3beta signaling pathway. Med Gas Res. 4:52014. View Article : Google Scholar
|
|
18
|
Chen L, Wei X, Hou Y, Liu X, Li S, Sun B,
Liu X and Liu H: Tetramethylpyrazine analogue CXC195 protects
against cerebral ischemia/reperfusion-induced apoptosis through PI3
K/Akt/GSK3β pathway in rats. Neurochem Int. 66:27–32. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhou RJ, Wang F, Zhang XH, Zhang JJ, Xu J,
Dong W and Zou ZQ: A-eleostearic acid inhibits growth and induces
apoptosis in breast cancer cells via HER2/HER3 signaling. Mol Med
Rep. 9:993–998. 2014.
|
|
20
|
Wang R, Li Z, Guo H, Shi W, Xin Y, Chang W
and Huang T: Caveolin 1 knockdown inhibits the proliferation,
migration and invasion of human breast cancer BT474 cells. Mol Med
Rep. 9:1723–1728. 2014.PubMed/NCBI
|
|
21
|
Yunqiao L, Vanke H, Jun X and Tangmeng G:
MicroRNA-206, down-regulated in hepatocellular carcinoma,
suppresses cell proliferation and promotes apoptosis.
Hepatogastroenterology. 61:1302–1307. 2014.PubMed/NCBI
|