Introduction
Breast cancer is a major threat to the health of
females and accounts for 7–10% of all incidences of systematic
malignancies in China; which is continuously increasing (1). There are a number of various
available interventions and ~80% of patients survive for >5
years (2), however, breast cancer
remains one of the leading causes of mortality in females. Vascular
endothelial growth factor (VEGF) has been recognized to be one of
the most potent pro-angiogenic factors. High expression of VEGF has
been observed in breast cancer, and was shown to be correlated with
prognosis (3).
NRP-1 was first identified from the axons of nerve
fibers in 1995 (4), and is a
130–135 kDa transmembrane glycoprotein, which consists of an
extracellular region of 860 amino acids, a transmembrane region of
23 amino acids and an intracellular region of 40 amino acids. The
extracellular region consists of five domains: a1/a2, bl/b2 and
ca1/a2, which are designated to be CUB domains; and a1/a2 and b1/b2
domains, which bind to the SEMA 3 family and VEGF family,
respectively (5).
NRP-1 was described to be a receptor corresponding
to SEMA that may regulate the development of the nervous system
(6). Recently, NRP-1 has also been
demonstrated to be involved in the regulation of vascular
endothelial cell migration and angiogenesis (7). Soker et al (8) observed that co-expression of VEGFR2
and NRP-1 in porcine aortic endothelial cells could increase the
binding affinity of VEGF165 to VEGFR2, by at least 4-fold. This
observation suggested that NRP-1 could promote the binding of
VEGF165 to VEGFR2, and promote VEGFR2 signal transduction,
thereafter increasing the chemotaxis of vascular endothelium. Thus,
NRP-1 has also been proposed to be a critical factor for the
regulation of angiogenesis.
NRP-1 is expressed in a number of types of
malignancies, such as pancreatic, lung, prostate, ovarian and
gastrointestinal cancer, and it was observed to promote
angiogenesis, tumor growth, invasion and metastasis (9–11).
Wang et al (12) observed
overexpression of NRP-1 in breast cancer tissues compared with
healthy breast tissue. However, the effects of NRP-1 on the
biological activities of breast cancer cells have never been
reported. In this study, the effects of stable suppression of NRP-1
expression on the biological activities of MCF-7 and SK-BR-3 breast
cancer cells were examined.
Materials and methods
Cell lines and reagents
MCF-7 cells were supplied by Nanjing KGI
Biotechnology Company (Nanjing, China). SK-BR-3 cells were
purchased from Shanghai Cell Bank of Chinese Academy of Sciences
(Shanghai, China). pLB lentiviral transfer vector, pCMVΔ8.91
packaging plasmid and pMD.G envelope protein plasmids were provided
by Professor Xu Kailin (Xuzhou Medical College, Xuzhou, China).
Interference sequence NRP-1/short hairpin (sh)RNA was synthesized
by Invitrogen Life Technologies (Carlsbad, CA, USA). Restriction
endonuclease HpaI, restriction endonuclease XhoI, and
T4DNA ligase were purchased from NEB Co., USA. A fluorescence
quantitative polymerase chain reaction kit was purchased from Roche
Diagnostics (Indianapolis, IN, USA). Rabbit Anti-human NRP-1
polyclonal antibody was purchased from Abcam (Cambridge, UK).
Epirubicin is supplied by Pfizer (USA); cell counting kit-8 kit was
purchased from Dojindo Laboratories (Kumamoto, Japan). An Annexin
V/propidium iodide apoptosis kit was purchased from Beijing Biosea
Biotechnology Co., Ltd (Beijing, China).
Culture of cell lines
MCF-7, SK-BR-3 and 293FT human breast cancer cell
lines (Invitrogen Life Technologies, Carlsbad, CA, USA), were
cultured in Dulbecco’s modified Eagle’s medium (DMEM; Invitrogen
Life Technologies) containing 10% fetal bovine serum (Invitrogen
Life Technologies) under 37°C/5% CO2/saturated humidity
conditions in an incubator until grown to 80–90% confluency and
passaged according to the principle of passage (1:2 or 1:3). When
stable growth was achieved, cells in their logarithmic growth phase
were selected for follow-up studies.
Design of short hairpin (sh)RNA
sequences
According to the full sequence of NRP-1 mRNA
retrieved from GeneBank (http://www.ncbi.nlm.nih.gov), three pairs of shRNA
sequences were selected based on the principle for shRNA design.
Another non-specific control sequence was also designed. All
sequences designed were submitted to Invitrogen Life Technologies
and the insertion sequences were described as follows:
5′-AACCCCAACAGCCTTGAATGCACTTATATTCAAGAGATATAAGTGCATTCAAGGCTGTTGGGTTTTTTC-3′;
5′-A ACCATTGGGCGTTACTGTGGACAGAAATTCAAGAGA
TTTCTGTCCACAGTAACGCCCAATGTTTTTTC-3′; 5′-AAC
CAGATCACAGCTTCTTCCCAGTATATTCAAGAGATAT
ACTGGGAAGAAGCTGTGATCTGTTTTTTC-3′; and
5′-AACGTTCTCCGAACGTGTCACGTTTCAAGAGAACGTG ACACGTTCGGAGAATTTTTTC-3′.
These sequences were designated shRNA1, 2, 3 and 4,
respectively.
Construction and identification of
lentiviral vectors carrying an RNAi sequence targeting human NRP-1
gene
The annealing products of sense and antisense
strands of pLB and NRP-1/shRNA were treated with restriction
endonucleases HpaI and XhoI. Subsequent to
purification, ligation, transformation and inoculation (13), they were cultured at 37°C
overnight. Isolated colonies were selected and inoculated with
ampicillin-resistant LB medium, followed by shaking at 220 rpm/min
at 37°C overnight to extract the recombinant plasmids. After
initial PCR identification, they were submitted to Invitrogen Life
Technologies for sequencing identification.
Identification of breast cancer cell
lines with high NRP-1 expression
Western blot analysis was used to identify the
breast cancer cell lines with high NRP-1 expression, using β-actin
as an internal reference. MCF-7 and SK-BR-3 breast cancer cell
lines in their logarithmic growth phase were treated to extract
total proteins. Equal quantities of protein were analyzed by
polyacrylamide gel electrophoresis, transferred to nitrocellulose
membranes under the condition of 300 mA current for 2 h, and
blocked with gelatin blocking solution for 1 h. Rabbit anti-human
NRP-1 polyclonal antibody (Abcam, St. Louis, MO, USA; 1:1,000
dilution) and mouse anti-human β-actin polyclonal antibody (Beijing
Zhongshan Golden Bridge Biotechnology Co.,. Ltd., Beijing, China;
1:500 dilution) were added, respectively, and incubated at 4°C
overnight. The membrane was washed with washing buffer three times
for 5 min, prior to the addition of goat anti-rabbit and horse
anti-mouse antibody (Beijing Zhongshan Golden Bridge Biotechnology
Co.,. Ltd., Beijing, China; 1:1,000 dilution), and the membrane was
then was incubated at room temperature for 1 h. After washing with
washing buffer a further three times for 5 min, the membrane was
impregnated in BCIP/NBT (Promega, Madison, WI, USA) in order to
develop color.
Packing and concentrating lentivirus
293FT cells (4×106) in the logarithmic
growth phase were inoculated to 100 mm culture plates treated with
polylysine and incubated at 37°C/5% CO2 for 1 h. The
plasmids (12 μg pLB-NRP-1/shRNA), pCMVΔ8.918 μg and
pMD.G 4 μg, were diluted in 1.5 ml serum-deprived Opti-MEM
medium. Following incubation for 5 min, diluted plasmids were mixed
with Lipofectamine 2000 (Invitrogen Life Technologies) prior to
cultivation for 25 min at room temperature. Post-incubation
plasmids and lipofectamine mixtures were transferred into cell
cultures and mixed gently. After cultivation at 37°C/5%
CO2 for 6 h. Culture medium was replaced with DMEM
containing 2% FBS and cultivated for another 18 h. Viral
supernatants were collected at 48 and 72 h, passed through a 0.45
μm filter and centrifuged at 70,000 × g at 4°C for 2 h. The
supernatant was discarded and the virus particles were re-suspended
in 0.5 ml Opti-MEM medium (Invitrogen Life Technologies) and stored
at −80°C for further use.
Infection of lentivirus into MCF-7 and
SK-BR-3 human breast cancer cells
MCF-7 and SK-BR-3 human breast cancer cells in their
logarithmic growth phase were inoculated in 6-well plates,
respectively, and cultured at 37°C/5% CO2 under normal
conditions. Concentrated lentivirus (three replicate wells each)
were added according to 3 multiplicities of infection (MOI) and
diluted with polybrene to a final concentration of 8 μg/l.
Green fluorescent protein (GFP) expression profiles were examined
under a fluorescence microscope (Olympus, Japan) at 24 and 48 h
after virus infection. After enrichment culture of post-infection
positive cells, flow cytometry was used to sort GFP-positive cells
in the Hematology Laboratory of Soochow University (Suzhou, China),
which were enriched for further experiments.
NRP-1 expression profile analyzed by
fluorescent qRT-PCR and western blot analysis
MCF-7 and SK-BR-3 breast cancer cell lines, were
divided into subgroups of blank controls, negative controls and
specific interference groups 1, 2 and 3.
Total RNA was extracted by TRIzol (Invitrogen) and
treated with DNase I (New England Biolabs), and then cDNA was
synthesized using GoScript Reverse Transcriptase (Promega, Madison,
WI, USA) with oligodT18 primer. For quantitative PCR, mRNA
expression was measured using SYBR Green I Master (Roche). The
relative quantification of mRNA levels was performed using the
comparative Ct method (Δ/ΔCt method) with β-actin as the reference
gene. The following primers were used: NRP-1, sense
5′-GGAAGCTCTGGGCATGGAAT-3′ and antisense
5′-AGGAATCCTCTCCGGGAGTC-3′; β-actin, sense
5′-CATGTACGTTGCTATCCAGGC-3′ and antisense
5′-CTCCTTAATGTCACGCACGAT-3′. The PCR reaction conditions were
specified as denaturation at 95°C for 5 min, 95°C for 20 sec and
60°C for 15 sec, for a total of 40 cycles. After extraction of
total proteins, equal quantities of proteins were transferred to
undergo SDS-PAGE analysis.
Cell proliferation by CCK-8 assay
MCF-7 and SK-BR-3 breast cancer cell lines were
divided into subgroups of blank controls, negative controls and
interference groups with the highest silencing efficiency.
Cells (2.5×104/ml) from all six groups
were inoculated into 96-well plates with three replicate wells and
blank control wells (with medium only) for each group.
Phosphate-buffered saline (PBS) was added along an arc of the plate
periphery. The medium was removed after cultivation for 24, 48, 72
and 96 h, respectively. Serum-deprived DMEM medium was added to
each well, followed by the addition of 10 μl CCK-8 reagent.
After cultivation for another 2 h, the optical density values at
450 nm wavelength were determined on a Universal Microplate
Spectrophotometer (Thermo Multiskan MK3; Thermo Fisher Scientific,
Waltham, MA, USA). Correct zero value from blank well to plot cell
growth curve of each group.
Apoptosis examination by Annexin
V-fluorescein isothiocyanate (FITC)/propidium iodide (PI) methods
in combination with flow cytometry
MCF-7 and SK-BR-3 breast cancer cell lines were
divided into subgroups of blank controls, negative controls and
interference groups with the highest silencing efficiency.
Cells (2.5×104/ml) of all six groups in
their logarithmic growth phase were cultured for 48 h and apoptosis
was examined using flow cytometry (BD Bioscience, San Jose, CA,
USA), according to the instructions of the Annexin V-FITC/PI
kit.
EPI-induced apoptosis
Effects of different concentrations of
EPI on the proliferation of MCF-7 and SK-BR-3 assayed by CCK-8
MCF-7 and SK-BR-3 breast cancer cells
(1.0×105/ml), were inoculated into 96-well plates with
three replicate wells and blank control wells (with medium only)
for each group. PBS was added along an arc of the plate periphery.
The medium was removed after cultivation for 24 h. Serum-deprived
DMEM medium containing 0.25, 0.5, 1.0, 2.0, 4.0 and 10.0
μg/ml EPI was added to each test group. Three parallel
replicates were established for each group. A blank control group
(free of cells) and a negative control group (containing cells and
no test substance) were also analyzed. Following cultivation for 24
h, 10 μl CCK-8 reagent was added to each well and cultivated
for another 2 h. The OD values were determined at 450 nm wavelength
on a Universal Microplate Spectrophotometer. Correct the zero value
according to the blank well and depict cell growth curve of each
group.
EPI induced apoptosis
In this study, MCF-7 and SK-BR-3 control groups and
cell groups with the most efficient silencing NRP-1 were
established. Cells in the logarithmic growth phase
(2×105 cells/well) were inoculated to 6-well plates
separately and cultured for 48 h. MCF-7 cells were inoculated with
serum-deprived medium containing 2.0 μg/ml EPI and SK-BR-3
cells were inoculated with serum-deprived medium containing 1.5
μg/ml EPI. Three replicates were used for each group. After
cultivation for 24 h, apoptosis was examined according to the
manufacturer’s instructions of the Annexin V-FITC/PI kit in
combination with flow cytometry.
Statistical analysis
Statistical analyses were conducted using SPSS 16.0
(SPSS, Inc., Chicago, IL, USA). Quantitative data are represented
as the mean ± standard deviation. Pair-wise inter-group comparison
was analyzed using a t-test. Multiple group comparisons were
analyzed using a one-way analysis of variance test. α=0.05 and
P<0.05 were considered to indicate a statistically significant
difference.
Results
Virus infection and flow cytometry of
MCF-7 and SK-BR-3 cells
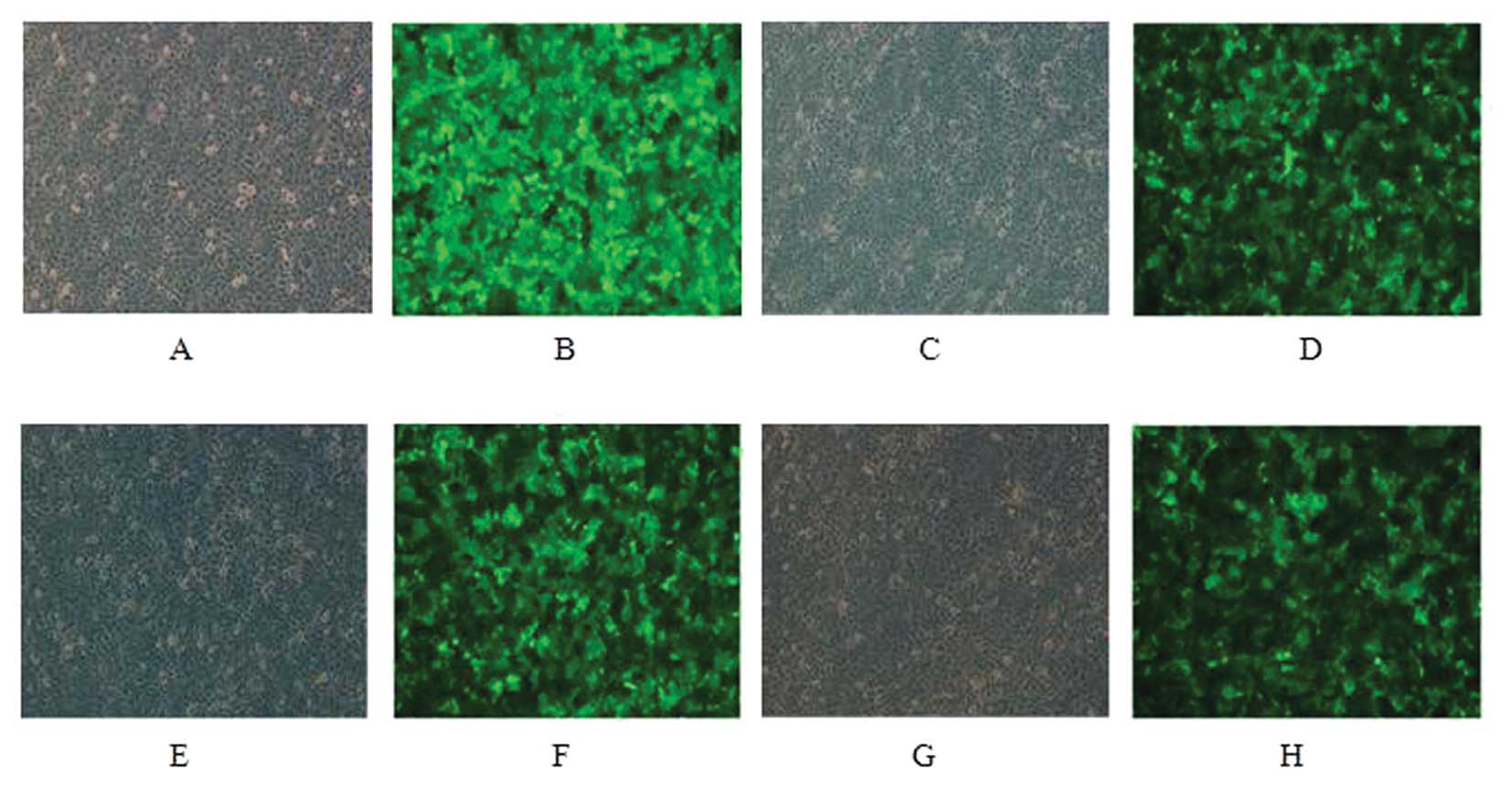
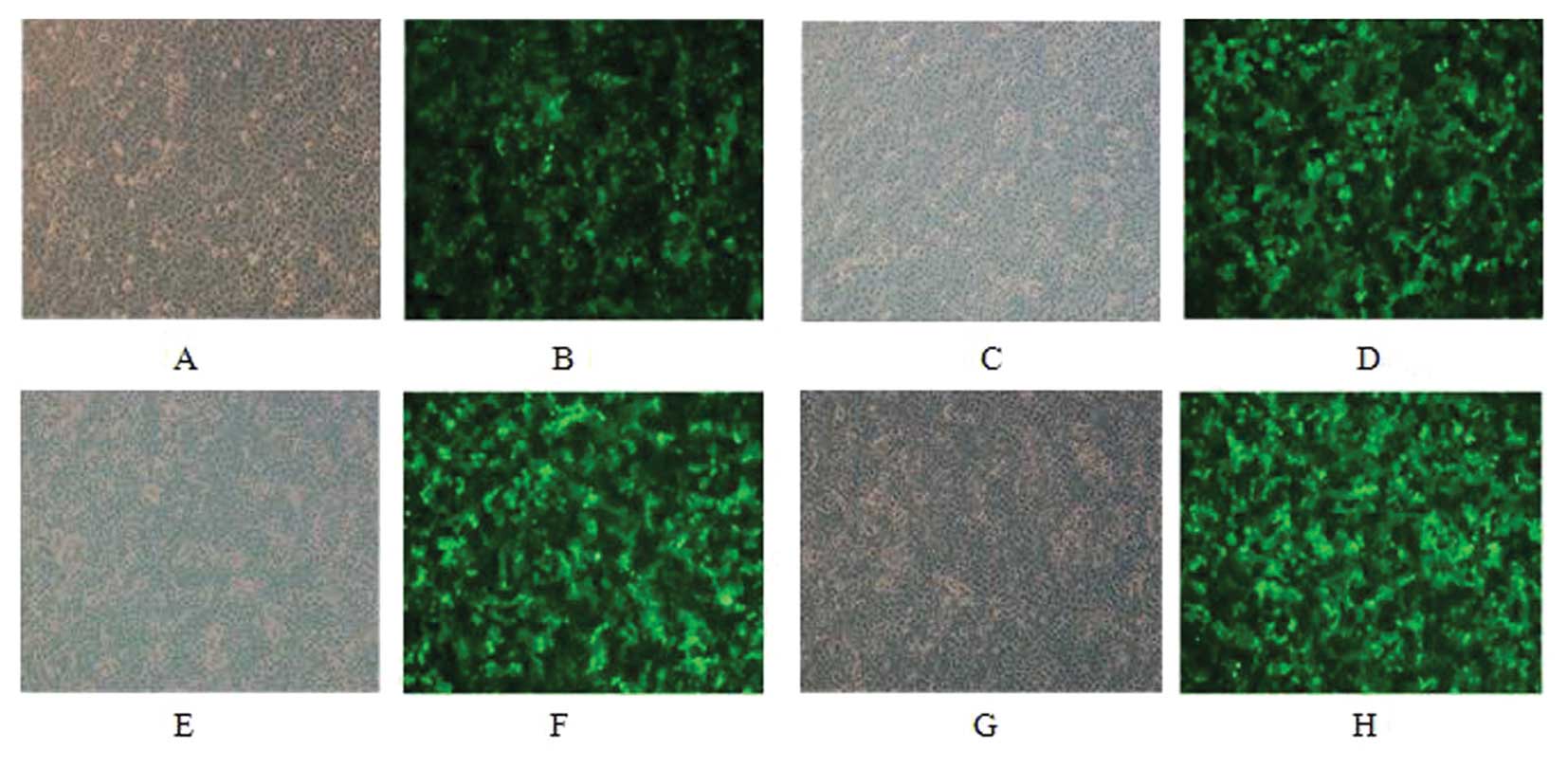
MCF-7 and SK-BR-3 cells were infected for 48 h and
only low GFP expression was observed under fluorescence microscopy.
GFP positive cells were sorted and re-examination suggested a
positive rate of 96% after sorting (Figs. 1 and 2).
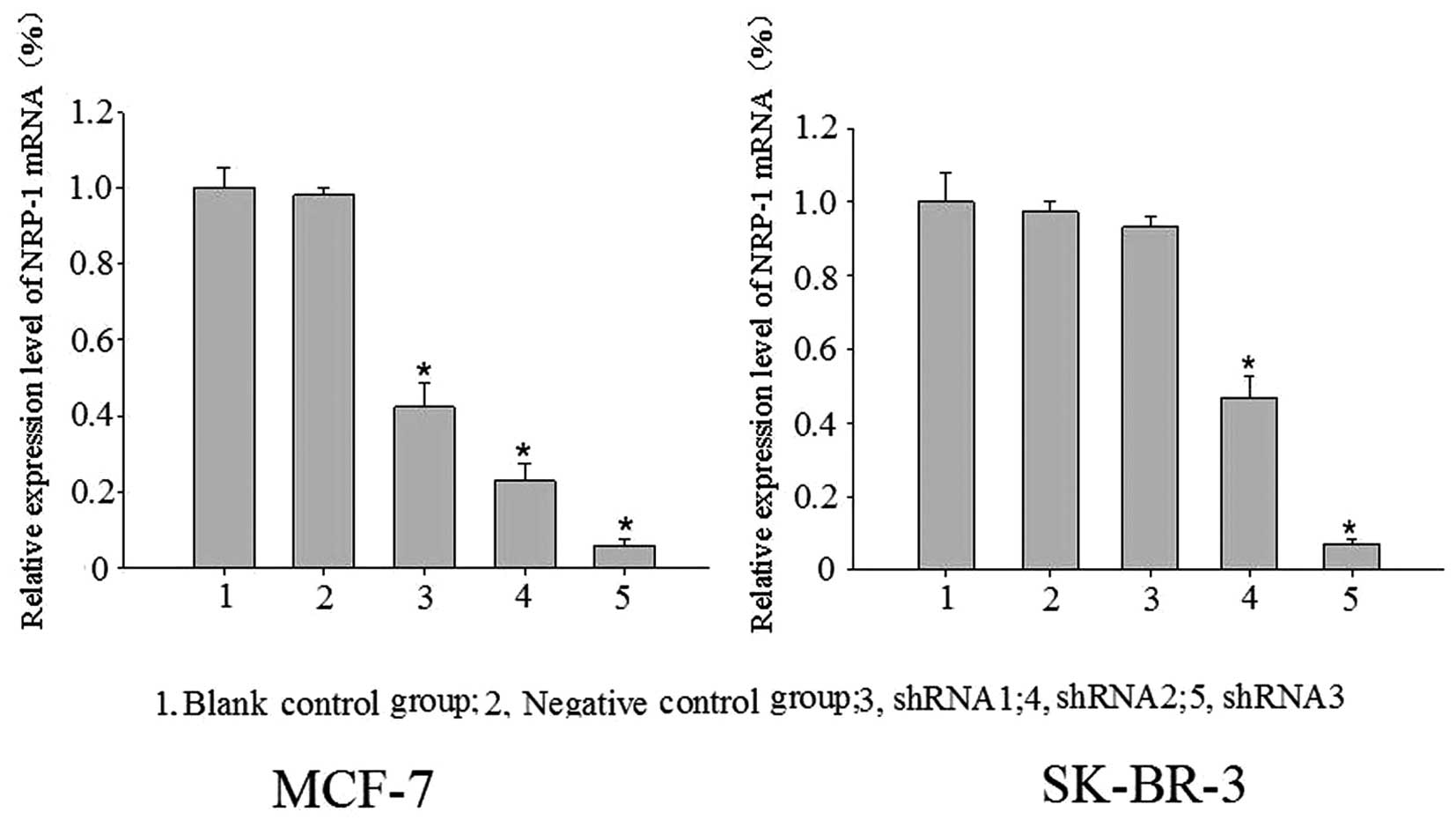
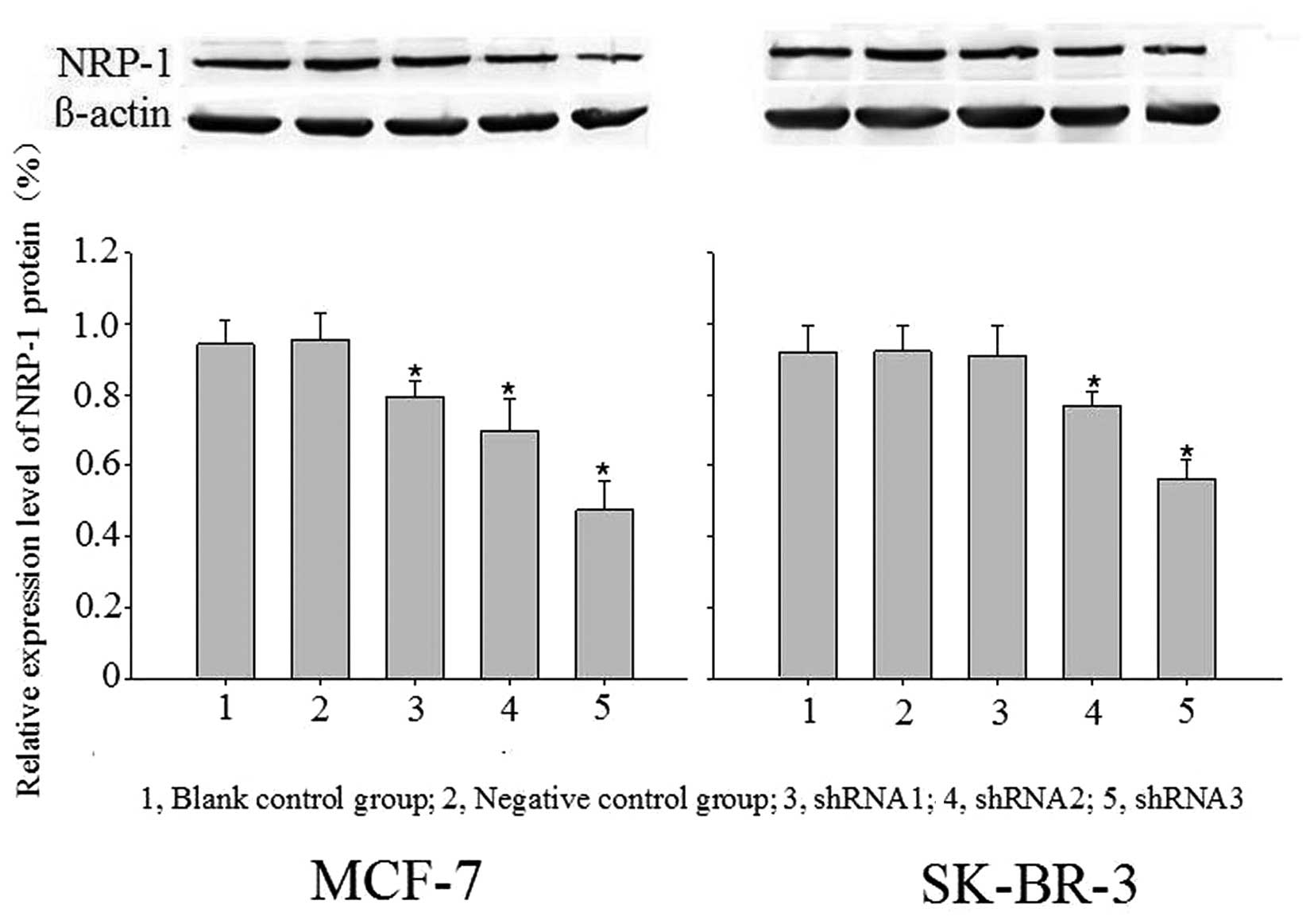
Significant expression suppression of
NRP-1 mRNA and protein following virus infection
Blank control, negative control and specific
interference groups (NRP-1/shRNA1, NRP-1/shRNA2, and NRP-1/shRNA3)
were established in this study. With β-actin as the internal
reference, the relative expression profiles of target gene was
estimated based on 2−ΔΔCt. Compared with the control
group, the expression profiles of NRP-1 mRNA and protein were
decreased significantly in the MCF-7 cell-specific interference
groups (pLB-NRP-1/shRNA1, 2 and 3), with mRNA expression decreased
by 57.73, 77.20 and 94.17% and protein expression decreased by
15.85, 26.25 and 49.29%, respectively. For the SK-BR-3 cell
specific interference group (pLB-NRP-1/shRNA1, 2 and 3), the
expression profiles of NRP-1 mRNA and protein also were also
significantly decreased, with mRNA expression decreased by 53.20
and 92.94%, protein expression decreased by 15.98 and 38.50%,
respectively. These results were statistically significant compared
with the control group (P<0.05); pLB-NRP-1/shRNA3 showed the
highest silencing efficiency among these two cell lines. The
pair-wise comparison between the negative control group and blank
control group was not statistically significant (P>0.05)
(Figs. 3 and 4).
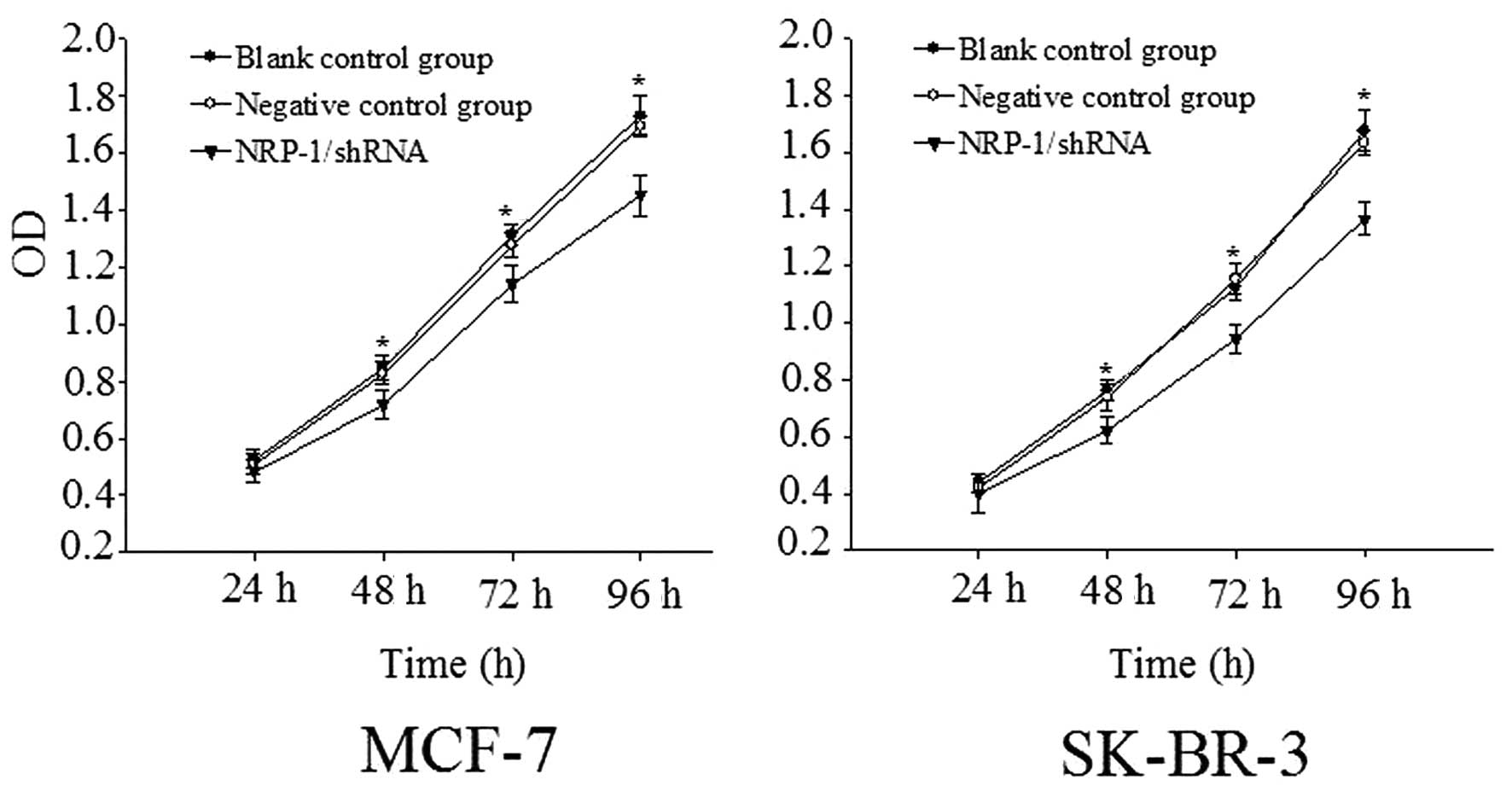
Determination of proliferation rate
According to the proliferation rates determined by
the CCK-8 method, the OD values of MCF-7 cell with NRP-1/shRNA
group were determined to be 0.717±0.051, 1.140±0.064 and
1.453±0.071 at 48, 72 and 96 h respectively. This difference was
statistically significant comparison to the control group
(P<0.05). The OD values of SK-BR-3 cell with NRP-1/shRN were
0.622±0.046, 0.944±0.051 and 1.365±0.058 at 48 h, 72 h, and 92 h
respectively. This difference was statistically significant
compared with the control group (P<0.05), but the inter-group
difference between the negative control group and the blank control
group was not statistically significant (P>0.05) (Fig. 5).
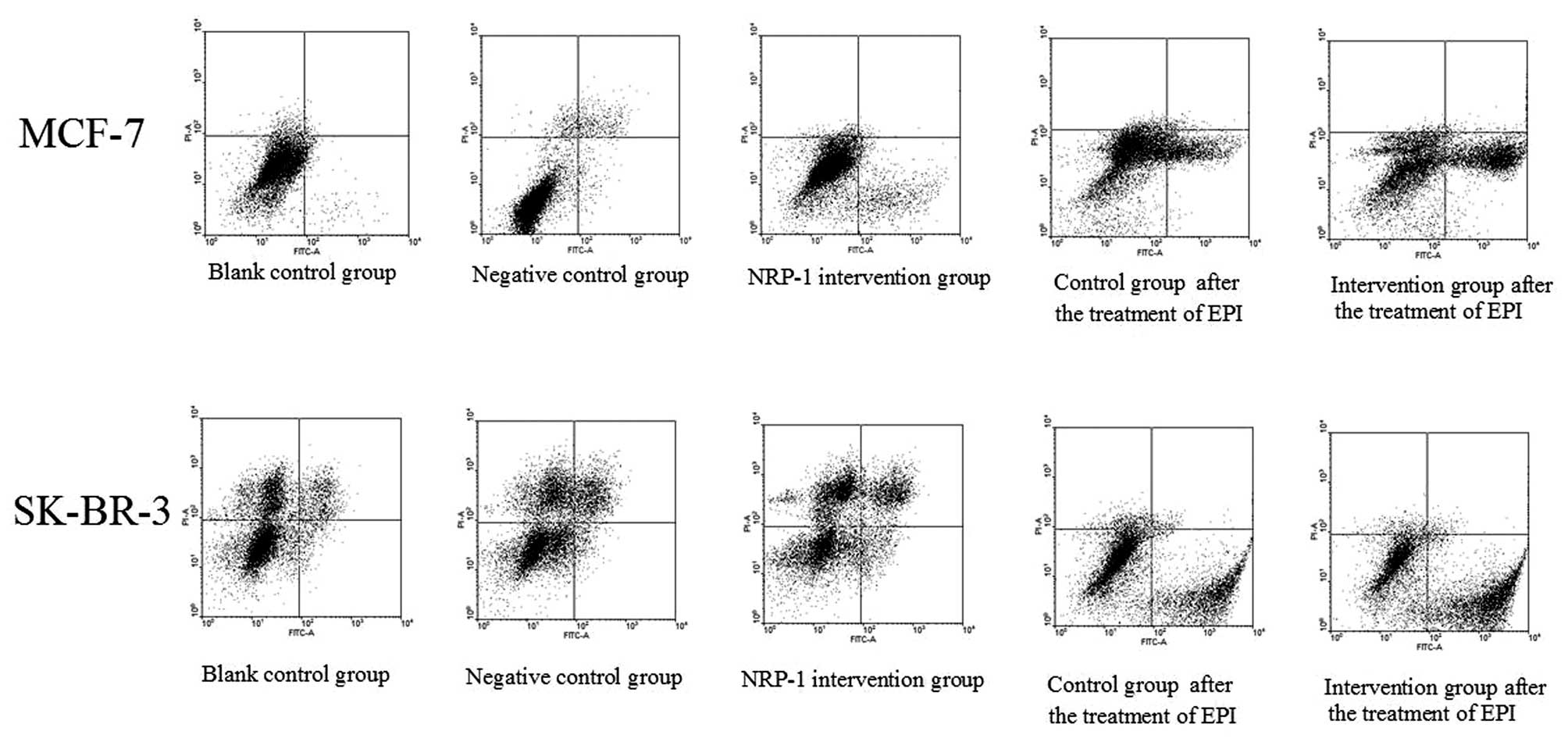
Determination of apoptotic rate
The apoptotic rates of MCF7 cells determined by the
Annexin V-propidium iodide method in combination with flow
cytometry were 2.05±0.18, 2.41±0.29 and 9.76±0.54% for the blank
control group, the negative control group and the NRP-1
intervention group; while the apoptotic rates of SK-BR-3 cells were
3.04±0.17, 3.22±0.26 and 5.98±0.17% for the blank control group,
the negative control group and the NRP-1 intervention group,
respectively. Compared with the control group, the rate of
apoptosis of cells in the NRP-1/shRNA groups was higher (P<0.05)
and no significant differences were observed in the negative group
and blank group (P>0.05) (Fig.
6).
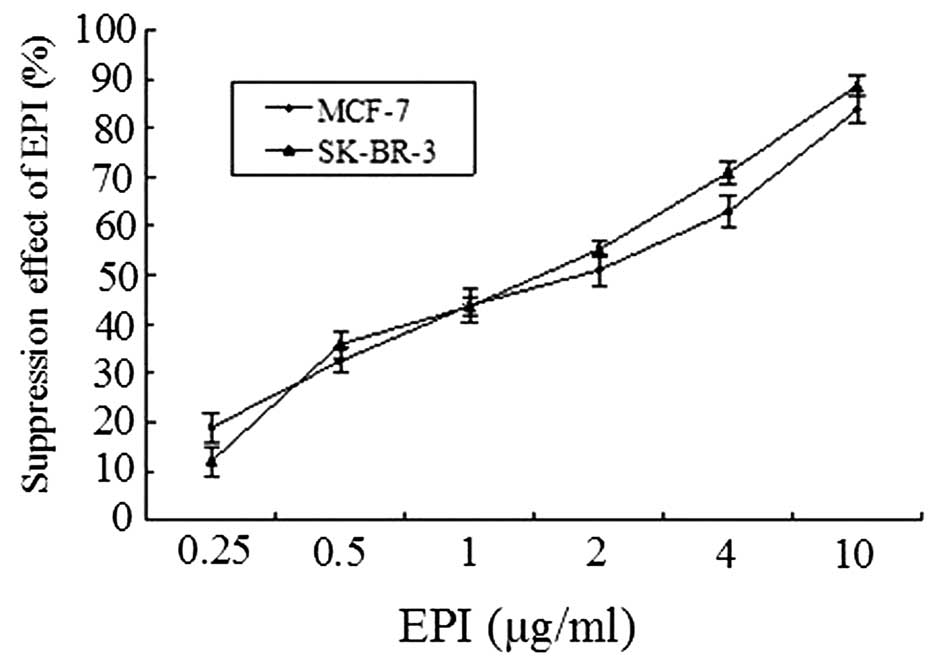
Selection of EPI concentration
CCK-8 assays demonstrated that the suppression
effect of EPI tends to increase with the elevation of concentration
in the range between 0 and 10.0 μg/ml. At 2 μg/ml,
the inhibitory rates of MCF-7 and SK-BR-3 cells were 50.99±3.07 and
55.18±1.58% respectively. The IC50s were determined to
be 2.0 and 1.5 μg/ml for MCF-7 and SK-BR-3 respectively.
Therefore, 2.0 and 1.5 μg/ml were selected as the EPI
concentrations for MCF-7 and SK-BR-3 assays, respectively (Fig. 7).
Determination of EPI-induced
apoptosis
As shown by the assays with Annexin V-FITC/PI in
combination with flow cytometry, the apoptotic rates of MCF-7 cells
with NRP-1 interference and the control group were 35.32±2.24 and
20.80±3.08% respectively after EPI treatment, while the apoptotic
rates of SK-BR-3 and the control group were 49.24±3.66 and
34.15±1.97%, respectively. The apoptotic rate of the interference
group was higher than the control group and the difference was
statistically significant (P<0.05) (Fig. 6).
Discussion
Breast cancer is the most common type of malignancy
in females and remains to be a common cause of cancer-related
mortality, secondary only to lung cancer (14). Although there are numerous
therapies available to treat metastatic breast cancer, poor
responses are observed for the majority of patients with
malignancies (15). Therefore,
current research is focused on relevant targeted therapies
(16). In patients with early
node-positive and -negative breast cancer, the VEGF expression
profile has been observed to be associated with the reduction of
PFS (progression-free survival) and OS (overall survival). Thus
VEGF has been recognized to be an effective target for
anti-angiogenic therapies (17).
Furthermore, the anti-VEGF monoclonal antibody, bevacizumab has
been approved by the FDA to treat breast cancer (18).
However, the results of AVADO and RIBBON-1 trials
demonstrated PFS other than OS could benefit from that the
treatment of bevacizumab in combination with cytotoxic chemotherapy
(19,20). The earlier AVF2119g trial,
evaluating the addition of bevacizumab to capecitabine in first to
fifth-lineMBC (including patients relapsing on therapy),
demonstrated a 10.7% improvement in response rate (P ¼0.001) and no
PFS benefit (21). Importantly,
bevacizumab have side-effects, the most important being
hypertension,proteinuria, bleeding and thromboembolic events. Some
concerns have also been raised that bevacizumab might increase the
rate of heart failure in breast cancer patients (22). Therefore, bevacizumab has limited
utility for the treatment of breast cancer due to its limited
efficacy.. Therefore, bevacizumab has limited utility for the
treatment of breast cancer due to its limited efficacy. The utility
of anti-angiogenic therapy in the treatment of breast cancer
requires further evaluation. Investigation into the mechanism
underlying anti-angiogenic therapies in breast cancer may
facilitate the development and application of targeted therapies
and individualized treatment.
As a multifunctional non-tyrosine kinase receptor,
NRP-1 is expressed in arterial endothelial cells and tumor cells.
NRP-1 expression profiles have been correlated with the progress
and prognosis of malignancies, while NRP-2 is predominantly
expressed in veins and lymphatic endothelium (23–25).
In 1998, Soker et al (8)
purified NRP-1 from human breast cancer cell lines and disclosed
the important roles of NRP-1 in the metastatic process of breast
cancer. Ghosh et al (23)
demonstrated that NRP-1 was highly expressed in metastatic breast
cancer. Another study also demonstrated that the NRP-1 expression
profile of breast cancer cell lines with high metastatic potential
in vitro was higher than that in lines with low metastatic
potential (12). High expression
of NRP-1 and VEGF in breast tissues was closely associated with
poor prognosis (23). This result
suggested that NRP-1 may be important for the biological behavior
of breast cancer and it has been proposed to be a novel target for
anti-angiogenic and individual therapies. Therefore, further
investigation into the association between NRP-1 and the biological
behavior of breast cancer.
In this study, MCF-7 and SK-BR-3 breast cancer cell
lines with high NRP-1 expression were selected. Lentiviral vectors
encoding the human NRP-1 gene were successfully constructed based
on lentiviral vectors carrying interference sequences. Highly
efficient NRP-1/shRNA interference sequences were selected. The
suppression of NRP-1 mRNA and protein mediated by NRP-1/shRNA
lentivirus resulted in inhibition of the proliferation of breast
cancer cells in vitro and promotion of apoptosis. The
results of this study were consistent with those reported in the
literature. Ochiumi et al (26) demonstrated the correlation between
the poor prognosis of colon cancer and high NRP-1 expression, and
NRP-1 interference was observed to suppress the migration of colon
cancer cells and induce apoptosis. Another study also demonstrated
that the post-NRP-1 interference inhibition of proliferation and
induction of apoptosis observed in glioma cells may be closely
associated with the decrease of Bcl-2 family expression and
inactivation of cancer development related signaling pathways, such
as extracellular signal-regulated kinases, c-Jun N-terminal kinases
and mitogen-activated protein kinase (27). However, the mechanisms of
proliferation inhibition and apoptosis induction observed in breast
cancer cells remain to be elucidated.
Chemotherapy containing anthracyclines are important
regimens in the treatment of breast cancer. Myelotoxicity and
cardiotoxicity have been identified to be the dose-limiting
toxicities (DLT) of these medications. These deleterious effects
(particularly cardiotoxicity) also restricted the improvement of
their clinical efficacy. Several studies demonstrated that high
NRP-1 expression has been recognized to be an independent
prognostic factor for breast cancer. Therefore, whether concomitant
administration of anthracyclines could increase the sensitivity to
chemotherapy following NRP-1 interference required further
investigation. As shown by this study, the apoptotic rate was
significantly higher than that of control group after concomitant
interventions of NRP-1 interference and epirubicin. This result
suggested that NRP-1 interference contributed to the increase of
sensitivity to epirubicin treatment. However, whether the NRP-1
targeted strategy targeting may increase the sensitivity of
epirubicin resistance cell lines to chemotherapies and whether the
synergic effects may exist when epirubicin is administered in
combination with other chemotherapeutic agents required further
investigation.
In conclusion, MCF-7 and SK-BR-3 cells with high
NRP-1 protein expression were identified in this study. Lentiviral
expression vectors encoding the NRP-1 gene were constructed
successfully using lentiviral vectors. RNA interference
significantly reduced the expression of NRP-1. In addition, NRP-1
silencing suppressed the proliferation of MCF-7 and SK-BR-3 cells,
promoted apoptosis and increased their sensitivity to epirubicin
chemotherapy. This evidence provides a theoretical basis for NRP-1
targeted chemotherapies against breast cancer. In this study, the
effects of NRP-1 over-expression on the biological behavior of
breast cancer have been investigated. As the co-receptor of
VEGF165, NRP-1 is highly expressed in breast cancer and the
signaling pathway of VEGFR mediated by VEGF has been extensively
investigated; however, the NRP-1 signaling pathway mediated by VEGF
requires further elucidation.
Acknowledgments
The authors would like to thank the Hematology
Laboratory of Soochow University (Suzhou, China) for their
assistance in sorting GFP-positive cells.
References
|
1
|
Yi W, Peng J, Zhang Y, et al: Differential
protein expressions in breast cancer between drug sensitive tissues
and drug resistant tissues. Gland Surg. 2:62–68. 2013.PubMed/NCBI
|
|
2
|
Barrett SV: Breast cancer. J R Coll
Physicians Edinb. 40:335–338. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Perez EA and Spano JP: Current and
emerging targeted therapies for metastatic breast cancer. Cancer.
118:3014–3025. 2012. View Article : Google Scholar
|
|
4
|
Satoda M, Takagi S, Ohta K, et al:
Differential expression of two cell surface proteins, neuropilin
and plexin, in Xenopus olfactory axon subclasses. J Neurosci.
15:942–955. 1995.PubMed/NCBI
|
|
5
|
Jubb AM, Strickland LA, Liu SD, et al:
Neuropilin-1 expression in cancer and development. J Pathol.
226:50–60. 2012. View Article : Google Scholar
|
|
6
|
Soker S, Takashima S, Miao HQ, Neufeld G
and Klagsbrun M: Neuropilin-1 is expressed by endothelial and tumor
cells as an isoform-specific receptor for vascular endothelial
growth factor. Cell. 92:735–745. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Miao HQ, Lee P, Lin H, Soker S and
Klgsbrun M: Neuropilin-1 expression by tumor cells promotes tumor
angiogenesis and progression. FASEB J. 14:2532–2539. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Soker S, Takashima S, Miao HQ, et al:
Neuropilin-1 is expressed by endothelial and tumor cells as an
isoform-specific receptor for vascular endothelial growth factor.
Cell. 92:735–745. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Guttmann-Raviv N, Kessler O, Shraga-Heled
N, et al: The neuropilins and their role in tumorigenesis and tumor
progression. Cancer Lett. 231:1–11. 2006. View Article : Google Scholar
|
|
10
|
Karjalainen K, Jaalouk DE, Bueso-Ramos CE,
et al: Targeting neuropilin-1 in human leukemia and lymphoma.
Blood. 117:920–927. 2011. View Article : Google Scholar
|
|
11
|
Pan Q, Chanthery Y, Liang WC, et al:
Blocking neuropilin-1 function has an additive effect with
anti-VEGF to inhibit tumor growth. Cancer Cell. 11:53–67. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang ZL, Tang ZC, Zhang Y, et al:
Neuropilin-1 down-regulation impairs cell migration and induces the
differentiation of human tongue squamous cell carcinoma cells. Head
Neck Oncol. 4:542012.
|
|
13
|
Cao J, Meng FJ, Li L, et al: Expression of
NANOG gene in acute lymphoblastic leukemia cells and construction
of lentiviralvector carrying NANOG specific shRNA. Zhongguo Shi Yan
Xue Ye Xue Za Zhi. 22:275–279. 2014.In Chinese. PubMed/NCBI
|
|
14
|
Jha K, Shukla M and Pandey M: Survivin
expression and targeting in breast cancer. Surg Oncol. 21:125–131.
2012. View Article : Google Scholar
|
|
15
|
Alvarez RH, Valero V and Hortobagyi GN:
Emerging targeted therapies for breast cancer. J Clin Oncol.
28:3366–3379. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Koutras AK, Fountzilas G, Makatsoris T, et
al: Bevacizumab in the treatment of breast cancer. Cancer Treat
Rev. 36:75–82. 2010. View Article : Google Scholar
|
|
17
|
Goldfarb SB, Traina TA and Dickler MN:
Bevacizumab for advanced breast cancer. Womens Health (Lond Engl).
6:17–25. 2010. View Article : Google Scholar
|
|
18
|
Alvarez RH, Guarneri V, Icli F, et al:
Bevacizumab treatment for advanced breast cancer. Oncologist.
16:1684–1697. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Miles DW, Chan A, Dirix LY, et al: Phase
III study of bevacizumab plus docetaxel compared with placebo plus
docetaxel for the first-line treatment of human epidermal growth
factor receptor 2-negative metastatic breast cancer. J Clin Oncol.
28:3239–3247. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Robert NJ, Diéras V, Glaspy J, et al:
RIBBON-1: randomized, double-blind, placebo-controlled, phase III
trial of chemotherapy with or without bevacizumab for first-line
treatment of human epidermal growth factor receptor 2-negative,
locally recurrent or metastatic breast cancer. J Clin Oncol.
29:1252–1260. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Miller KD, Chap LI, Holmes FA, et al:
Randomized phase III trial of capecitabine compared with
bevacizumab plus capecitabine in patients with previously
treatedmetastatic breast cancer. J Clin Oncol. 23:792–799. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Choueiri TK, Mayer EL, Je Y, et al:
Congestive heart failure risk inpatients with breast cancer treated
with bevacizumab. J Clin Oncol. 29:632–638. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ghosh S, Sullivan CA, Zerkowski MP, et al:
High levels of vascular endothelial growth factor and its receptors
(VEGFR-1, VEGFR-2, neuropilin-1) are associated with worse outcome
in breast cancer. Hum Pathol. 39:1835–1843. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Herzog Y, Kalcheim C, Kahane N, et al:
Differential expression of neuropilin-1 and neuropilin-2 in
arteries and veins. Mech. 109:115–119. 2001.
|
|
25
|
Yuan L, Moyon D, Pardanaud L, et al:
Abnormal lymphatic vessel development in neuropilin 2 mutant mice.
Development. 129:4797–4806. 2002.PubMed/NCBI
|
|
26
|
Ochiumi T, Kitadai Y, Tanaka S, et al:
Neuropilin-1 is involved in regulation of apoptosis and migration
of human colon cancer. Int J Oncol. 29:105–116. 2006.PubMed/NCBI
|
|
27
|
Li X, Tang T, Lu X, et al: RNA
interference targeting NRP-1 inhibits human glioma cell
proliferation and enhances cell apoptosis. Mol Med Rep.
4:1261–1266. 2011.PubMed/NCBI
|