Introduction
Diabetes is the primary cause of end-stage renal
disease (ESRD), which accounts for almost half of all new patients
diagnosed with ESRD (1). The
pathogenesis of diabetic nephropathy (DN) is complex and
indefinite, involving metabolic disorder, activation of the
renin-angiotensin-aldosterone system, alteration in glomerular
hemodynamics, genetic susceptibility, oxidative stress, an
inflammatory reaction and cytokine overexpression (2–8).
Hyperglycemia has been reported to promote oxidative stress and the
generation of ROS in vivo and in vitro (9,10).
Increased levels of ROS result in direct oxidation and damage to
DNA, protein, lipid and carbohydrate, and are hypothesized to have
a key role in the pathogenesis of chronic diabetic complications
(11,12), particularly DN (2). In addition, ROS have been reported to
function as signaling molecules, mediating high glucose-induced
activation of signal transduction cascades and transcription
factors, which in turns leads to transcriptional activation of
profibrotic genes in the kidney that are essential for the
development and progression of DN (13). A key protective mechanism against
oxidative stress-induced DN is mediated via antioxidant gene
induction and/or oxidative gene inhibition (14). Previous studies have indicated that
p38MAPK is involved in multiple signal transduction channels in the
pathogenesis of DN, activation of which may promote cell
proliferation, growth and differentiation (15,16).
p38MAPK has been reported to be an essential downstream effector of
NADPH oxidase 4 (Nox4) in the signaling pathway that is involved in
high glucose and tubular cell injury (17). Metformin is an aminoguanidine
derived hypoglycemic agent, which is commonly used in the
management of diabetes (18).
Multiple clinical trials have confirmed that metformin is able to
reduce levels of protein oxidative end-products, advanced glycation
end products and ROS, in addition to its hypoglycemic activity
(19–21). However, it has not been previously
reported whether metformin is able to reduce oxidative stress by
inhibiting the activity of p38MAPK. The aim of the present study
was to investigate the inhibitory effects of metformin on high
glucose-induced oxidative stress and p38MAPK expression in rat
glomerular mesangial cells (MCs), in order to elucidate its
underlying reno-protective mechanisms in vitro.
Materials and methods
Materials
Rat glomerular mesangial cells (HBZY-1) were
obtained from China Center for Type Culture Collection (Wuhan,
China). The following material, reagents and kits were used in the
present study: Dulbecco’s modified Eagle’s medium (DMEM; HyClone
Laboratories, Inc., Logan, UT, USA), fetal calf serum (HyClone
Laboratories, Inc.), trypsin (HyClone Laboratories, Inc.),
polyvinylidene fluoride (PVDF) membranes (EMD Millipore, Billerica,
MA, USA), metformin (Sigma-Aldrich, St. Louis, MO, USA),
4-(4-fluorophenyl)-2-(4-methylsulfinylphenyl)-5-(4-pyridyl)-1H-imidazole
(SB203580; Sigma-Aldrich), N-acetylcysteine (NAC; Sigma-Aldrich),
2′,7′-dichloro-f luoresceindiacetate (DCFH-DA; Sigma-Aldrich),
phenylmethanesulfonyl fluoride (Sigma-Aldrich), RevertAid First
Strand cDNA Synthesis kit and PCR Master Mix (Thermo Fisher
Scientific, Waltham, MA, USA), total superoxide dismutase (SOD)
assay kits (Nanjing Jiancheng Bioengineering Institute, Nanjing,
China), ELISA kit for malondialdehyde (MDA) kits (USCN Life Science
Inc., Wuhan, China), p22phox and GAPDH primers (Sangon Biotech Co.,
Ltd., Shanghai, China), MTT
[(3-4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide;
Invitrogen Life Technologies, Carlsbad, CA, USA], TRIzol reagent
(Invitrogen Life Technologies) and electrochemiluminescence (ECL)
color developing reagent (Pierce Biotechnology, Inc., Rockford, IL,
USA). In addition the following primary antibodies were used in the
present study: Rabbit monoclonal primary anti-p38MAPK (1:1,000;
cat. no. 8690S; Cell Signaling Technology, Inc., Danvers, MA, USA),
rabbit monoclonal anti-phosphorylated p38MAPK (p-p38MAPK; 1:1,000;
cat. no. 4631S; Cell Signaling Technology, Inc.), rabbit polyclonal
anti-p22phox (1:500; cat. no. sc-20781; Santa Cruz Biotechnology,
Inc. (Dallas, TX, USA), and monoclonal rabbit GAPDH (1:1,000; cat.
no. 2118S; Cell Signaling Technology, Inc.). Goat anti-rabbit
horseradish peroxidase (HRP)-conjugated secondary antibodies
(1:1,000; cat. no. 7074) were purchased from Cell Signaling
Technology, Inc.
Cell viability MTT assay
Rat MCs in the exponential growth phase were
cultured in complete medium (DMEM supplemented with 10% fetal calf
serum, 100 U/ml penicillin and 100 μg/ml streptomycin; North
China Pharmaceutical Group Corporation, Shijiazhuang, China) and
then seeded into 96-well plates at a density of 5×103
cells/well. Different concentrations of metformin (2.5, 5.0, 10.0
and 20.0 mmol/l) were then added. The viability of cells was
assessed using the MTT assay, as previously described (22) and the half maximal inhibitory
concentration (IC50) of metformin was determined.
Cell culture and specimen collection
Rat MCs were cultured in complete medium (as above)
in a humidified incubator at 37°C and 5% CO2 and were
subcultured every two days. The cells were seeded into 6-well
plates at a density of 1×106 cells/well and were divided
into the following five groups: Normal control (NC; glucose
concentration 5.6 mmol/l), high glucose (HG; glucose concentration
30 mmol/l), metformin-treated (MET; glucose concentration 30 mmol/l
+ metformin final concentration 6.8 mmol/l), SB203580-treated (SB;
glucose concentration 30 mmol/l + SB203580 final concentration 10
μmol/l) and NAC-treated (NAC; glucose concentration 30
mmol/l + NAC final concentration 100 μmol/l). Rat MCs and
the supernatant were collected after centrifugation at 1,500 × g
for 5 min at 20°C, following cell culture for 48 h at 37°C and 5%
CO2.
Detection of intracellular ROS production
in rat MCs by flow cytometry
Intracellular ROS production in rat MCs was analyzed
fluorometrically by detecting the oxidation of the non-fluorescent
probes 2′,7′ -dichloro-fluoresceindiacetate (DCFH) to the
fluorescent metabolites dichlorofluorescein (DCF). Briefly, 10
μM DCFH-DA was added to each well of MCs and the wells were
incubated for 30 min at 37°C. The oxidation of DCFH by ROS was
determined by measuring the percentage of cells marked by DCF in a
minimum of 10,000 cells using a flow cytometer (Cytomics FC 500
MPL; Beckman Coulter, Brea, CA, USA) at excitation and emission
wave lengths of 488 and 525 nm, respectively.
Colorimetric analysis of SOD activity in
supernatant fluid
SOD activity in the supernatant fluid was determined
by the xanthine oxidase method using SOD kits according to the
manufacturer’s instructions. Absorbance values of samples were
determined at 550 nm using an ultraviolet spectrophotometer
(UVmini-1240; Shimadzu Corporation, Kyoto, Japan). According to
enzymatic definition, when the inhibitory ratio of SOD in 1 ml
supernatant reached 50%, the corresponding dose of SOD was
considered a vitality unit, which represents the quantity of enzyme
that can transform 1 μmol substrate in a minute under
certain conditions.
Detection of MDA content in the
supernatant fluid by ELISA
MDA content in the supernatant fluid was determined
using ELISA kits for MDA according to the manufacturer’s
instructions. Absorbance values of samples were measured at 450 nm
using an ultraviolet spectrophotometer (UVmini-1240) and the MDA
content (ng/ml) was determined.
Quantification of p22phox mRNA expression
in rat MCs by reverse transcription-quantitative polymerase chain
reaction (RT-qPCR)
Total RNA was extracted using the TRIzol reagent kit
according to the manufacturer’s instructions and quantified by
measuring the absorbance at 260 nm. RNA quality was then determined
by measuring the 260/280 nm ratio. Subsequently, first-strand cDNA
was synthesized from total RNA using a RevertAid First Strand cDNA
Synthesis kit according to the manufacturer’s instructions. The
sequences of PCR primers used in the assays are as follows: p22phox
forward, 5′-TCC ACT TAC TGC TGT CCG T-3′ and reverse, 5′-TGG TAG
GTG GCT GCT TGA T-3′ (NM_024160; 185 bp); and GAPDH forward, 5′-ACA
GCA ACT CCC ATT CTT C-3′ and reverse, 5′-TGG TCC AGG GTT TCT TAC-3′
(163 bp). RT-qPCR was subsequently performed using SsoFast EvaGreen
Supermix (Bio-Rad Laboratories, Inc., Hercules, USA) and a CFX96™
Real-Time PCR system (Bio-Rad Laboratories, Inc.) according to the
manufacturer’s instructions. Reaction conditions for the PCR were
as follows: Enzyme activation at 95°C for 30 sec followed by 35
cycles at 95°C for 5 sec, 56°C for 5 sec and 72°C for 5 sec. The
threshold cycle (Ct) value for each gene was normalized to the Ct
value of GAPDH. The relative mRNA expression was calculated using
the comparative Ct (2−ΔΔCt) method as previously
described (23,24).
Detection of p22phox and p-p38MAPK
protein expression in rat MCs by western blot analysis
Rat MCs were rinsed twice with ice-cold
phosphate-buffered saline (Bailunsi Biotechnology Co., Ltd.,
Tianjin, China) and lysed on ice with Cellytic M lysis buffer
(Sigma-Aldrich, St. Louis, MO, USA) supplemented with protease
inhibitors (Sigma-Aldrich) and phosphorylase inhibitors
(Phosphatase Inhibitor Cocktail 2; Sigma-Aldrich) for 30 min.
Following centrifugation at 12,000 x g for 5 min at 4°C, total cell
lysate extracts were collected and the protein content was
determined using the protein assay (Beyotime Institute of
Biotechnology, Shanghai, China). A total of 50 μg total
proteins were separated using SDS-PAGE and transferred onto PVDF
membranes. The membranes were blocked with 5% non-fat milk in
Tris-buffered saline containing 0.05% Tween 20 (Bailunsi
Biotechnology Co., Ltd.) for 1 h and were then incubated with
p22phox, GAPDH, p38MAPK or p-p38MAPK monoclonal antibodies at 4°C
overnight, followed by incubation with a HRP-conjugated secondary
antibody for 1 h. Immunoblots were developed using ECL color
developing reagent and the chemiluminescence image analysis system
(Tanon 5500 Gel Imaging System; Tanon Science and Technology Co.,
Ltd, Shanghai, China) was used to quantitatively analyze the
immu-noblot results. The band intensity ratio of p22phox to GAPDH
and p-p38MAPK to p38MAPK represented the p22phox and p-p38MAPK
protein relative content respectively, while GAPDH or p38MAPK
served as the internal reference.
Statistical analysis
A minimum of three repeats were conducted for each
experiment. All data are expressed as the mean ± standard deviation
and were analyzed with an analysis of variance using SPSS software,
version 13.0 (SPSS, Inc., Chicago, IL, USA). For experiments in
which only single experimental and control groups were used, the
difference between groups was examined using an unpaired Student’s
t-test. P<0.05 was considered to indicate a statistically
signifi-cant difference.
Results
ROS production in rat MCs
Compared with that of the NC group, ROS production
in rat MCs was significantly increased in the HG group (P<0.05).
In addition, ROS production was significantly reduced in the MET,
SB and NAC groups, compared with that of the HG group (P<0.05)
(Fig. 1).
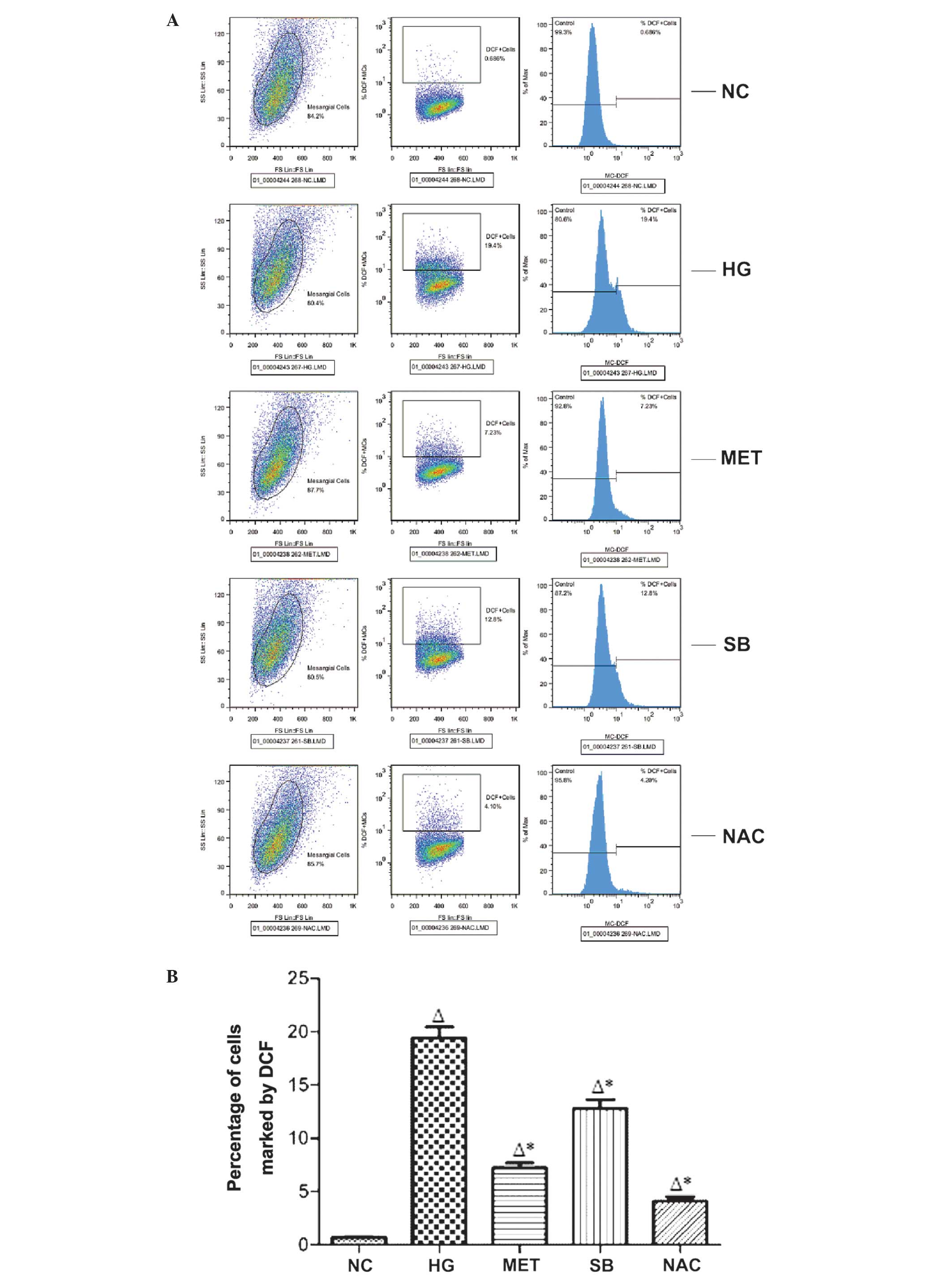 | Figure 1Flow cytometric analysis of rective
oxygen species production in different experimental and control
groups. (A) The oxidation of the non-fluorescent probes DCFH to the
fluorescent metabolites DCF in every group. (B) The percentage of
cells marked by DCF in a minimum of 10,000 cells in every group.
The values are expressed as the mean ± standard deviation
(∆P<0.05, vs. NC group; *P<0.05, vs. HG
group). HG, high glucose; MET, metformin-treated; SB,
SB203580-treated; NAC, N-acetylcysteine-treated; NC, normal
control; DCF, dichlorofuorescein; DCFH,
2′,7′-dichloro-fluoresceindiacetate. |
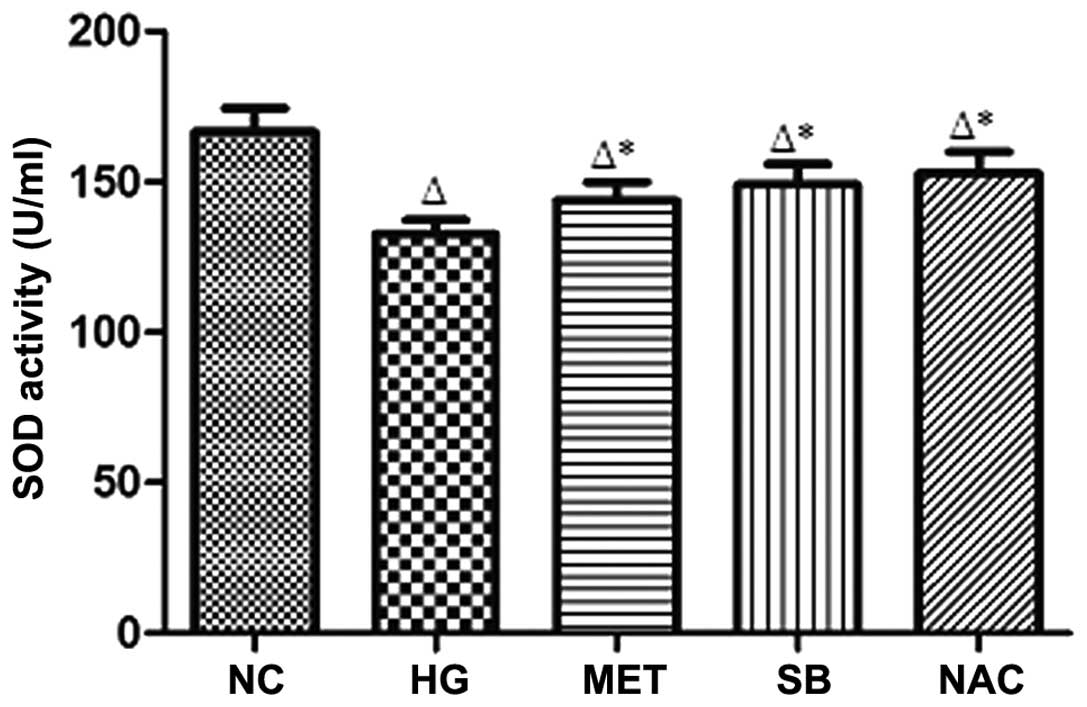
SOD activity in supernatant fluid
Compared with that of the NC group, SOD activity in
the supernatant was significantly reduced in the HG group
(P<0.05). By contrast, SOD activity was significantly higher in
the MET, SB and NAC groups, compared with that of the HG group
(P<0.05) (Fig. 2).
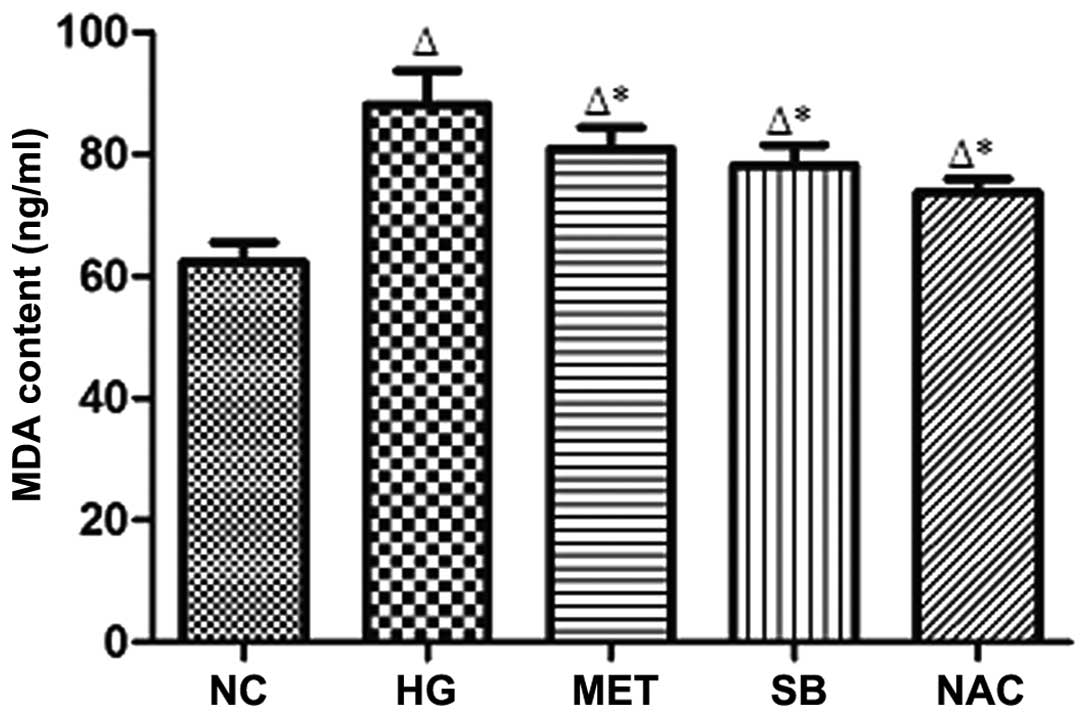
MDA content in the supernatant fluid
Compared with that of the NC group, MDA content in
the supernatant was signifi-cantly increased in the HG group
(P<0.05), whereas MDA content was significantly reduced in the
supernatant of the MET, SB and NAC groups compared with that of the
HG group (*P<0.05) (Fig.
3).
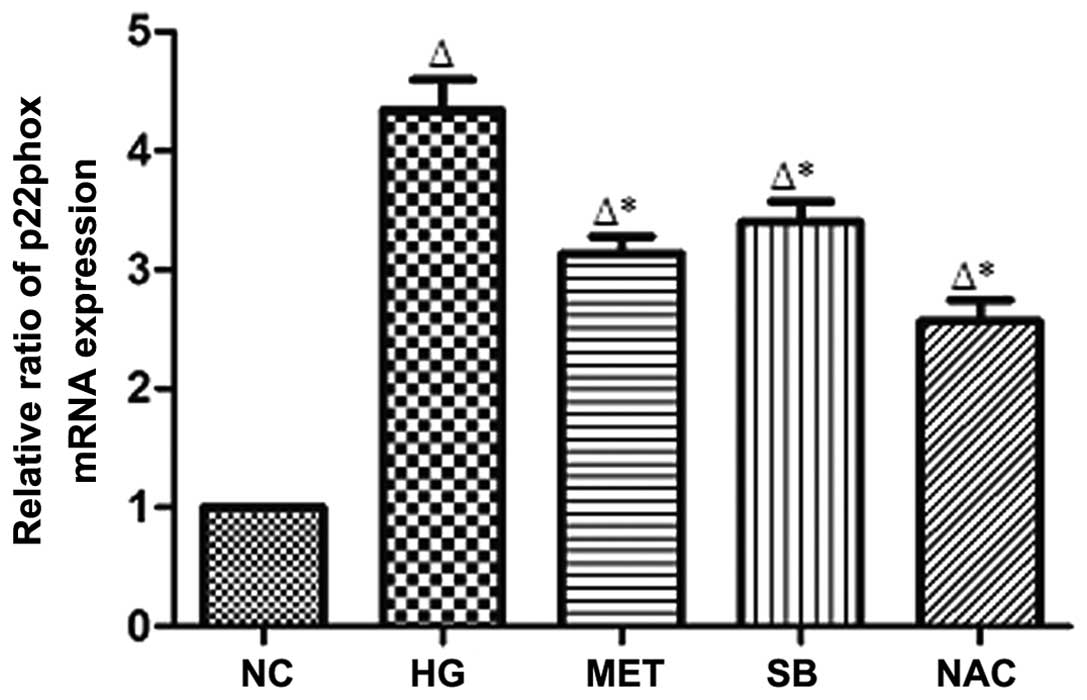
p22phox mRNA expression in rat MCs
Compared with that of the NC group, p22phox mRNA
expression in rat MCs was identified to be significantly increased
in the HG group (P<0.05); however, p22phox mRNA expression was
significantly reduced in the MET, SB and NAC groups compared with
that of the HG group (P<0.05) (Fig.
4).
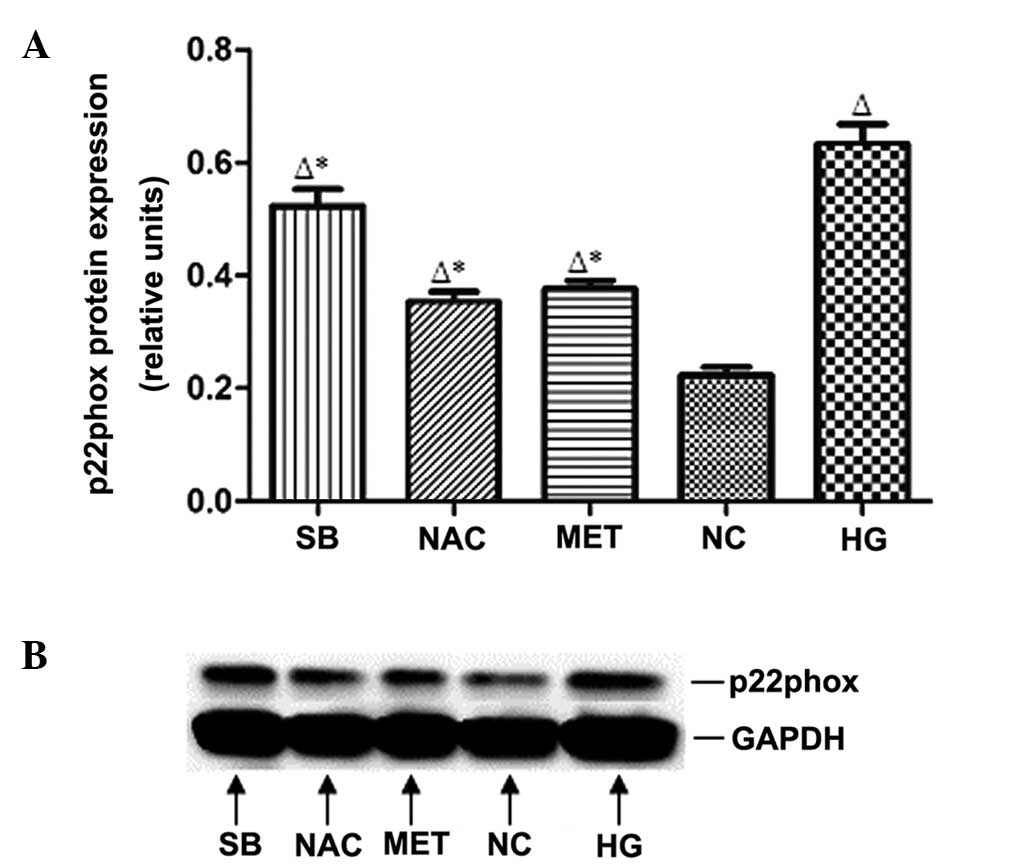
p22phox protein expression in rat
MCs
Compared with that of the NC group, p22phox protein
expression in rat MCs was significantly increased in the HG group
(P<0.05). By contrast, p22phox protein expression was
significantly reduced in the MET, SB and NAC groups compared with
that of the HG group (P<0.05) (Fig.
5).
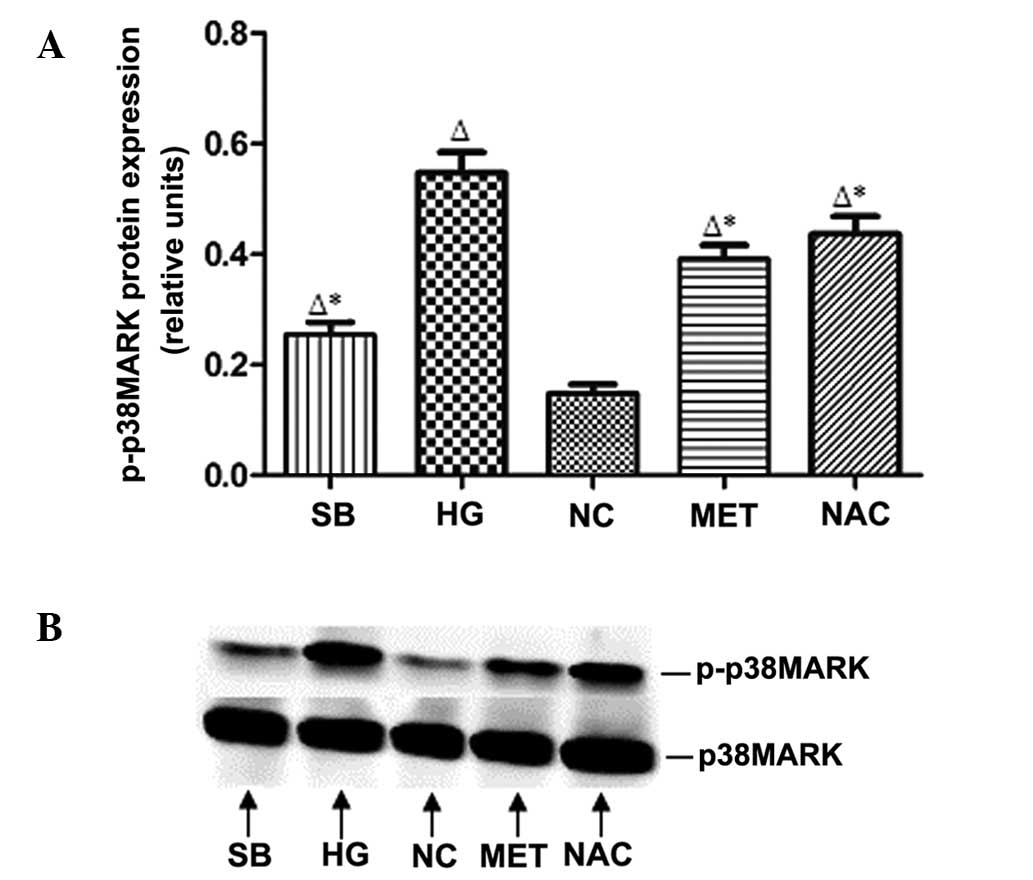
p-p38MAPK protein expression in rat
MCs
Compared with that of the NC group, p-p38MAPK
protein expression in rat MCs was observed to increase in the HG
group (P<0.05); however, p-p38MAPK protein expression was
significantly reduced in the MET, SB and NAC groups compared with
that of the HG group (P<0.05) (Fig.
6).
Discussion
Oxidative stress has been reported to be an
important factor involved in the pathogenesis of diabetic
complications, such as DN (25–29).
Diabetes is associated with an increase in the generation of ROS in
the kidney (25,27–29),
which is involved in the accumulation of extracellular matrix in
the MCs and glomerulosclerosis. Although there are various sources
of ROS in cells and tissues, the major sources of ROS in renal
cells were found to be the mitochondrial electron transport chain
(30,31) and the Nox family (32,33).
Nox4 and p22phox have been identified to be involved in
high-glucose-dependent oxidative stress and fibronectin or collagen
IV accumulation in MCs (34,35).
p22phox mRNA and protein expression were observed to be upregulated
by high glucose in these cells in addition to glomeruli from
diabetic animals (34,35). Xia et al (36) additionally reported that
high-glucose-induced oxidative stress involved the ROS production
by p22phox-based Nox, which occurs due to the upregulation of
p22phox protein in MCs. Increased cellular oxidative stress was
previously demonstrated to act as a second messenger for cellular
signaling pathways, which in turn activates numerous
redox-sensitive transcription factors, and results in cell membrane
damage and the inactivation of enzymes (37). Human (2) and animal (38) studies have reported a significant
reduction in the levels of SOD and catalase were observed in
uncontrolled diabetes, whereas serum levels of MDA were found to be
increased. In the current study, the activity of SOD in the
supernatant of rat MCs was significantly reduced, whereas the level
of MDA, intracellular ROS production, p22phox mRNA and protein
expression were all identified to increase when rat MCs were
cultured in high glucose. This indicated that high glucose induced
oxidative stress.
Previous studies have demonstrated that metformin,
an oral hypoglycemic drug, exhibits antioxidative effects (19,39).
A direct scavenging effect of metformin has also been reported,
with studies identifying that metformin acts to remove oxygenated
free radicals and ROS generated in aortic endothelial cells, the
mechanism of which was found to proceed via the reduction of Nox
and/or mitochondrial respiratory chain pathways (40,41).
In the current study, in vitro administration of metformin
to cultured rat MCs significantly reversed the high
glucose-mediated over-production of MDA and intracellular ROS,
reversed the overexpression of p22phox mRNA and protein levels as
well as improved SOD activity. These results suggested that
metformin may act to reduce oxidative stress in high
glucose-stimulated rat MCs, thus leading to reno-protection, which
is in agreement with the results of previous studies (39,42).
Nishida et al (43) reported that ROS are able to induce
the activation of p38MAPK in rat neonatal cardiomyocytes, which was
in agreement with an additional study using 3T3-L1 adipose cells
(44). ROS are also able to
promote the activation of p38MAPK in rat MCs cultured in a high
glucose medium, which was reversed by antioxidants (45). Song et al (46) identified that SB203580, a p38MAPK
inhibitor, reduced MDA content and enhanced SOD activity in rats
with spinal cord injury (46). In
addition, various previous studies have observed that exposure of
cells to H2O2 results in the activation of
p38MAPK, which in turn mediates ROS-induced senescence and
oxidative stress-induced autophagy (47–49).
The current study observed that oxidative stress marker expression
levels and p-p38MAPK protein in rat MCs cultured in a high glucose
medium were increased compared with those of the NC group. These
effects were reduced by the addition of SB203580 (p38MAPK
inhibitor) and NAC (antioxidant), indicating that ROS promotes
activation of p38MAPK in rat MCs, which in turn mediates oxidative
stress.
Bae et al (50) reported that metformin resulted in
the phosphorylation of p38MAPK in NCI-H292 airway epithelial cells,
while an additional study demonstrated that high-dose metformin
increased phosphorylation of p38MAPK protein (51). However, metformin has been observed
to reduce paclitaxel-elicited p38MAPK phosphorylation in non-small
cell lung cancer cells (52).
Kappe et al (53) also
confirmed that metformin significantly reduces the expression of
p38MAPK in glucagon-like peptide-1-secreting cells. The present
study demonstrated that metformin alleviated the phosphorylation of
p38MAPK protein in rat MCs, which may be partly responsible for its
effect in inhibiting oxidative stress in rat MCs stimulated by high
glucose.
In conclusion, the current study demonstrated that
oxidative stress results in activation of the p38MAPK signaling
pathway, which amplifies its cascade reaction to in turn stimulate
oxidative stress. Metformin was suggested to be able to alleviate
oxidative stress and phosphorylation of p38MAPK protein in MCs
cultured with high glucose, which may explain the preventative
ability of metformin in DN.
Acknowledgments
The current study was supported by grants from the
Natural Science Foundation of Anhui Province (grant no.
11040606M159) and Anhui Province Natural Science Research Project
of China (grant no. KJ2011A157).
References
|
1
|
Collins AJ, Kasiske B, Herzog C, et al:
Excerpts from the United States renal data system 2006 annual data
report. Am J Kidney Dis. 49(Suppl 1): S1–S296. 2007. View Article : Google Scholar
|
|
2
|
Pan HZ, Zhang L, Guo M, et al: The
oxidative stress status in diabetes mellitus and diabetic
nephropathy. Acta Diabetol. 47(Suppl 1): 71–76. 2010. View Article : Google Scholar
|
|
3
|
Kiss E, Kränzlin B, Wagenblaβ K, et al:
Lipid droplet accumulation is associated with an increase in
hyperglycemia-induced renal damage: Prevention by liver X
receptors. Am J Pathol. 182:727–741. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zain M and Awan FR: Renin Angiotensin
Aldosterone System (RAAS): Its biology and drug targets for
treating diabetic nephropathy. Pak J Pharm Sci. 27:1379–1391.
2014.PubMed/NCBI
|
|
5
|
Bhaskar LV, Mahin S, Ginila RT and
Soundararajan P: Role of the ACE ID and PPARG P12A polymorphisms in
genetic susceptibility of diabetic nephropathy in a South Indian
population. Nephrourol Mon. 5:813–817. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Duran-Salgado MB and Rubio-Guerra AF:
Diabetic nephropathy and inflammation. World J Diabetes. 5:393–398.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zikou X, Tellis CC, Rousouli K, Dounousi
E, Siamopoulos KC and Tselepis AD: Differential membrane expression
of toll-like receptors and intracellular cytokine induction in
peripheral blood monocytes of patients with chronic kidney disease
and diabetic nephropathy. Nephron Clin Pract. 128:399–406. 2014.
View Article : Google Scholar
|
|
8
|
Ots M, Pechter U and Tamm A:
Characteristics of progressive renal disease. Clin Chim Acta.
297:29–41. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kumar P, Rao GN, Pal BB and Pal A:
Hyperglycemia-induced oxidative stress induces apoptosis by
inhibiting PI3-kinase/Akt and ERK1/2 MAPK mediated signaling
pathway causing down-regulation of 8-oxoG-DNA glycosylase levels in
glial cells. Int J Biochem Cell Biol. 53:302–319. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jayakumar P, Pugalendi KV and Sankaran M:
Attenuation of hyperglycemia -mediated oxidative stress by
indole-3-carbinol and its metabolite 3, 3′-diindolylmethane in
C57BL/6J mice. J Physiol Biochem. 70:525–534. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Swaminathan S and Shah SV: Novel
approaches targeted toward oxidative stress for the treatment of
chronic kidney disease. Curr Opin Nephrol Hypertens. 17:143–148.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rösen P, Nawroth PP, King G, Möller W,
Tritschler HJ and Packer L: The role of oxidative stress in the
onset and progression of diabetes and its complications: a summary
of a congress series sponsored by UNESCO-MCBN, the American
Diabetes Association and the German Diabetes Society. Diabetes
Metab Res Rev. 17:189–212. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ha H, Hwang IA, Park JH and Lee HB: Role
of reactive oxygen species in the pathogenesis of diabetic
nephropathy. Diabetes Res Clin Pract. 82(Suppl 1): S42–S45. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li H, Zhang L, Wang F, et al: Attenuation
of glomerular injury in diabetic mice with tertbutylhydroquinone
through nuclear factor erythroid 2-related factor 2-dependent
antioxidant gene activation. Am J Nephrol. 33:289–297. 2011.
View Article : Google Scholar
|
|
15
|
Knizhnik AV, Kovaleva OV, Komelkov AV, et
al: Arf6 promotes cell proliferation via the PLD-mTORC1 and p38MAPK
pathways. J Cell Biochem. 113:360–371. 2012. View Article : Google Scholar
|
|
16
|
Lee YL, Chen CW, Liu FH, Huang YW and
Huang HM: Aclacinomycin A sensitizes K562 chronic myeloid leukemia
cells to imatinib through p38MAPK-mediated erythroid
differentiation. PLoS One. 8:e619392013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sedeek M, Callera G, Montezano A, et al:
Critical role of Nox4-based NADPH oxidase in glucose-induced
oxidative stress in the kidney: implications in type 2 diabetic
nephropathy. Am J Physiol Renal Physiol. 299:F1348–F1358. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mediavilla Bravo JJ: Guidelines for the
management of diabetes mellitus type 2. Semergen. 40:11–18. 2014.In
Spanish. View Article : Google Scholar
|
|
19
|
Chakraborty A, Chowdhury S and
Bhattacharyya M: Effect of metformin on oxidative stress,
nitrosative stress and inflammatory biomarkers in type 2 diabetes
patients. Diabetes Res Clin Pract. 93:56–62. 2011. View Article : Google Scholar
|
|
20
|
Esteghamati A, Eskandari D, Mirmiranpour
H, Noshad S, Mousavizadeh M, Hedayati M and Nakhjavani M: Effects
of metformin on markers of oxidative stress and antioxidant reserve
in patients with newly diagnosed type 2 diabetes: A randomized
clinical trial. Clin Nutr. 32:179–185. 2013. View Article : Google Scholar
|
|
21
|
Abdulkadir AA and Thanoon IA: Comparative
effects of glibenclamide and metformin on C-reactive protein and
oxidant/antioxidant status in patients with type II diabetes
mellitus. Sultan Qaboos Univ Med J. 12:55–61. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Marks DC, Belov L, Davey MW, Davey RA and
Kidman AD: The MTT cell viability assay for cytotoxicity testing in
multidrug-resistant human leukemic cells. Leuk Res. 16:1165–1173.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Schmittgen TD and Livak KJ: Analyzing
real-time PCR data by the comparative C(T) method. Nat Protoc.
3:1101–1108. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
25
|
Forbes JM, Coughlan MT and Cooper ME:
Oxidative stress as a major culprit in kidney disease in diabetes.
Diabetes. 57:1446–1454. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Giacco F and Brownlee M: Oxidative stress
and diabetic complications. Circ Res. 107:1058–1070. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kashihara N, Haruna Y, Kondeti VK and
Kanwar YS: Oxidative stress in diabetic nephropathy. Curr Med Chem.
17:4256–4269. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Singh DK, Winocour P and Farrington K:
Oxidative stress in early diabetic nephropathy: fueling the fire.
Nat Rev Endocrinol. 7:176–184. 2011. View Article : Google Scholar
|
|
29
|
Stanton RC: Oxidative stress and diabetic
kidney disease. Curr Diab Rep. 11:330–336. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kiritoshi S, Nishikawa T, Sonoda K, et al:
Reactive oxygen species from mitochondria induce cyclooxygenase-2
gene expression in human mesangial cells: potential role in
diabetic nephropathy. Diabetes. 52:2570–2577. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kitada M, Kume S, Imaizumi N and Koya D:
Resveratrol improves oxidative stress and protects against diabetic
nephropathy through normalization of Mn-SOD dysfunction in
AMPK/SIRT1-independent pathway. Diabetes. 60:634–643. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Barnes JL and Gorin Y: Myofibroblast
differentiation during fibrosis: role of NAD(P)H oxidases. Kidney
Int. 79:944–956. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bedard K and Krause KH: The NOX family of
ROS-generating NADPH oxidases: physiology and pathophysiology.
Physiol Rev. 87:245–313. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Xia L, Wang H, Goldberg HJ, Munk S, Fantus
IG and Whiteside CI: Mesangial cell NADPH oxidase upregulation in
high glucose is protein kinase C dependent and required for
collagen IV expression. Am J Physiol Renal Physiol. 290:F345–F356.
2006. View Article : Google Scholar
|
|
35
|
Zhang L, Pang S, Deng B, et al: High
glucose induces renal mesangial cell proliferation and fibronectin
expression through JNK/NF-κB/NADPH oxidase/ROS pathway, which is
inhibited by resveratrol. Int J Biochem Cell Biol. 44:629–638.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Xia L, Wang H, Munk S, Kwan J, Goldberg
HJ, Fantus IG and Whiteside CI: High glucose activates PKC-zeta and
NADPH oxidase through autocrine TGF-beta1 signaling in mesangial
cells. Am J Physiol Renal Physiol. 295:F1705–F1714. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Korashy HM and El-Kadi AO: The role of
aryl hydrocarbon receptor and the reactive oxygen species in the
modulation of glutathione transferase by heavy metals in murine
hepatoma cell lines. Chem Biol Interact. 162:237–248. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Nam SM, Lee MY, Koh JH, et al: Effects of
NADPH oxidase inhibitor on diabetic nephropathy in OLETF rats: the
role of reducing oxidative stress in its protective property.
Diabetes Res Clin Pract. 83:176–182. 2009. View Article : Google Scholar
|
|
39
|
Alhaider AA, Korashy HM, Sayed-Ahmed MM,
Mobark M, Kfoury H and Mansour MA: Metformin attenuates
streptozotocin-induced diabetic nephropathy in rats through
modulation of oxidative stress genes expression. Chem Biol
Interact. 192:233–242. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Bellin C, de Wiza DH, Wiernsperger NF and
Rösen P: Generation of reactive oxygen species by endothelial and
smooth muscle cells: influence of hyperglycemia and metformin. Horm
Metab Res. 38:732–739. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Mahrouf M, Ouslimani N, Peynet J, et al:
Metformin reduces angiotensin-mediated intracellular production of
reactive oxygen species in endothelial cells through the inhibition
of protein kinase C. Biochem Pharmacol. 72:176–183. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ouslimani N, Peynet J, Bonnefont-Rousselot
D, Thérond P, Legrand A and Beaudeux JL: Metformin decreases
intracellular production of reactive oxygen species in aortic
endothelial cells. Metabolism. 54:829–834. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Nishida M, Tanabe S, Maruyama Y, et al: G
alpha 12/13- and reactive oxygen species-dependent activation of
c-Jun NH2-terminal kinase and p38 mitogen-activated protein kinase
by angiotensin receptor stimulation in rat neonatal cardiomyocytes.
J Biol Chem. 280:18434–18441. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zarrouki B, Soares AF, Guichardant M,
Lagarde M and Géloën A: The lipid peroxidation end-product 4-HNE
induces COX-2 expression through p38MAPK activation in 3T3-L1
adipose cell. FEBS Lett. 581:2394–2400. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Nowak JZ and Wiktorowska-Owczarek A:
Neovascularization in ocular tissues: mechanisms and role of
proangiogenic and antiangiogenic factors. Klin Oczna. 106:90–97.
2004.In Polish.
|
|
46
|
Song Y, Liu J, Zhang F, Zhang J, Shi T and
Zeng Z: Antioxidant effect of quercetin against acute spinal cord
injury in rats and its correlation with the p38MAPK/iNOS signaling
pathway. Life Sci. 92:1215–1221. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Luo Y, Zou P, Zou J, Wang J, Zhou D and
Liu L: Autophagy regulates ROS-induced cellular senescence via p21
in a p38 MAPKα dependent manner. Exp Gerontol. 46:860–867. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Frippiat C, Dewelle J, Remacle J and
Toussaint O: Signal transduction in H2O2-induced senescence-like
phenotype in human diploid fibroblasts. Free Radic Biol Med.
33:1334–1346. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Tamagno E, Robino G, Obbili A, Bardini P,
Aragno M, Parola M and Danni O: H2O2 and 4-hydroxynonenal mediate
amyloid-induced neuronal apoptosis by activating JNKs and p38MAPK.
Exp Neurol. 180:144–155. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Bae CH, Kim JW, Ye SB, Song SY, Kim YW,
Park SY and Kim YD: AMPK induces MUC5B expression via p38 MAPK in
NCI-H292 airway epithelial cells. Biochem Biophys Res Commun.
409:669–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Hauton D: Does long-term metformin
treatment increase cardiac lipoprotein lipase? Metabolism.
60:32–42. 2011. View Article : Google Scholar :
|
|
52
|
Tseng SC, Huang YC, Chen HJ, et al:
Metformin-mediated down-regulation of p38 mitogen-activated protein
kinase-dependent excision repair cross-complementing 1 decreases
DNA repair capacity and sensitizes human lung cancer cells to
paclitaxel. Biochem Pharmacol. 85:583–594. 2013. View Article : Google Scholar
|
|
53
|
Kappe C, Holst JJ, Zhang Q and Sjöholm A:
Molecular mechanisms of lipoapoptosis and metformin protection in
GLP-1 secreting cells. Biochem Biophys Res Commun. 427:91–95. 2012.
View Article : Google Scholar : PubMed/NCBI
|