Introduction
Pancreatic ductal adenocarcinoma (PDAC), usually
referred to as pancreatic cancer, is a highly aggressive malignant
tumor. It is the fourth leading cause of cancer-associated
mortality with an estimated 37,390 fatalities in the USA and
227,000 fatalities globally per year (1). Patients with PDAC usually present
with locally advanced, unresectable or metastatic disease and the
majority of patients suffer significant pain (2). Despite the developments in the
detection and management of PDAC, the five-year relative survival
rate has not changed (3). Thus,
further investigation into this malignant tumor is required.
Trophoblast glycoprotein (TPBG), also termed 5T4, is
a 72 kDa transmembrane glycoprotein and an extensively investigated
oncofetal antigen, which is limited in normal adult tissues, but
highly expressed in various types of cancer in humans (4,5).
This expression pattern renders it an attractive target for cancer
therapy (6). Southall et al
(5) observed that TPBG is highly
expressed in tumor tissues, including carcinomas of the bladder,
breast, ovaries, pancreas and stomach, as elucidated using
immunohistochemical analysis, and was closely associated with poor
clinical outcome in colorectal, ovarian and gastric cancer
(7–9). The authors further demonstrated that
TPBG not only disrupts cell-cell contacts and induces cellular
motility in epithelial cells (10), but also interacts with
GAIP-interacting protein C terminus 1, which has implications for
tumor metastasis (11).
However, the roles of TPBG in PDAC progression
remain to be elucidated. The present study aimed to explore the
cellular functions of TPBG in PDAC, and to investigate the
associated mechanisms.
Materials and methods
Immunohistochemical staining
The PDAC tissue microarray (OD-CT-DgPan01-006),
which contains 81 PDAC tissues, 44 normal pancreatic tissues and 32
chronic pancreatitis tissues was purchased from Shanghai Outdo
Biotech Co., Ltd. (Shanghai, China). The immunohistochemical
staining was performed as previously described (12). Briefly, tissue sections were
deparaffinized gradually using 50% xylene (Meryer, Shanghai, China)
and rehydrated. The sections were then incubated with 0.3% hydrogen
peroxide (Jianglaibio, Shanghai, China) for 30 min and blocked with
10% bovine serum albumin (BSA; Sangon, Shanghai, China). The slides
were initially incubated with an antibody targeting TPBG (cat. no.
HPA010554; Sigma-Aldrich, St. Louis, MO, USA) at 4°C overnight with
optimal dilution (1:200). The slides were then incubated with a
horsradish peroxidase-conjugated rabbit secondary antibody (cat.
no. 65-6120; Thermo Fisher Scientific, Waltham, MA, USA) at room
temperature for 1 hour. Subsequently, the slides were incubated
with diaminobenizidine substrate liquid (Gene Tech Ltd., Shanghai,
China), for 2 min and counterstained with hematoxylin (Beyotime
Institue of Biotechnology, Haimen, China). Then these sections were
ehydrated sequentially using 50, 80, 90 and 100% ethanol (Meryer),
and 50 and 100% xylene, and covered. The slides were subsequently
visualized using Primostar FL2 microscope (CarlZeiss, Oberkochen,
Germany).
Cell culture
The PANC-1 and BxPC-3 human PDAC cell lines were
obtained from the Cell Bank of the Chinese Academy of Sciences
(Shanghai, China), and the hTERT-HPNE normal pancreatic duct cell
line was purchased from the American Type Culture Collection (ATCC;
Manassas, VA, USA). PANC-1 cells were cultured with Dulbecco’s
modified Eagle’s medium (Beijing Solarbio Science & Technology
Co., Ltd., Beijing, China), BxPC-3 cells were cultured with RMPI
1640 (Beijing Solarbio Science & Technology Co., Ltd.) and
hTERT-HPNE cells were cultured with specific medium recommended by
the ATCC [75% glucose-free Dulbecco’s modified Eagle’s medium
(Gibco Life Technologies, Carlsbad, CA, USA), 25% Medium M3 Base
(Gibco Life Technologies)] supplemented with 5% fetal bovine serum
(FBS), 10 ng/ml human recombinant epidermal growth factor
(PeproTech, New Jersey, NJ, USA), 5.5 mM D-glucose (Gibco Life
Technologies) and 750 ng/ml puromycin (Sangon, Shanghai, China)].
All cells were supplemented with 10% FBS (v/v; Gibco Life
Technologies, Carlsbad, CA, USA), 100 units penicillin (Gibco Life
Technologies) and 100 μg streptomycin (Gibco Life
Technologies) and incubated at 37°C in a humidified incubator with
5% CO2.
Construction of stably interfered TPBG
cell lines
Short hairpin (sh)RNAs and control vector were
purchased from GenePharma (Shanghai, China). The packaging of
lentiviral particles was performed in HEK293T cells (ATCC,
Manassas, VA, USA)following cotransfection with shRNAs with the
following sequences: sh-1,
5′-CCGGGGATCACATGGAAGGGTATCACTCGAGTGATACCCTTCCA TGTGATCCTTTTTG-3′
and sh-2,
5′-CCGGGCACAGTCAAGTGCGTTAACCCTCGAGGGTTAACGCACTTGACTGTGCTTTTTG-3′.
Control vectors were transfected using Lipofectamine 2000
(Invitrogen Life Technologies, Carlsbad, CA, USA). The viruses were
harvested at 24 and 48 h after transfection. The PANC-1 and BxPC-3
cells (1×105) were seeded into 12-well plates and
infected with lentivirus in the presence of 6 μg/ml
polybrene (Sigma-Aldrich) on the following day. The infected PANC-1
and BxPC-3 cells were then selected with medium containing 2
μg/ml puromycin (Sangon Biotech) for 7 days. The puromycin
resistant cells were considered to be cells that had undergone
stable knockdown of TPBG as verified using reverse
transcription-quantitative polymerase chain reaction (RT-qPCR) and
western blot analysis.
RT-qPCR
Total RNA was extracted using TRIzol reagent (Takara
Bio Inc., Otsu, Japan) and reverse transcribed using the
PrimeScript RT-PCR kit (Takara Bio Inc.) according to the
manufacturer’s instructions. RT-qPCR analysis was performed using
SYBR Premix Ex Taq (Takara Bio Inc.) with the ViiA 7 Real-time PCR
system (Applied Biosystems, Foster City, CA, USA), with the
following program: 95°C for 30 sec, followed by 40 cycles at 95°C
for 5 sec and 60°C for 31 sec, and finally 95°C for 15 sec, 60°C
for 1 min an d 95°C for 15 sec. The primer sequences for TPBG and
18S RNA in the present study were as follows: forward:
5′-TGGGTATTGTTTTAGCCCTGAT-3′ and reverse:
5′-GTTGTCCTTGGTCTGTCCTCTA-3′ for TPBG, and forward:
5′-TGCGAGTACTCAACACCAACA-3′ and reverse: 5′-GCATATCTTCGGCCCACA-3′
for 18S RNA. The relative expression of TPBG was calculated using
the 2−ΔΔCT method with 18S RNA as the reference
gene.
Western blotting
Total proteins were extracted using an
immunoprecipitation lysis buffer (P0013; Beyotime Institute of
Biotechnology, Haimen, China) according to the manufacturer’s
instructions and proteins were then separated using reducing
SDS-PAGE and transferred onto a nitrocellulose membrane (GE
Healthcare Life Sciences, Pittsburgh, PA, USA). Subsequently, the
membrane was blocked in Tris-buffered saline (Beijing Solarbio
Science & Technology Co.) containing 5% BSA for 1 h. The
membrane was incubated with primary antibodies against TPBG (1:500;
cat. no. HPA010554; Sigma-Aldrich), Wnt5a (1:1,000; cat. no. 2392;
Cell Signaling Technology, Danvers, MA, USA), ROR2 (1:1,000; cat.
no. 4105; Cell Signaling Technology) c-Jun N-terminal kinase (JNK;
1:1,000; cat. no. 9252; Cell Signaling Technology), phospho-JNK
(1:1,000; cat. no. 4668; Cell Signaling Technology) and β-actin
(1:5,000; cat. no. 20536-1-AP; Proteintech, Chicago, IL, USA)
overnight and then the membranes were incubated with
species-specific secondary antibodies, IRDye 680 anti-mouse
(1:20,000; cat. no. 926-68072; LI-COR, Lincoln, NE, USA) or IRDye
800 anti-rabbit (1:10,000; cat. no. 926-32213L; LI-COR) to probe
the primary antibodies while signals were detected using the
Odyssey infrared imaging system (LI-COR) as described previously
(13).
In vitro cell migration and invasion
assays
Transwell migration and invasion assays were
performed. For the cell migration assay, 4×104 cells
were seeded into the upper compartment of the Transwell inserts
(Millipore, Billerica, MA, USA) and the lower compartment was
supplemented with 700 μl conditioned medium containing 5%
FBS (v/v). The cells remaining in the top chambers or on the upper
membrane of the inserts were carefully removed after incubation for
10 h. The cells that migrated to the lower membrane of the inserts
were fixed with 2% glutaraldehyde and stained with 0.1% crystal
violet (Beyotime Institute of Biotechnology). Subsequently,
migrated cells were counted by capturing images of six randomly
selected fields through an IX71 inverted microscope (Olympus Corp.,
Tokyo, Japan). A cell invasion assay was performed by adding 100
μl Matrigel (BD Biosciences, Mountain View, CA, USA) into
the top chamber of the Transwell inserts and placing
4×104 primary cells onto the Matrigel. The cell invasion
assay was allowed to continue for 14–20 h and was then terminated
and treated according to the cell migration assay described
above.
Luciferase reporter assay
Cells were seeded into the 96-well plates and
transfected with a mixture of 100 ng ATF2 plasmid (Wnt/PCP
signaling) and 10 ng Renilla plasmid according to the
manufacturer’s instructions of the Lipofectamine 2000 transfection
system. After 48 h of incubation, firefly and Renilla
luciferase activity of cell lysates was measured using the
dual-luciferase reporter assay system (Promega Corporation,
Madison, WI, USA).
Statistical analysis
Data are expressed as the mean ± standard deviation.
The correlation between TPBG expression and the clinicopathological
parameters was evaluated using the χ2 test. Student’s
t-test was used for comparisons between groups. P<0.05 was
considered to indicate a statistically significant difference.
Results
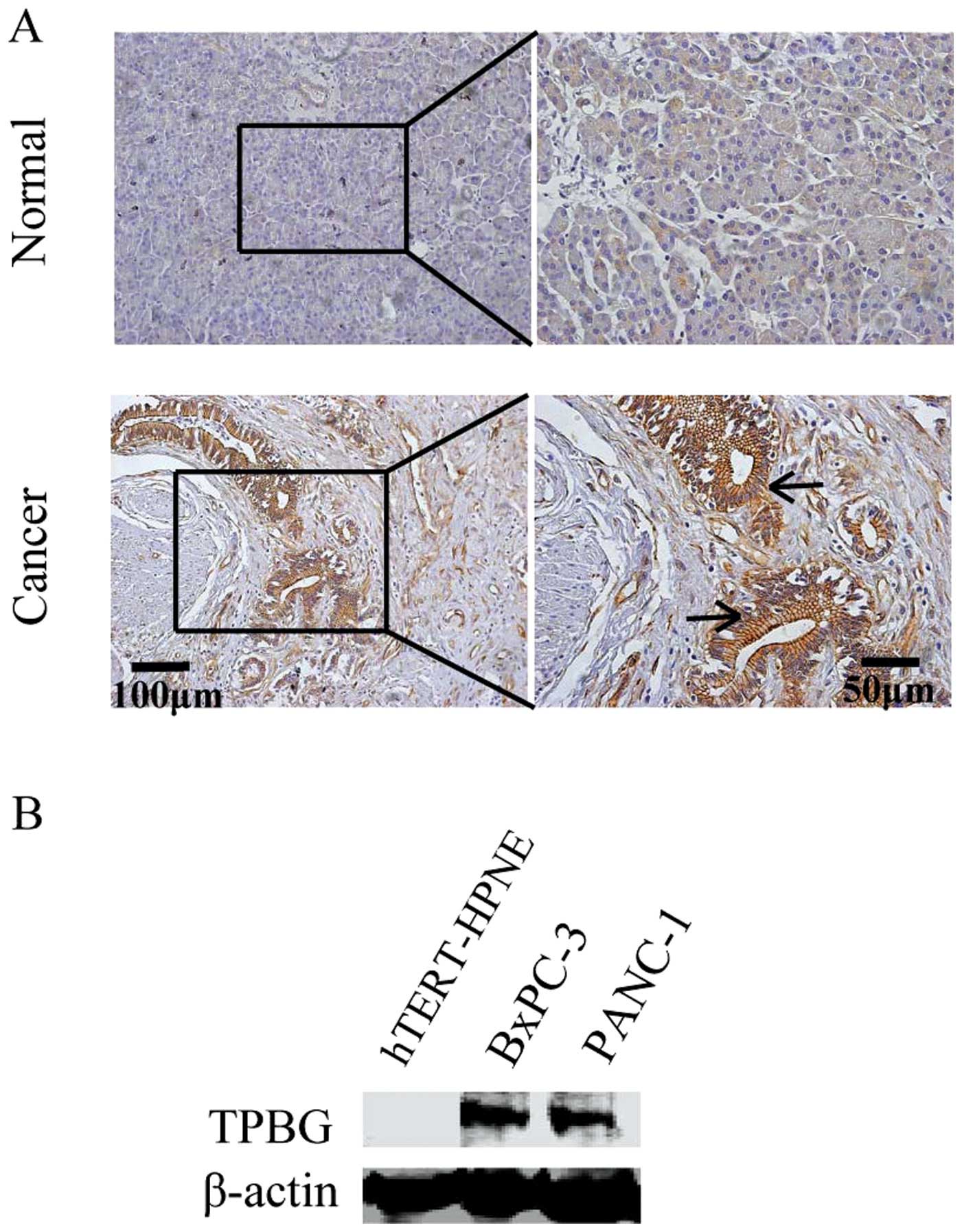
TPBG is highly expressed in PDAC tissues
and cell lines
To observe the change in expression occurring in
PDAC, the expression of TPBG was initially identified in a tissue
microarray, which contained 81 cases of PDAC. Through
immunohistochemical staining, it was revealed that TPBG is highly
expressed in cancer tissues compared with the normal pancreatic
tissues (Fig. 1). In addition, a
high expression of TPBG was closely correlated with
tumor-node-metastasis (TNM) stage and the age of the patient
(Table I). In addition, the
expression of TPBG was detected in the hTERT-HPNE normal pancreatic
duct cell line and the PANC-1 and BxPC-3 PDAC cell lines. The
expression of TPBG was detected at extremely low levels in
hTERT-HPNE, while it was highly expressed in the PANC-1 and BxPC-3
cell lines (Fig. 1B).
 | Table IStatistical analysis of TPBG
expression against clinicopathological parameters of the
patient. |
Table I
Statistical analysis of TPBG
expression against clinicopathological parameters of the
patient.
| Variable | TPBG, n (%)
| P-value |
|---|
| High | Low |
|---|
| Sample |
| Carcinoma | 49 (60.49) | 32 (39.51) | |
| Normal | 10 (22.73) | 34 (77.27) | |
| Pancreatitis | 19 (59.38) | 13 (40.62) | 0.913 |
| Age (years) | | | 0.022 |
| ≤60 | 28 (73.68) | 10 (26.32) | |
| >60 | 21 (48.84) | 22 (51.16) | |
| Gender | | | 0.062 |
| Female | 24 (72.73) | 9 (27.27) | |
| Male | 25 (52.08) | 23 (47.91) | |
| Tumor size (cm) | | | 0.051 |
| ≤4 | 14 (46.67) | 16 (53.33) | |
| >4 | 35 (68.63) | 16 (31.37) | |
| Tumor location | | | 0.45 |
| Pancreas head | 36 (84.00) | 21 (16.00) | |
| Pancreas body and
tail | 13 (54.17) | 11 (45.83) | |
| TNM stage | | | 0.005 |
| I | 18 (45.00) | 22 (55.00) | |
| II–III | 31 (75.61) | 10 (24.39) | |
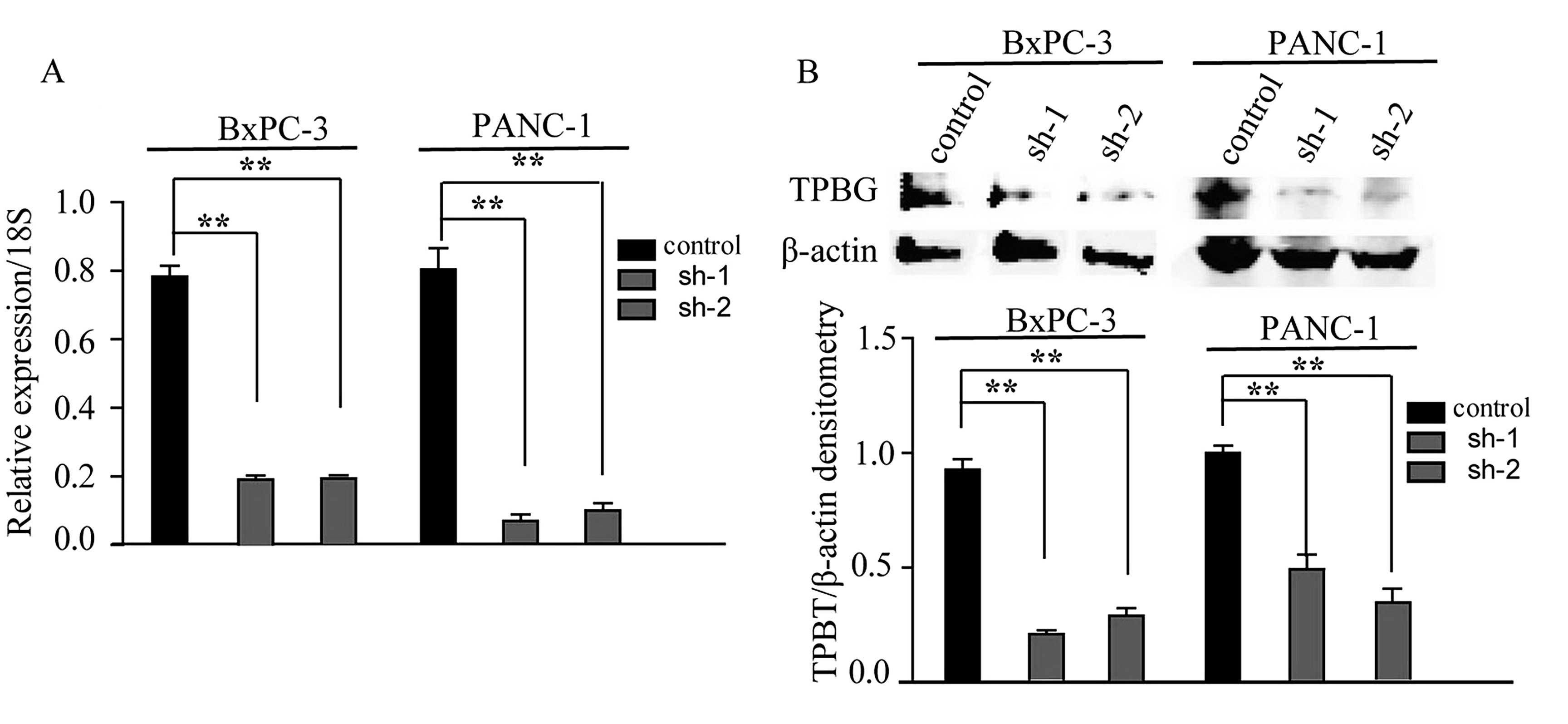
Expression of TPBG in BxPC-3 and PANC-1
cell lines is significantly decreased following stable
silencing
To further investigate the role of TPBG in PDAC
carcinogenesis and progression, the BxPC-3 and PANC-1 PDAC cell
lines were selected, which highly expressed TPBG, to construct cell
lines exhibiting stable knockdown of TPBG. The silencing of TPBG by
two shRNAs (sh-1 and sh-2) in the BxPC-3 and PANC-1 cells resulted
in significant decreases in the expression of TPBG (Fig. 2).
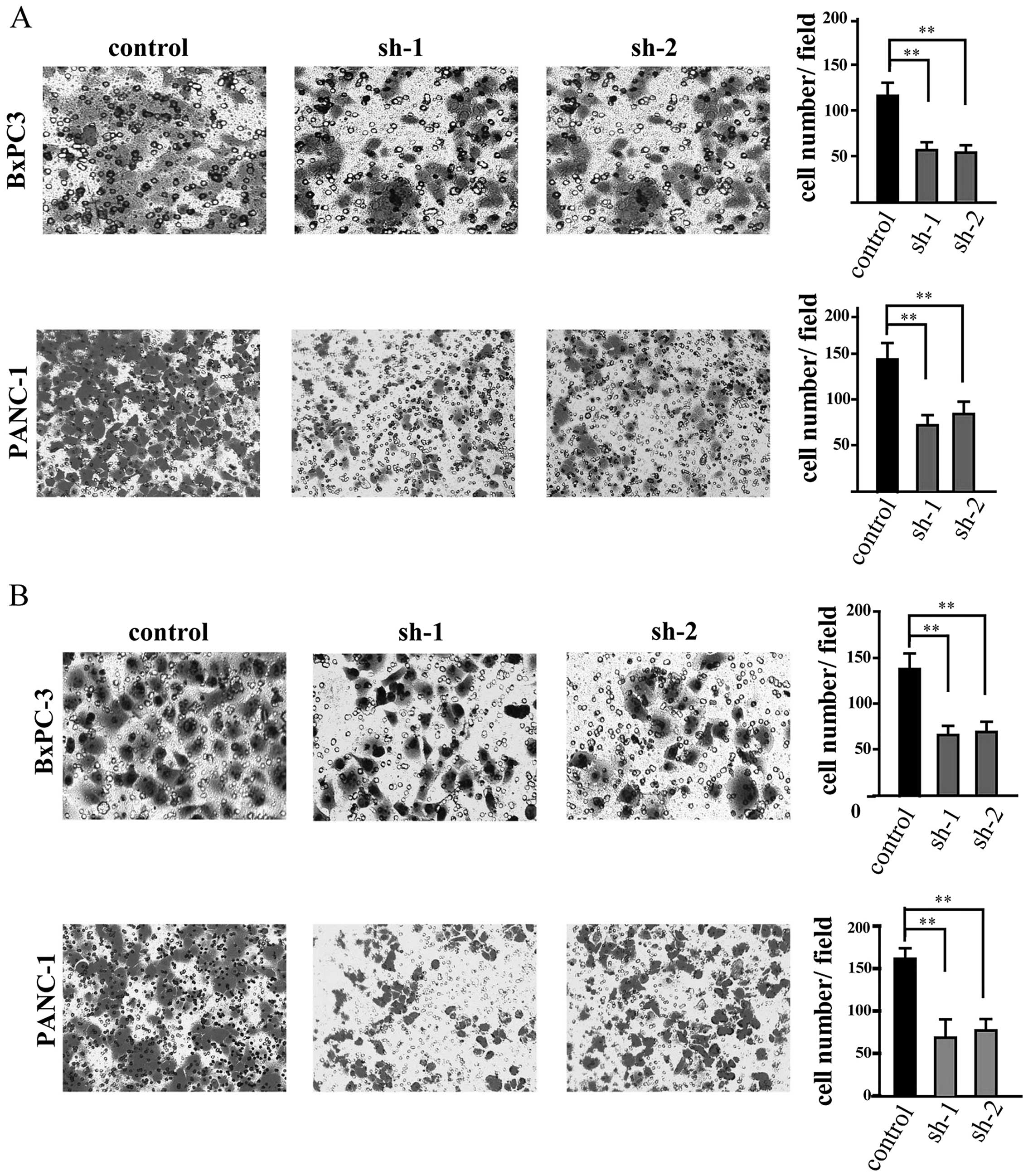
Silencing of TPBG inhibits PDAC cell
migration and invasion
Given that TPBG is a gene that is associated with
cell motility, a Transwell assay was performed. In the migration
assay, it was observed that the motility of the cells was notably
decreased following TPBG knockdown (Fig. 3A). In addition, to examine whether
TPBG has an affect on the invasive ability of the cells, a cell
invasion assay was performed and it was identified that number of
cells that invaded through the membrane was also decreased
significantly following silencing of TPBG (Fig. 3B).
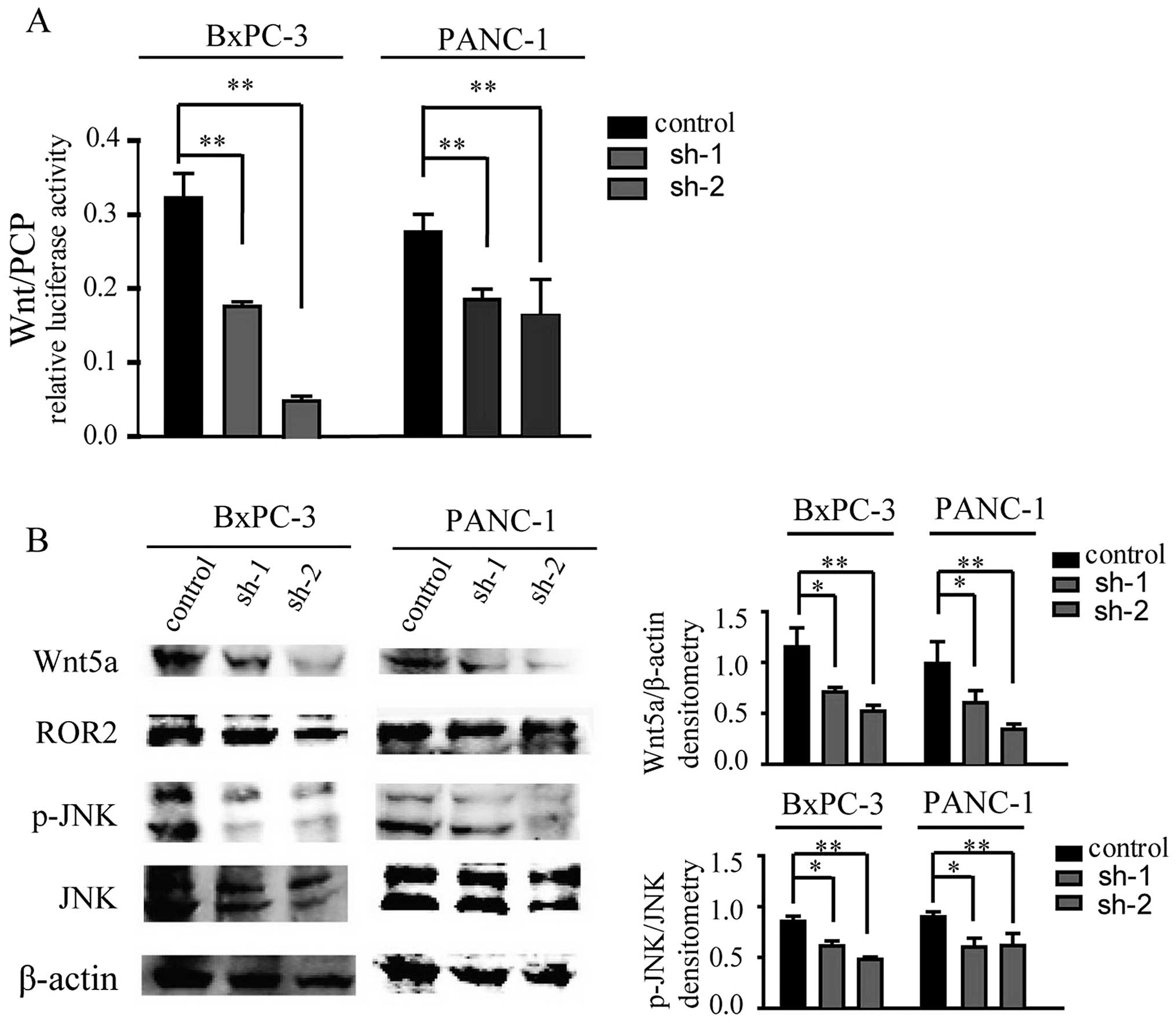
Wnt/PCP pathway is suppressed following
silencing of TPBG
To further investigate the underlying mechanism
involved in cell migration and invasion in PDAC, Wnt/PCP signaling
was examined, which has been observed to promote cell migration in
cancer metastasis (14). Using a
luciferase reporter assay, it was identified that Wnt/PCP signaling
was inhibited in the cells subjected to TPBG knockdown compared
with the control cells (Fig. 4A).
Collectively, the present results suggested that TPBG may affect
cell migration and invasion through the Wnt/PCP signaling pathway.
To further confirm these results, several molecules associated with
the Wnt/PCP pathway were investigated. It was identified that the
expression of Wnt5a, an extensively investigated Wnt ligand of the
Wnt/PCP pathway (15,16) was inhibited (Fig. 4B). The activation of JNK, a
downstream molecule of the Wnt/PCP pathway, was also reduced
markedly (Fig. 4B). Thus, the
present data indicated that TPBG may affect cell migration and
invasion through the Wnt/PCP pathway.
Discussion
In the present study, the aberrant expression of
TPBG in PDAC was described and the potential effect of highly
expressed TPBG in PDAC cell lines was investigated. In previous
studies, immunohistochemical analysis has revealed that TPBG is
overexpressed in numerous types of cancer (5). In addition, the expression of TPBG
usually indicates a poor prognosis, with TPBG expression
correlating with the likelihood of cancer metastasis (7–9,17).
Consistent with these observations, the present results provide
evidence that TPBG may be a critical factor that facilitates PDAC
progression and metastasis. In the present study,
immunohistochemical analysis of a PDAC tissue microarray revealed
that TPBG is closely associated with the TNM stage of the tumor.
The cell migration and invasion abilities were significantly
decreased following TPBG knockdown. TPBG expression is, therefore,
correlated with the aggressiveness of PDAC, including the
metastatic properties of the malignancy.
However, how TPBG regulates PDAC cell migration
remains to be fully elucidated. The Wnt/PCP signaling pathway has
been revealed to be involved in cell movement through the
activation of the RhoA, JNK and nemo-like kinase signaling cascades
(18). In addition, accumulating
evidence has suggested that Wnt/PCP signaling is closely associated
with tumors and has diverse roles in tumorigenesis (18,19).
Previous studies have demonstrated that TPBG activates the Wnt/PCP
pathways by modifying the subcellular localization of low density
lipoprotein receptor-related protein 6 during Zebrafish development
(20). These findings imply that
TPBG may affect Wnt/PCP signaling in PDAC, thus the correlation
between TPBG and Wnt/PCP signaling was further investigated.
It was identified that Wnt/PCP signaling was
inhibited significantly in the PDAC cell lines following TPBG
knockdown via a luciferase reporter assay. Numerous studies have
suggested that Wnt5a, a typical noncanonical Wnt ligand, promotes
the metastasis of melanoma, gastric cancer and breast cancer by
activating the Rac and JNK signaling pathways (14,21,22).
The expression of Wnt5a, Ror2 (a receptor for Wnt5a) (23) and a change in phospho-JNK were also
detected and it was observed that the expression of Wnt5a and the
activation of phospho-JNK were decreased markedly in the cell lines
in which TPBG expression was silenced. Collectively, the present
results demonstrated that TPBG promotes cancer metastasis partly
through Wnt/PCP signaling. However, numerous factors remain to be
elucidated, including how TPBG regulates the expression of Wnt5a
and how TPBG affects the downstream molecules of the Wnt/PCP
pathway.
In conclusion, the present study demonstrated that
TPBG expression is upregulated in human PDAC tissues and the highly
expressed TPBG may regulate the Wnt/PCP signaling to enhance the
abilities of cancer cell migration and invasion. The findings of
the present study indicate that the distinctive expression pattern
of TPBG may render it a suitable target for the treatment of
PDAC.
Acknowledgments
The present study was supported by the National High
Technology Research and Development Program of China (863 Program;
grant no. 2014AA020609) and the State Key Laboratory Project (grant
no. 91-14-08, 91-14-14).
References
|
1
|
Vincent A, Herman J, Schulick R, Hruban RH
and Goggins M: Pancreatic cancer. Lancet. 378:607–620. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Helm JF, Centeno BA, Coppola D, et al:
Outcomes following resection of pancreatic adenocarcinoma: 20-year
experience at a single institution. Cancer Control. 15:288–294.
2008.PubMed/NCBI
|
|
3
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hole N and Stern PL: A 72 kD trophoblast
glycoprotein defined by a monoclonal antibody. Br J Cancer.
57:239–246. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Southall PJ, Boxer GM, Bagshawe KD, Hole
N, Bromley M and Stern PL: Immunohistological distribution of 5T4
antigen in normal and malignant tissues. Br J Cancer. 61:89–95.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Harrop R, Drury N, Shingler W, et al:
Vaccination of colorectal cancer patients with modified vaccinia
ankara encoding the tumor antigen 5T4 (TroVax) given alongside
chemotherapy induces potent immune responses. Clin Cancer Res.
13:4487–4494. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Starzynska T, Marsh PJ, Schofield PF,
Roberts SA, Myers KA and Stern PL: Prognostic significance of 5T4
oncofetal antigen expression in colorectal carcinoma. Br J Cancer.
69:899–902. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wrigley E, McGown AT, Rennison J, et al:
5T4 oncofetal antigen expression in ovarian carcinoma. Int J
Gynecol Cancer. 5:269–274. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Naganuma H, Kono K, Mori Y, et al:
Oncofetal antigen 5T4 expression as a prognostic factor in patients
with gastric cancer. Anticancer Res. 22:1033–1038. 2002.PubMed/NCBI
|
|
10
|
Carsberg CJ, Myers KA and Stern PL:
Metastasis-associated 5T4 antigen disrupts cell-cell contacts and
induces cellular motility in epithelial cells. Int J Cancer.
68:84–92. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Awan A, Lucic MR, Shaw DM, et al: 5T4
interacts with TIP-2/GIPC, a PDZ protein, with implications for
metastasis. Biochem Biophys Res Commun. 290:1030–1036. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ma M-Z, Zhuang C, Yang X-M, et al: CTHRC1
acts as a prognostic factor and promotes invasiveness of
gastrointestinal stromal tumors by activating Wnt/PCP-Rho
signaling. Neoplasia. 16:265–278. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li J, Yang X-M, Wang Y-H, et al: Monoamine
oxidase a suppresses hepatocellular carcinoma metastasis by
inhibiting the adrenergic system and its transactivation of EGFR
signaling. J Hepatol. 60:1225–1234. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Weeraratna AT, Jiang Y, Hostetter G, et
al: Wnt5a signaling directly affects cell motility and invasion of
metastatic melanoma. Cancer Cell. 1:279–288. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yamamoto H, Oue N, Sato A, et al: Wnt5a
signaling is involved in the aggressiveness of prostate cancer and
expression of metal-loproteinase. Oncogene. 29:2036–2046. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Vuga LJ, Ben-Yehudah A,
Kovkarova-Naumovski E, et al: WNT5A is a regulator of fibroblast
proliferation and resistance to apoptosis. Am J Respir Cell Mol
Biol. 41:583–589. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Castro FV, McGinn OJ, Krishnan S, et al:
5T4 oncofetal antigen is expressed in high risk of relapse
childhood pre-B acute lymphoblastic leukemia and is associated with
a more invasive and chemotactic phenotype. Leukemia. 26:1487–1498.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Katoh M: WNT/PCP signaling pathway and
human cancer (review). Oncol Rep. 14:1583–1588. 2005.PubMed/NCBI
|
|
19
|
Wang Y: Wnt/Planar cell polarity
signaling: a new paradigm for cancer therapy. Mol Cancer Ther.
8:2103–2109. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kagermeier-Schenk B, Wehner D, Özhan-Kizil
G, et al: Waif1/5T4 inhibits Wnt/β-catenin signaling and activates
noncanonical Wnt pathways by modifying LRP6 subcellular
localization. Dev Cell. 21:1129–1143. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kurayoshi M, Oue N, Yamamoto H, et al:
Expression of Wnt-5a is correlated with aggressiveness of gastric
cancer by stimulating cell migration and invasion. Cancer Res.
66:10439–10448. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pukrop T, Klemm F, Hagemann T, et al: Wnt
5a signaling is critical for macrophage-induced invasion of breast
cancer cell lines. Proc Natl Acad Sci USA. 103:5454–5459. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Oishi I, Suzuki H, Onishi N, et al: The
receptor tyrosine kinase Ror2 is involved in non-canonical
Wnt5a/JNK signalling pathway. Genes Cells. 8:645–654. 2003.
View Article : Google Scholar : PubMed/NCBI
|