Introduction
Cardiovascular diseases associated with oxidative
stress, including atherosclerosis, have become the leading cause of
mortality worldwide (1). The
accelerated proliferation and migration of vascular smooth muscle
cells (VSMCs) are the predominant characteristics of atherogenesis,
and endothelial dysfunction is a major risk factor for the
pathogenesis of atherosclerosis (2). Low-density-lipoprotein
(LDL)-cholesterol is a clear predictor of cardiovascular disease
and platelet-derived growth factor (PDGF), secreted by macrophages,
exacerbates atherogenesis-stimulated VSMC proliferation and plaque
neovascularisation (3).
Podocalyxin (PODXL), a type I member of the cluster of
differentiation 34 family of sialomucins, was originally observed
in the epithelial cells of renal glomeruli (podocytes), which acted
as a marker for the progression of kidney inflammatory diseases
(4,5). PODXL is also observed in vascular
endothelium and in breast cancer, in which the expression levels
appear to correlate with the metastatic capacity (6). A previous study reported that the
overexpression of PODXL in Chinese hamster ovary cells increase
their adhesion, migration and cellular interaction; indicating that
PODXL acts as a pro-adhesive molecule (7). However, PODXL has also been reported
to confer cell anti-adhesive properties in ovarian carcinoma
(8). Despite this, it has been
suggested that PODXL is associated with cell adhesion and migration
(9,10), indicating that it may be important
in atherosclerosis.
Micro (mi)RNAs are 22 nucleotides long, non-coding
RNA molecules, which can regulate gene expression either through
translational inhibition or destabilization of target mRNA
(11). Emerging evidence has
revealed the importance of miRNAs in the cardiovascular system and
a previous study demonstrated that let-7g miRNA downregulated the
expression of the lectin-type oxidized LDL receptor-1 (Lox-1)
resulting in the inhibition of vascular endothelial cell
proliferation and migration (12).
miRNA (miR)-21 has a pro-proliferative and anti-apoptotic effect on
VSMCs and is involved in arteriosclerosis obliterans by targeting
tropomyosin 1 (13). A previous
study demonstrated, using an miRNA microarray analysis, that
miR-125b was significantly downregulated in arteriosclerosis
obliterans (14). However, its
precise function in atherosclerosis obliterans remains to be
elucidated.
The present study aimed to investigate the role of
miR-125b in atherosclerosis obliterans and examine the association
between miR-125b and PODXL.
Materials and methods
Cell culture and treatment
HUVECs and HAVSMCs were obtained from American Type
Culture Collection (ATCC; Manassas, VA, USA) and cultivated in
ATCC-formulated F-12K basic medium. The HUVECs were cultured in the
complete growth medium supplemented with 10% fetal bovine serum
(FBS; Gibco Life Technologies, Carlsbad, CA, USA), 0.1 mg/ml
heparin (Sigma-Aldrich, St. Louis, MO, USA) and 0.04 mg/ml
endothelial cell growth supplement (ECGS; Sigma-Aldrich). The
HAVSMCs were cultured in complete growth medium supplemented with
10% FBS, 0.05 mg/ml ascorbic acid (Sigma-Aldrich), 0.01 mg/ml
insulin (Sigma-Aldrich), 0.01 mg/ml transferrin (Sigma-Aldrich), 10
ng/ml sodium selenite (Sigma-Aldrich), 0.03 mg/ml ECGS, 10 mM HEPES
(Sigma-Aldrich) and 10 mM triethylsilane (Sigma-Aldrich). All the
cells were cultured in 95% air and 5% CO2 at 37°C.
The HUVECs and HAVSMCs were treated with ox-LDL (75
μg/ml; Union Biotechnology, Hangzhou, China) and PDGF-BB (20
ng/ml; Union Biotechnology) for 24 or 48 h at 37°C, to create a
model of arteriosclerotic-like pathology. Ectopic expression of
miR-125b in the cells was achieved by transfection with miR-125b
mimics (GeneCopoeia, Inc. Rockville, MD, USA) using Lipofectamine
2000 (Invitrogen Life Technologies, Carlsbad, CA, USA). The pre-
and anti-scramble controls were obtained from GeneCopoeia, Inc. The
HUVECs and HAVSMCs were plated into six-well plates (Nest
Biotechnology Co., Ltd., Shanghai, China) at a density of
1×105 cells/ml and transfected for 24 or 48 h. These
transfected cells were used for total RNA or protein extraction and
further analysis.
Reagents
Rabbit polyclonal antibodies against VE-cadherin
(1:500 dilution; cat. no. B8251) and ICAM-1 (1:500 dilution; cat.
no. B7113) were purchased from Assay Biotech Co., Inc. (Sunnyvale,
CA, USA). Mouse monoclonal antibodies against PODXL (1:500
dilution; cat. no. BM0595), MCP-1 (1:1,000 dilution; cat. no.
BM0186), IL-6 (1:1,000 dilution; cat. no. BM0009) and β-actin
(1:2,000 dilution; cat. no. BM0272) were purchased from Abzoom
Biolabs, Inc. (Dallas, TX, USA). Additional mouse monoclonal
antibodies against SM-22 (1:1,000 dilution; cat. no. ab77442) and
Lox-1 (1:1,500 dilution; cat. no. ab53202) were obtained from Abcam
(Cambridge, MA, USA). Ox-LDL and PDGF-BB were obtained from Beijing
Union Biotechnology (Beijing, China).
Lentiviral (Lv) transfection
The Lv-PODXL was purchased from GeneChem Co., Ltd.
(Shanghai, China). The titer of the lentiviral recombinant vectors
were 2×1010 titer units (TU)/ml. The cells were plated
and cultured in 6-well plates at a density of 5×105
cells/ml at 37°C, until cell fusion reached between 60 and 70%.
Subsequently, 2.5×104 TU/well Lv- PODXL lentivirus
(conjugated to green fluorescent protein) were added to the cells
at a multiplicity of infection of 50 for 48 h. After 48 h, the
green fluorescence of the cells was observed using a AE31
microscope (MOTIC China Group Co., Ltd., Hong Kong) and the
relative mRNA expression levels of PODXL were measured by reverse
transcription quantitative polymerase chain reaction (RT-qPCR); the
cells exhibiting >90% fluorescence and a 15-fold increase in
PODXL expression levels were considered to be sufficiently infected
with the lentivirus. The infected cells were harvested for further
analysis.
RT-qPCR
The total RNA was extracted from the control cells,
the cells treated with ox-LDL and the cells treated with PDGF-BB,
using TRIzol reagent (Invitrogen Life Technologies), according to
the manufacturer’s instructions. The mRNA expression levels of
PODXL, MCP-1 and IL-6 were detected using a SYBR green qPCR assay
kit (Bio-Rad Laboratories, Inc., Hercules, CA, USA). The expression
of β-actin was used as an endogenous control. The specific primers
used were as follows: PODXL, forward 5′-TTTTACTCTTGCCCTCTC-3′ and
reverse 5′-CTTTCTTTCTGCCAAGAAAC-3′ and β-actin, forward
5′-AGGGGCCGGACTCGTCATACT-3′ and reverse
5′-GGCGGCACCACCATGTACCCT-3′. The relative expression of miR-125b
was measured using an All-in-One™ miRNA qRT-PCR Detection kit
(GeneCopoeia, Inc.). The specific primer sets for miRNA-103 and U6
and the qPCR mix were purchased from GeneCopoeia. The expression of
U6 was used as an endogenous control. The PCR cycling conditions
were set as follows: 95°C for 1 min, followed by 35 cycles of 95°C
for 15 sec and 58°C for 15 sec. All experiments were performed in
triplicate. Data were processed using the 2−ΔΔCT method
(15).
Western blotting
The total cellular protein extracts (50 μg)
were prepared from the control cells, the cells treated with ox-LDL
and the cells treated with PDGF-BB, separated on 10%
SDS-polyacrylamide gels (EMD Millipore, Billerica, MA, USA) and
transferred onto nitrocellulose membranes (EMD Millipore). The
membranes were treated with tris-buffered saline-Tween 20 (TBST;
Auragene, Changsha, China) containing 50 g/ml skimmed milk (BD
Biosciences, Franklin Lakes, NJ, USA) overnight at 4°C. The
membranes were then incubated with antibodies targeting
VE-cadherin, ICAM-1, PODXL, MCP-1, SM-22 and Lox-1 in the
recommended dilutions overnight at 4°C, and with an antibody
targeting β-actin (1:2,000 dilution) at room temperature for 2 h.
Following the incubation with primary antibodies, the membranes
were washed with TBST four times. The membranes were then washed
and incubated with horseradish peroxidase-conjugated goat
anti-mouse (cat. no. SA001) and goat anti-rabbit (cat. no. SA009)
secondary antibodies (1:3,000 dilutions; Auragene) at room
temperature for 1 h. The signals were visualized using enhanced
chemiluminescence substrate (EMD Millipore). β-actin was used as a
loading control.
3-(4,5-dimethylthiazol-2-yl)-2,5 diphenyl
tetrazolium bromide (MTT) assay
An MTT assay was used to assess the cell growth
ability of the cells transfected with pre-miR125b or pre-scramble,
following PDGF-BB treatment. An MTT assay kit (Sigma-Aldrich) was
used according to the manufacturer’s instructions. Briefly,
5×103 cells were plated into each well of 96-well plates
and cultured for 0, 1, 2 and 3 days at 37°C. The cells were
incubated with 20 μl MTT (5mg/ml) at 37°C for 4 h at the end
of each time-point and, subsequently, the reaction was inhibited by
adding 150 μl dimethyl sulfoxide (Amresco LLC, Solon, OH,
USA) for 10 min at room temperature. Formazan production was
detected at 570 nm using an enzyme immunoassay analyzer (MK3;
Thermo Fisher Scientific, Waltham, MA, USA).
Transwell assay
The HAVSMCs (1×106 cells), trafnsfected
with either pre-miR125b or pre-scramble, were treated with 20 ng/ml
PDGF-BB for 48 h and starved in serum-free medium for 24 h at 37°C,
prior to being resuspended in serum-free medium (5×104
cells). The cells were added to the upper chamber, while the lower
chamber was filled with Dulbecco’s modified Eagle’s medium (GE
Healthcare Life Technologies, Logan, UT, USA) containing 10% FBS.
The chambers were BioCoat Matrigel Invasion Chambers from BD
Biosciences. Following incubation for 24 h, the cells attached to
the lower chamber were fixed and stained with crystal violet (BD
Biosciences) for 20 min. The redundant crystal violet was washed
with water and dried in air. The crystal violet in the membrane was
dissolved in 10% acetic acid and the optical density at 570 nm was
detected using an enzyme immunoassay analyzer.
Dual luciferase reporter assay
A fragment of the 3′ untranslated region (UTR) of
PODXL, containing the putative miR-125b binding site, was amplified
and recombined immediately into a psiCHECK-2 vector (Promega
Corporation, Madison, WI, USA) downstream of the luciferase gene
sequence. A psiCHECK-2 construct containing the 3′UTR of PODXL,
with a mutant sequence of miR-125b, was synthesized. Wild-type
(Wt)-PODXL and mutant (Mut)-PODXL primers were purchased from
Promega Corporation. HUVECs and HAVSMCs were plated into 24-well
plates 1 day prior to transfection, at a density of
2×104 cells/well and either the
Wt-PODXL-3′UTR-psi-CHECK2 or Mut-PODXL-3′UTR-psi-CHECK2 were
co-transfected with the miR-125b mimics, miR-125b inhibitor
(GeneCopoeia, Inc.), pre-scramble and pre-scramble inhibitor,
respectively, using Lipofectamine 2000 (Invitrogen Life
Technologies). The cells were incubated for 48 h and the luciferase
activity was detected using a dual-luciferase reporter assay system
(Promega Corporation) and normalized to the activity of
Renilla.
Statistical analysis
Statistical analyses were performed using
independent samples t-tests to compare between two groups or the
one-way analysis of variance to compare differences between more
than two groups with SPSS 13.0 statistical software (SPSS, Inc.,
Chicago, IL, USA), depending on the experimental conditions. The
data are expressed as the mean ± standard deviation. Compared with
the respective controls, P<0.05 was considered to indicate a
statistically significant difference.
Results
Expression levels of miR-125b and PODXL
in HUVECs and HAVSMCs treated with ox-LDL and PDGF-BB
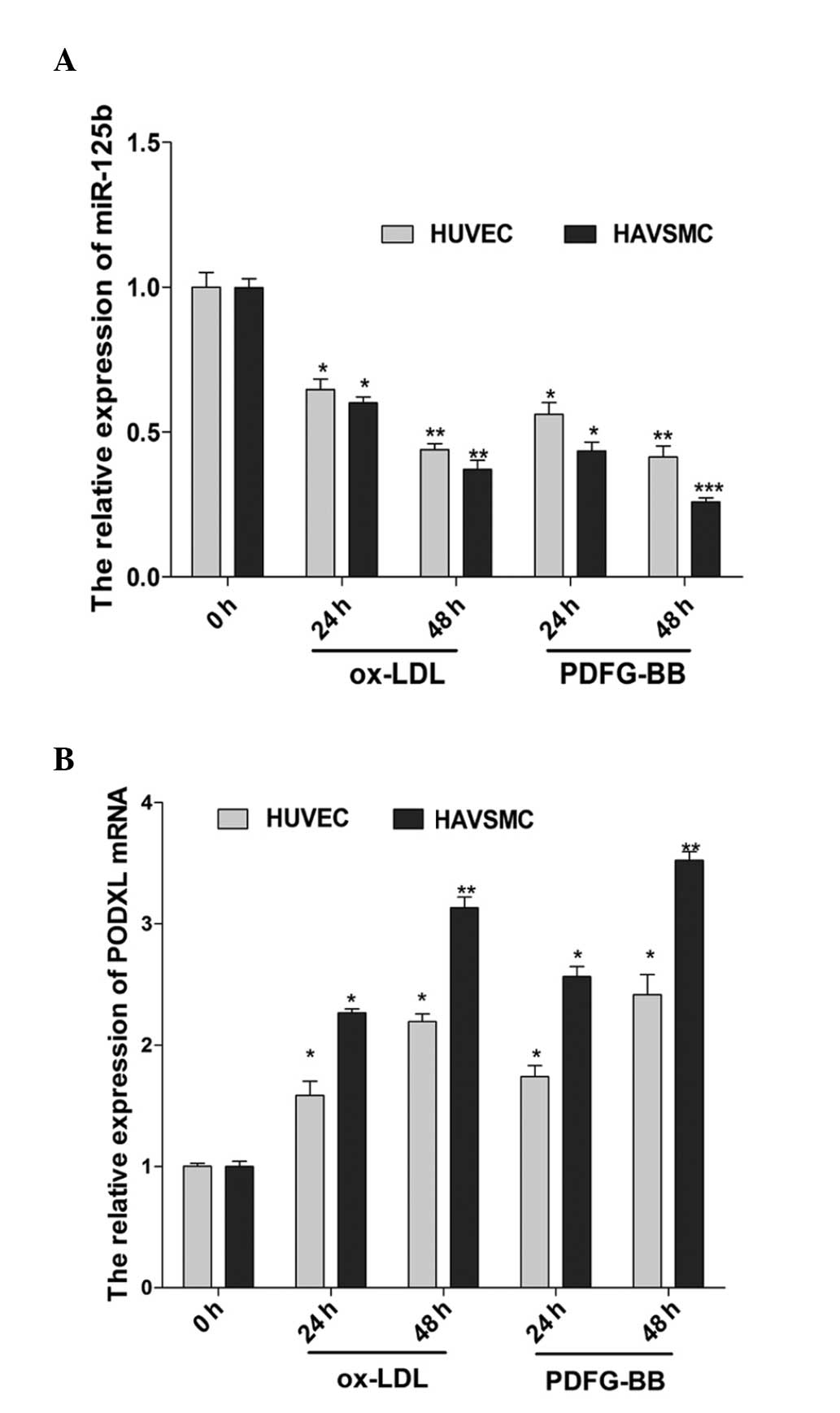
LDL and PDGF are effective predictors of
atherosclerosis. Following treatment with high concentrations of
ox-LDL and PDGF-BB, certain characteristics of atherosclerosis were
observed. To elucidate the association between the expression of
miR-125b and PODXL in atherosclerosis, HUVECs and HAVSMCs were
treated with ox-LDL (75 μg/ml) and PDGF-BB (20 ng/ml) to
create an atherosclerotic-like pathological model. As shown in
Fig. 1, a significant upregulation
of the mRNA expression of PODXL was observed in the cells treated
with ox-LDL and PDGF-BB, whereas the expression of miR-125b was
significantly downregulated compared with that of the 0 h group.
The results demonstrated that there was an inverse association
between miR-125b and PODXL in the HUVECs and HAVSMCs with
atherosclerotic-like pathological changes following treatment with
ox-LDL and PDGF-BB.
miR-125b directly targets PODXL
According to an online miRNA target prediction
database (TargetScan; www.targetscan.org), it was hypothesized that PODXL
was a target of miR-125b (Fig.
2A). The present study revealed that there was an inverse
association between the expression profiles of PODXL and miR-125b
in the HUVECs and HAVSMCs treated with ox-LDL and PDGF-BB. To
confirm whether PODXL was targeted by miR-125b, the HUVECs and
HAVSMCs were transfected with miR-125b mimics or miR-scramble
(Fig. 2B). The mRNA expression of
PODXL was measured by RT-qPCR (Fig.
2D) and indicated the potential regulation of PODXL by
miR-125b. Western blot analysis revealed that the upregulation of
miR-125b significantly reduced the protein expression of PODXL
compared with the cells transfected with the pre-scramble control
(Fig. 2E and F). To further
confirm whether the predicted binding site of miR-125b to the 3′UTR
of PODXL was required for this regulation, the Wt-PODXL vector and
miR-125b mimics or the scramble control were transfected into
HUVECs and HAVSMCs. The relative luciferase activity of the
miR-125b transfected cells was significantly repressed compared
with the pre-scramble, anti-scramble and anti-miR-125b control
groups (Fig. 2C). In addition, the
miR-125b-mediated repression of the luciferase activity was
eliminated by the mutant putative binding site (Fig. 2C). These results confirmed that
miR-125b directly targeted PODXL and regulated its expression at
the transcriptional level.
 | Figure 2miR-125b directly targets PODXL. (A)
Predicted miR-125b binding site within PODXL 3′UTR and its mutated
version. (B) RT-qPCR was performed to detect the expression of
miR-125b following transfection with pre-miR-125b and pre-scramble
in the HUVECs and HAVSMCs. (C) Repression of luciferase activity by
PODXL 3′UTR was dependent on miR-125b. Mutated PODXL 3′UTR
abrogated the miR-125b mediated repression of luciferase activity.
(D) RT-qPCR was performed to detect the mRNA expression of PODXL
following transfection with pre-miR-125b and pre-scramble in the
HUVECs and HAVSMCs. (E) Western blot analysis was performed to
detect the protein expression of PODXL in the HUVECs and HAVSMCs
treated with pre-miR-125b or pre-scramble. (F) Quantification of
the protein expression of PODXL. (*P<0.05,
**P<0.01, ***P<0.001, vs. control).
Data are expressed as the mean ± standard deviation. UTR,
untranslated region; wt, wild-type; miR, microRNA; mut, mutant;
PODXL, podocalyxin; HUVEC, human umbilical vein endothelial cell;
HAVSMC, human aortic vascular smooth muscle cell; RT-qPCR, reverse
transcription quantitative polymerase chain reaction. |
Effect of miR-125b on the growth of
HAVSMCs
To investigate whether miR-125b regulates the growth
of HAVSMCs, cell growth was measured using an MTT assay following
transfection with miR-125b mimics or scramble control. As shown in
Fig. 3D, the HAVSMCs transfected
with the miR-125b mimics exhibited significant inhibition of cell
proliferation, compared with the control. However, when the HAVSMCs
were co-transfected with pre-miR-125b and Lv-PODXL, the cell
proliferation rate was moderately rescued. To elucidate the
underlying mechanism of miR-125b in the HUVECs and HAVSMCs with
atherosclerotic-like pathological changes, the protein expression
levels of the inflammatory factors, IL-6 and MCP-1, were
characterized in the HAVSMCs. The upregulation of miR-125b
significantly decreased the protein expression levels of IL-6 and
MCP-1 (Fig. 3E–G) and this was
reversed by the overexpression of PODXL (Fig. 4). The expression levels of SM-22
and Lox-1 were also detected by western blotting and, as shown in
Fig. 3A–C, ectopic expression of
miR-125b decreased the expression of SM-22 and increased the
expression of Lox-1.
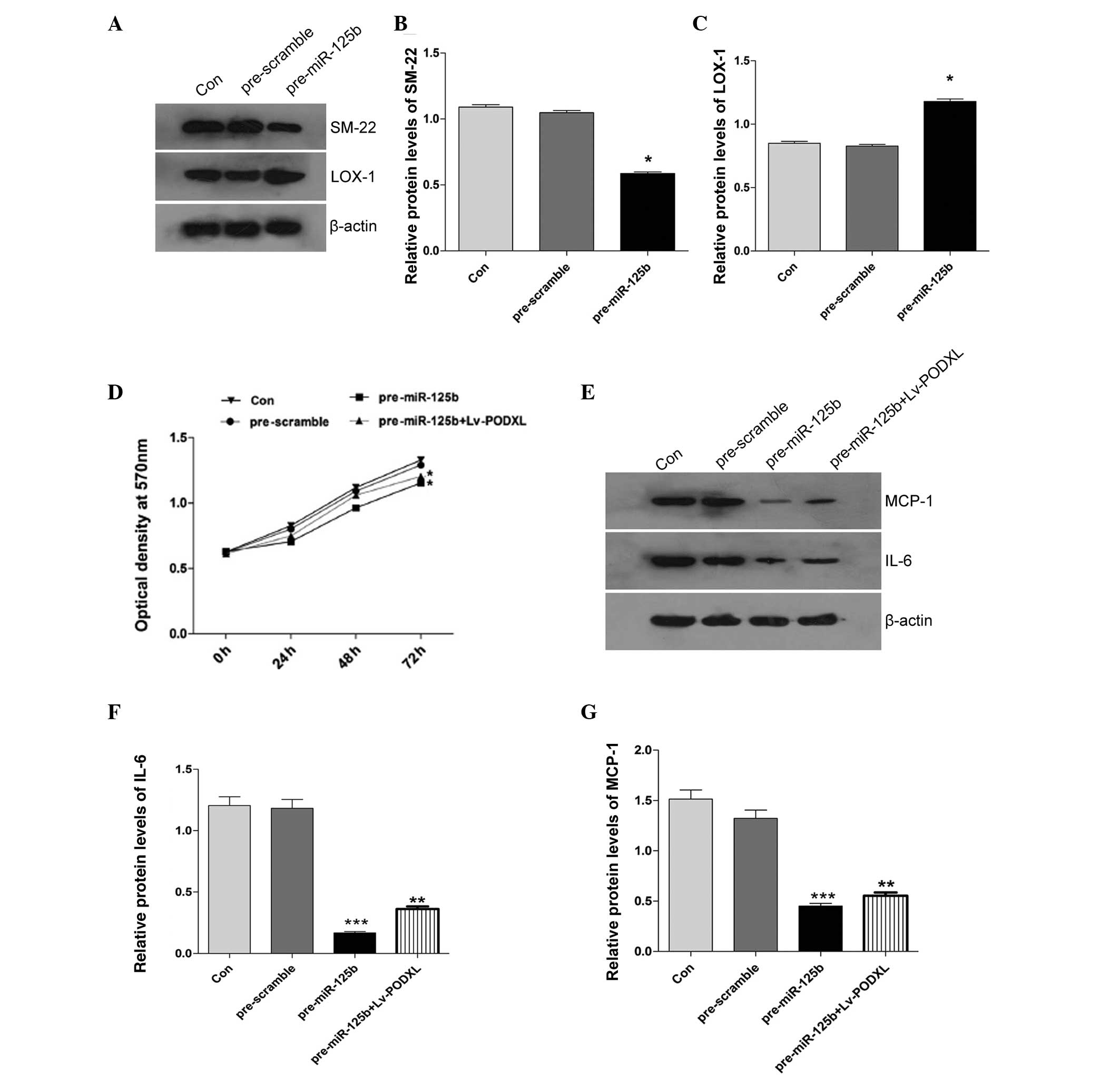 | Figure 3Effects of ectopic miR-125b on the
expression levels of SM-22, Lox-1, IL-6 and MCP-1, and cell
proliferation in the HAVSMCs treated with PDGF-BB. (A) Western
blotting was performed to detect the expression levels of (B) SM-22
and (C) Lox-1 and
the band intensity was quantified. (D) Cell growth was evaluated
using an MTT assay. The absorbance was measured at an optical
density of 570 nm. (E) Western blotting was performed to detect the
expression levels of (F) IL-6 and (G) MCP-1 in HAVSMCs treated with
PDGF-BB and the band intensity was quantified.
(*P<0.05, **P<0.01 and
***P<0.001, vs. control). The data are expressed as
the mean ± standard deviation. Con, control; miR, microRNA; Lox-1,
lectin-type oxidized low-density lipoprotein receptor-1; MCP-1,
monocyte chemotactic protein-1; IL-6, interleukin-6; HAVSMC, human
aortic vascular smooth muscle cell; PDGF, platelet-derived growth
factor; LV, lentivirus. |
 | Figure 4Effects of ectopic miR-125b on cell
migration in HAVSMCs and the expression levels of VE-cadherin and
ICAM-1 in HUVECs treated with PDGF-BB. (A) Representative images
captured by a light microscope of migrated cells stained with
crystal violet (magnification, ×100). (B) Cell migration was
determined using a Transwell assay and quantification. The
absorbance was measured at an optical density of 570 nm. (C)
Western blotting was performed to detect the expression levels of
(D) VE-cadherin and (E) ICAM-1 in the HUVECs treated with PDGF-BB
and band intensity was quantified (*P<0.05,
**P<0.01 and ***P<0.001, vs. control).
The data are expressed as the mean ± standard deviation. Con,
control; miR, microRNA; VE, vascular endothelial; ICAM-1,
intercellular adhesion molecule-1; HAVSMC, human aortic vascular
smooth muscle cell; PDGF, platelet-derived growth factor; LV,
lentivirus. |
Transfection with miR-125b affects the
migration of HAVSMCs and affects the expression levels of
VE-cadherin and ICAM-1 in HUVECs
To validate whether miR-125b regulates the migration
of HAVSMCs, a Transwell assay was performed by transfecting either
the miR-125b mimics or scramble control into HAVSMCs and treating
with PDGF-BB. As shown in Fig. 4A,
the HAVSMCs transfected with the miR-125b mimics exhibited
significant inhibition of the PDGF-BB-induced migratory ability
inhibition, compared with the pre-scramble control. However, when
the HAVSMCs were co-transfected with pre-miR-125b and Lv-PODXL, the
migratory ability was rescued. To elucidate the underlying
mechanism, the protein expression levels of VE-cadherin and ICAM-1
were characterized in the HUVECs. Following transfection with the
miR-125b mimics, it was found that the upregulation of miR-125b
significantly decreased the expression levels of VE-cadherin and
ICAM-1. However, the downregulation of VE-cadherin and ICAM-1,
induced by miR-125b, was reversed by the overexpression of PODXL
(Fig. 4).
Discussion
In the present study, upregulation of the mRNA
expression of PODXL was observed in HUVECs and HAVSMCs treated with
ox-LDL and PDGF-BB, which induced the overproliferation and
migration of smooth muscle cells. PODXL, a cell surface
glycoprotein, is closely associated with endoglycan and cell
adhesion. It has been reported that PODXL increases the in
vitro migration and invasion of breast and prostate cancer
cells (16) and knock-down of
PODXL significantly increases cell apoptosis in the presence of
temozolamide in glioblastomas (17). In the endothelial venules, PODXL
functions as an adhesive ligand for L-selectin-expressing
leukocytes (18), indicating that
PODXL has a pro-proliferative and pro-adhesive role. Results from
the present study supported the hypothesis that PODXL acts as a
pro-adhesive molecule.
Each miRNA can target the mRNA of several genes and
can be involved in several physiological and pathophysiological
processes. Emerging evidence suggests that mRNAs are important in
vascular diseases through regulating the expression of target
molecules in endothelial cells or smooth muscle cells. It was
reported that the proliferation and migration of endothelial cells
or smooth muscle cells was regulated by miR-424, miR-17-92 and
miR-26a (19–21). Additionally, certain miRNAs are
involved in ox-LDL- or PDGF-BB-induced atherosclerosis (22). Ox-LDL and PDGF-BB, effective
predictors of cardiovascular disease, are important in the
development of atherosclerosis by inducing overproliferation,
monocyte adhesion and inflammation (23). The underlying mechanism and
therapeutic strategies of atherosclerosis obliterans may be
revealed by identification of the miRNAs involved and their
targets. Although it has been confirmed that miR-125b is important
in various types of cancer (24),
few studies have investigated its functions in cardiovascular
diseases, including atherosclerosis obliterans. Previous studies
have demonstrated that miR-125b has a novel upstream role in the
epigenetic regulation of inflammatory genes in VSMCs of diabetic
mice, in that miR-125b targets the transcription factor, SP7, to
regulate the transdifferentiation of VSMCs into osteoblast-like
cells (14), and that is involved
in the inhibition of osteoblastic differentiation by downregulating
cell proliferation (25). The
present study revealed that miR-125b was significantly
downregulated in the HUVECs and HAVSMCs treated with ox-LDL or
PDGF-BB, suggesting an association with overproliferation and
migration of smooth muscle cells in the pathological process of
atherosclerosis obliterans. To investigate the precise association
and underlying mechanism between miR-125b and PODXL, the present
study confirmed, using a luciferase reporter assay, that miR-125b
directly targeted the PODXL gene through binding to a specific
complementary site within its 3′UTR. Additionally, the results
revealed that PODXL was effectively inhibited by the upregulation
of the mRNA and protein expression of miR-125b in the HUVECs and
HAVSMCs. Therefore, the decreased expression of miR-125b may
account for the upregulation of PODXL in the pathological process
of atherosclerosis obliterans.
The present study also demonstrated that the
upregulation of miR-125b inhibited cell proliferation and migration
in the HAVSMCs. However, cell proliferation was only moderately
rescued by the overexpression of PODXL, suggesting that miR-125b
had a suppressive function in cell proliferation, at least
partially by targeting PODXL. Chronic inflammation in the arterial
walls causes monocyte/macrophage recruitment and the migration and
proliferation of VSMCs, promoting atherosclerosis obliterans
(26). Upregulation of the
inflammatory cytokines, IL-6 and MCP-1, has been associated with
atherosclerosis obliterans (27–29).
The dysregulation of miRNAs have been involved in several
pathological processes of the cardiovascular diseases, including
vascular atherosclerosis (30).
Downregulation of miRNAs is associated with inflammation and is
observed in patients with coronary artery disease (29). MicroRNAs, including miR-26a,
miR-30b and miR-195, in the aortic valves of patients with aortic
stenosis are decreased compared with those with aortic
insufficiency (31). It was also
reported that miR-125b is downregulated in calcified vessels in
atherosclerotic mice and during vascular neointima formation
(32). The present study revealed
that the PDGF-BB-induced overexpression of miR-125b decreased the
upregulation of IL-6 and MCP-1, which was consistent with a
previous study suggesting that miR-125b was downregu-lated in
response to lipopolysaccharide and tumor necrosis factor-α
(33). In arteries, miR-125b is
one of the most abundant microRNAs. This may explain why the
overexpression of miR-125b significantly altered the gene
expression of IL-6 and MCP-1, consistent with the hypothesis that
small changes in miRNAs lead to widespread effects in cell
function.
SM22-α, a marker of cell differentiation, is
associated with the transdifferentiation of ox-LDL-induced smooth
muscle progenitor cell-derived smooth muscle-like cells into
foam-like cells and, Lox-1 mediates this process (34). SM22-α has been used to identify
vascular adventitial fibroblasts differentiating into
myofibroblasts following vascular adventitial damage. Foam cell
formation is a critical step in the development of atherosclerosis.
In the present study, it was demonstrated that an increase in
miR-125b induced the expression of Lox-1 and reduced the expression
of SM-22 in HAVSMCs. It is possible that miR-125b regulates the
expression of Lox-1 and SM-22 to inhibit the formation of foam
cells.
Furthermore, the results revealed that the miR-125b
mimic repressed the expression levels of VE-cadherin and ICAM-1,
the master regulators of endothelial permeability and leukocyte
transendothelial migration, in several vascular beds in the HUVECs.
A previous study reported that ox-LDL induces a
concentration-dependent upregulation of protein expression levels
of MCP-1 and ICAM-1 genes, promoting the atherosclerosis obliterans
process (35). The results from
the present study demonstrated that miR-125b exhibited significant
inhibition of the migratory ability of HAVSMCs, compared with the
pre-scramble control. However, co-transfection with pre-miR-125b
and Lv-PODXL, rescued the migratory ability of the HAVSMCs. These
findings suggested that miR-125b inhibited the HAVSMCs from
migrating into the blood vessel intima, most likely by repressing
the expression of vascular cell adhesion molecules.
In conclusion, the present study demonstrated for
the first time, to the best of our knowledge, that miR-125b is
involved in atherosclerosis obliterans, at least partially by
targeting PODXL and signalling for the inflammatory cytokines and
adhesion molecules involved in the pathological process of vascular
atherosclerosis. These findings indicated that miR-125b is a
potential small molecular target to prevent atherosclerosis
obliterans. The underlying pathophysiological mechanisms of
atherosclerosis remain to be elucidated and require further
investigation.
References
|
1
|
Di Pietro M, Filardo S, De Santis F,
Mastromarino P and Sessa R: Chlamydia pneumoniae and oxidative
stress in cardiovascular disease: State of the art and prevention
strategies. Int J Mol Sci. 16:724–735. 2014. View Article : Google Scholar
|
|
2
|
Endemann DH and Schiffrin EL: Endothelial
dysfunction. J Am Soc Nephrol. 15:1983–1992. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Guo J, Li L, Wu YJ, et al: Inhibitory
effects of Brazilin on the vascular smooth muscle cell
proliferation and migration induced by PDGF-BB. Am J Chin Med.
41:1283–1296. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Michael AF, Blau E and Vernier RL:
Glomerular polyanion. Alteration in aminonucleoside nephrosis. Lab
Invest. 23:649–657. 1970.PubMed/NCBI
|
|
5
|
Hara M, Yanagihara T, Takada T, et al:
Podocalyxin on the glomerular epithelial cells is preserved well in
various glomerular diseases. Nephron. 67:123–124. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Somasiri A, Nielsen JS, Makretsov N, et
al: Overexpression of the anti-adhesin podocalyxin is an
independent predictor of breast cancer progression. Cancer Res.
64:5068–5073. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Larrucea S, Butta N, Rodriguez RB, et al:
Podocalyxin enhances the adherence of cells to platelets. Cell Mol
Life Sci. 64:2965–2974. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cipollone JA, Graves ML, Köbel M, et al:
The anti-adhesive mucin podocalyxin may help initiate the
transperitoneal metastasis of high grade serous ovarian carcinoma.
Clin Exp Metastasis. 29:239–252. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fernández D, Horrillo A, Alquezar C,
González-Manchón C, Parrilla R and Ayuso MS: Control of cell
adhesion and migration by podocalyxin. Implication of Rac1 and
Cdc42. Biochem Biophys Res Commun. 432:302–307. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lin CW, Sun MS and Wu HC: Podocalyxin-like
1 is associated with tumor aggressiveness and metastatic gene
expression in human oral squamous cell carcinoma. Int J Oncol.
45:710–718. 2014.PubMed/NCBI
|
|
11
|
Huang BS, Luo QZ, Han Y, Li XB, Cao LJ and
Wu LX: microRNA-223 promotes the growth and invasion of
glioblastoma cells by targeting tumor suppressor PAX6. Oncol Rep.
30:2263–2269. 2013.PubMed/NCBI
|
|
12
|
Chen KC, Hsieh IC, Hsi E, et al: Negative
feedback regulation between microRNA let-7g and the oxLDL receptor
LOX-1. J Cell Sci. 124:4115–4124. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang M, Li W, Chang GQ, et al: MicroRNA-21
regulates vascular smooth muscle cell function via targeting
tropomyosin 1 in arteriosclerosis obliterans of lower extremities.
Arterioscler Thromb Vasc Biol. 31:2044–2053. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Goettsch C, Rauner M, Pacyna N, Hempel U,
Bornstein SR and Hofbauer LC: miR-125b regulates calcification of
vascular smooth muscle cells. Am J Pathol. 179:1594–1600. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Arocho A, Chen B, Ladanyi M and Pan Q:
Validation of the 2-DeltaDeltaCt calculation as an alternate method
of data analysis for quantitative PCR of BCR-ABL P210 transcripts.
Diagn Mol Pathol. 15:56–61. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sizemore S, Cicek M, Sizemore N, et al:
Podocalyxin increases the aggressive phenotype of breast and
prostate cancer cells in vitro through its interaction with ezrin.
Cancer Res. 67:6183–6191. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wu H, Yang L, Liao D, Chen Y, Wang W and
Fang J: Podocalyxin regulates astrocytoma cell invasion and
survival against temozolomide. Exp Ther Med. 5:1025–1029.
2013.PubMed/NCBI
|
|
18
|
Sassetti C, Tangemann K, Singer MS,
Kershaw DB and Rosen SD: Identification of podocalyxin-like protein
as a high endothelial venule ligand for L-selectin: parallels to
CD34. J Exp Med. 187:1965–1975. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ghosh G, Subramanian IV, Adhikari N, et
al: Hypoxia-induced microRNA-424 expression in human endothelial
cells regulates HIF-α isoforms and promotes angiogenesis. J Clin
Invest. 120:4141–4154. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kuhnert F and Kuo CJ: miR-17-92
angiogenesis micromanagement. Blood. 115:4631–4633. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Staszel T, Zapala B, Polus A, et al: Role
of microRNAs in endothelial cell pathophysiology. Pol Arch Med
Wewn. 121:361–366. 2011.PubMed/NCBI
|
|
22
|
Wu C, Gong Y, Yuan J, et al: microRNA-181a
represses ox-LDL-stimulated inflammatory response in dendritic cell
by targeting c-Fos. J Lipid Res. 53:2355–2363. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li D and Mehta JL: Antisense to LOX-1
inhibits oxidized LDL-mediated upregulation of monocyte
chemoattractant protein-1 and monocyte adhesion to human coronary
artery endothelial cells. Circulation. 101:2889–2895. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhou M, Liu Z, Zhao Y, et al:
MicroRNA-125b confers the resistance of breast cancer cells to
paclitaxel through suppression of pro-apoptotic Bcl-2 antagonist
killer 1 (Bak1) expression. J Biol Chem. 285:21496–21507. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mizuno Y, Yagi K, Tokuzawa Y, et al:
miR-125b inhibits osteoblastic differentiation by down-regulation
of cell proliferation. Biochem Biophys Res Commun. 368:267–272.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Williams KJ and Tabas I: Atherosclerosis
and inflammation. Science. 297:521–522. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cushing SD, Berliner JA, Valente AJ, et
al: Minimially modified low density lipoprotein induces monocyte
chemotactic protein 1 in human endothelial cells and smooth muscle
cells. Proc Natl Acad Sci USA. 87:5134–5138. 1990. View Article : Google Scholar
|
|
28
|
Rose CE Jr, Sung SS and Fu SM: Significant
involvement of CCL2 (MCP-1) in inflammatory disorders of the lung.
Microcirculation. 10:273–288. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Villeneuve LM, Kato M, Reddy MA, Wang M,
Lanting L and Natarjan R: Enchanced levels of microRNA-125b in
vascular smooth muscle cells of diabetic db/db mice lead to
increased inflammatory gene expression by targeting the histone
methyltransferase Suv39h1. Diabetes. 59:2904–2915. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
de Swiniarski R, Beatty D, Donoghue E, et
al: Comparison of Schrodinger and Dirac coupled-channels analyses
of the 28Si(p,p’)28Si reaction at 500 MeV. Phys Rev C Nucl Phys.
42:1137–1140. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Nigam V, Sievers HH, Jensen BC, et al:
Altered microRNAs in bicuspid aortic valve: a comparison between
stenotic and insufficient valves. J Heart Valve Dis. 19:459–465.
2010.PubMed/NCBI
|
|
32
|
Ji R, Cheng Y, Yue J, et al: MicroRNA
expression signature and antisense-mediated depletion reveal an
essential role of MicroRNA in vascular neointimal lesion formation.
Circ Res. 100:1579–1588. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tili E, Michaille JJ, Cimino A, et al:
Modulation of miR-155 and miR-125b levels following
lipopolysaccharide/TNF-α stimulation and their possible roles in
regulating the response to endotoxin shock. J Immunol.
179:5082–5089. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yu J, Li Y, Li M, Qu Z and Ruan Q:
Oxidized low density lipoprotein-induced transdifferentiation of
bone marrow-derived smooth muscle-like cells into foam-like cells
in vitro. Int J Exp Pathol. 91:24–33. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Feng Y, Cai ZR, Tang Y, et al: TLR4/NF-κB
signaling pathway-mediated and oxLDL-induced up-regulation of
LOX-1, MCP-1, and VCAM-1 expressions in human umbilical vein
endothelial cells. Genet Mol Res. 13:680–695. 2014. View Article : Google Scholar : PubMed/NCBI
|