Introduction
Renal cell carcinoma (RCC) is a malignant tumor,
which originates in the renal tubular epithelial system. It is one
of the most common tumors in the urinary system, and clear cell RCC
(CCRCC) is the commonest pathological type of RCC (85–90%)
(1). The incidence of RCC is
comparable with that of bladder cancer, and ranks second among
urinary tumors, accounting for ~2–3% of all malignant human tumors
(1). The incidence of RCC and the
mortality due to RCC is growing, with a yearly increase of the
incidence of RCC of ~2% worldwide (2–3).
However, the pathogenesis of RCC remains poorly understood.
Oncogene activation, and anti-oncogene mutation and inactivation
may lead to RCC tumor development. Therefore, further research may
help to provide novel methods for tumor prevention and
treatment.
Nodal is a member of the transforming growth factor
β (TGF-β) superfamily. It is involved in the development of
embryonic stem cells, via regulation of the induction of embryonic
tissues to form a complete axis (4,5).
Nodal is a primary element that regulates early embryo-inducing
signals, which involves a series of events, such as the formation
of the mesoderm and entoderm, the determination of the location of
the front-rear axis, and the specialization of the left-right axis.
Therefore, nodal exhibits important functions in the early
developmental stages of vertebrates (4–5). The
nodal signaling pathway involves the secretion of nodal into the
cytoplasm, where it binds with the co-receptor cripto-1, which
forms a bond with II-type receptor activin receptor type-2B and
I-type receptor ALK4/7 (5). This
leads to the formation and phosphorylation of the ALK receptor
complex, which activate smad2 and smad3 expression in the
cytoplasm. Subsequently, the smad2/3 compound and smad4 develop
into active trimers, which pass into the nucleus. In the nucleus
the smad2/3 compound and smad4 bind with forkhead box protein H1,
mix paired-like homeobox, and other transcription factors and
co-activators or co-repressors, which regulate the transcription of
Nodal responsive genes, such as Lefty (5). Lefty is a member of the TGF β
superfamily and is an important cytokine. It is involved in the
regulation of embryonic development and stem cell differentiation,
and its expression inhibits the nodal signaling pathway (6). Lefty inhibits nodal signaling by
binding directly with nodal or with cripto, thereby preventing the
formation of an active nodal/activin receptor complex (7). A balance between lefty and nodal
activity is important for a number of developmental processes, as
demonstrated by the severe and often fatal phenotypes observed in
lefty- or nodal-deficient embryos (4).
Nodal protein expression has been reported in
malignant melanoma, testicular cancer, breast cancer, and brain
glioma. Its expression is closely associated with tumor invasion,
metastasis and poor prognosis (8–10). A
number of studies have shown that nodal may be developed as a
biomarker for monitoring the malignant progression of tumors and as
a target molecule for clinical intervention (9). Furthermore, nodal is capable of
regulating tumor cell plasticity; the downregulation of nodal
expression abolishes tumor cell plasticity, which prevents tumor
cells from undergoing vasculogenic mimicry (VM) (9). McAllister et al (11) demonstrated that nodal gene
expression is associated with the formation of VM-like structures
in a physiological model of human melanoma tumorigenesis, providing
further support for an association between nodal expression and the
formation of channel-like structures. Studies have demonstrated an
association between nodal expression and human malignant melanoma
(12). A previous study
demonstrated that nodal downregulation may reduce the occurrence of
VM in human malignant melanoma cells, induce cell apoptosis, and
inhibit the development of tumors (9).
Previous studies have demonstrated that tumor
metastasis and embryonic development exhibit a similar activation
of nodal signaling pathways (5).
However, regulation of the nodal signaling pathways in tumor
metastasis and embryonic development is different. Embryonic tissue
is capable of secreting the following endogenous nodal signaling
pathway inhibitors: I-smads (smad6, smad7), lefty A/B and other
proteins that regulate developmental processes. These endogenous
nodal signaling pathway inhibitors, however, were not detected in
certain invasive tumor cells, such as melanoma cells (13–14).
The lack of these endogenous nodal signaling pathway inhibitors in
tumor cells may therefore be associated with tumor invasion and
metastasis. Studies have shown that the embryonic microenvironment,
in particular the microenvironment of embryonic stem cells, may
enable tumor cells to obtain more differentiated phenotypes and to
markedly reduce the malignancy grade (12,13).
Cucina et al (15) exposed
metastatic melanoma cells to an embryonic microenvironment prior to
zebrafish gastrulation, which led to gene rearrangement and the
formation of non-oncogenic phenotypes. Metastatic melanoma cells
transplanted into developing chick embryos are capable of following
the neural crest migration pathway. The cells therefore lose
tumorigenicity and exhibit a phenotype similar to that of healthy
neural crest cells (16). A high
expression of nodal in migrating melanoma cells and breast cancer
cells may inhibit cell differentiation, whereas the presence of
glycosylated lefty in the embryonic stem cell microenvironment may
inhibit nodal expression, thereby reducing the degree of malignancy
of tumor cells (15,16). Previous research has shown that the
extraction of a small concentration of lefty (20–50 ng/ml) from
human embryonic stem cells (hESCs) is capable of reducing melanoma
C8161 cell proliferation and increasing apoptosis, leading to
reduced tumor invasiveness (14).
Therefore, restoring the balance of the nodal signaling pathway may
aid in the control of tumor cell proliferation.
To the best of our knowledge, few studies have
investigated the role of lefty and nodal in RCC. The present study
examined whether the expression of lefty and nodal in RCC cells is
similar to that of other types tumor cells. The regulatory
mechanisms underlying lefty and nodal expression in RCC cells were
also investigated.
Materials and methods
Tissue samples and cell culture
Tumor and adjacent non-tumor tissues (45 pairs) were
obtained from patients with CCRCC. The tissue samples had been
resected at the First Affiliated Hospital of Dalian Medical
University (Dalian, China) between February 2012 and April 2013.
The patients were of Chinese origin and they fulfilled the RCC
criteria of the World Health Organization (WHO; 17). Tissue samples
were subjected to pathological examinations in order to confirm the
diagnosis of RCC. RCC staging was assessed using the TNM staging
system for kidney cancer revised by the American Joint Committee On
Cancer (AJCC; 18). The present study was approved by the ethics
committee of First Affiliated Hospital of Dalian Medical
University. All patients gave informed written consent prior to the
initiation of the study. The demographic data and
clinicopathological features of the patients are summarized in
Table I. Tissue samples were
snap-frozen in liquid nitrogen immediately following resection, and
stored at −80°C. Human A498 and 786-O cell lines were obtained from
the American Type Culture Collection (Rockville, MD, USA) and
cultured in high glucose Dulbecco’s modified Eagle’s medium (GE
Healthcare Life Sciences, Beijing, China) with 10% fetal bovine
serum (Gibco, China) in <5% CO2 at 37°C.
 | Table IClinicopathological parameters of
patients with renal cell carcinoma (n=45). |
Table I
Clinicopathological parameters of
patients with renal cell carcinoma (n=45).
| Characteristic | Frequency (%) |
|---|
| Gender |
| Male | 23 (51.1) |
| Female | 22 (48.9) |
| Age (years) |
| ≤50 | 7 (15.6) |
| >50 | 38 (84.4) |
| Size of tumor
(length, cm) |
| ≤7 | 27 (60.0) |
| >7 | 18 (40.0) |
| TNM staging |
| I | 15 (33.3) |
| II | 12 (26.7) |
| III | 14 (31.1) |
| IV | 4 (8.9) |
| Fuhrman grade |
| High
differentiation | 27 (60.0) |
| Moderate
differentiation | 14 (31.1) |
| Poor
differentiation | 4 (8.9) |
Lefty and nodal overexpression vector
construction and small interfering RNA (siRNA) design
RCC cells and hESCs were collected in order to
extract RNA, using TRIzol® (Invitrogen Life
Technologies, Carlsbad, CA, USA) according to the manufacturer’s
instructions. Subsequently, reverse transcription polymerase chain
reaction (RT-PCR) was conducted in order to amplify the coding
regions of lefty and nodal. The products were digested with
Kpn I and EcoR I (Takara Bio, Inc., Shiga, Japan),
cloned into pcDNA3.1 vectors (Promega, Beijing, China) sequenced
and verified using ABI3730xl DNA Analyzer (Applied Biosystems,
Forster City, CA, USA). The following primers were used for PCR:
Forward: 5′-GGGGTACCGCCACCATGCAGCCCCTGTGGCTC-3′ and reverse:
5′-CGGAATTCCTATGGCTGGAGCCTCCTTG-3′ for lefty, forward:
5′-GGGGTACCGCCACCATGCACGCCCACTGCCTG-3′ and reverse:
5′-CGGAATTCTCAGAGGCACCCACATTCTTC-3′ for nodal, forward:
5′-AGACAUGAUCGUGGAAGAATT-3′ and reverse:
5′-UUCUUCCACGAUCAUGUCUTT-3′ for nodal siRNA (19), forward:
5′-CUGUGUGAGUUCGCCUUCAUUTT-3′, and reverse:
5′-UGAAGGCGAACUCACACAGUUTT-3′ for smad3 siRNA (20) and forward:
5′-UUCUCCGAACGUGUCACGUTT-3′ and reverse:
5′-ACGUGACACGUUCGGAGAATT-3′ for control siRNA, which were taken
from a previous publication (19).
Transfection and signaling pathway
inhibition
Cells (1×105 cells/ml) were seed into
6-well plates and incubated for 24 h at 37°C. Once cells had
reached ~70% confluence, plasmid and siRNA transfection were
conducted using Lipofectamine 2000® according to the
manufacturer’s instructions (Invitrogen Life Technologies).
Following 4–6 h of transfection the medium was changed.
Extracellular signal-related kinase (Erk) inhibitor II was added to
786-O cells post transfection (FR180204; 10 μM; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA). Following 24 h of
transfection, all cells were cultured for a further 24–48 h at
37°C.
RT-qPCR
Total RNA was extracted from the RCC cells and hESCs
using TRIzol (Invitrogen Life Technologies). The concentration of
extracted total RNA was determined by measuring the absorbance at
260 nm using a Cary 8454 UV-Visible Spectrophotometer. Total RNA (1
μg) was used for first-strand cDNA synthesis using
RevertAid™, a first strand cDNA Synthesis kit (Fermentas™, Logan,
UT, USA). quantitative PCR (qPCR) was performed on 100 ng of cDNA
in 20 μl of reaction mixture using SYBR Premix Ex Taq
(Takara). The following primers were used for PCR: Forward:
5′-GCGAGTGTCCTAATCCTGTTG-3′ and reverse: 5′-CAGCGGCTTGGTCTTCAC-3′
for nodal-QT, forward: 5′-AACCGCACCTCCCTCATC-3′ and reverse:
5′-GCTGCTGCCAGAAGTTCAC-3′ for lefty-QT and forward:
5′-GGTATCGTGGAAGGACTC-3′ and reverse: 5′-GTAGAGGCAGGGATGATG-3′ for
glyceraldehyde 3-phosphate dehydrogenase (GAPDH). The following PCR
protocol was performed: One cycle of 95°C for 5 min, and 40 cycles
of 95°C for 30 sec, 55°C for 30 sec and 72°C for 30 sec. Three
independent experiments were conducted for each sample. Data were
analyzed by comparing the 2−ΔΔCt values.
Western blotting
Total cellular proteins were extracted by incubating
cells in lysis buffer (Pierce Biotechnology, Inc., Rockford, IL,
USA) The protein concentrations in the cell lysates were determined
using a bicinchoninic acid assay (Pierce Biotechnology, Inc.).
SDS-PAGE was conducted using 8% glycine gels (Bio-Rad, Hercules,
CA, USA) loading equal quantities of proteins (20 μg) per
lane. Following electrophoresis, separated protein bands were
transferred to a nitrocellulose membrane (Pierce Biotechnology,
Inc.) and blocked using 5% non-fat milk in tris-buffered saline
with Tween-20 buffer for 1 h. Subsequently, the membranes were
incubated with rabbit polyclonal immunoglobulin (Ig)G anti-nodal
(sc-28913; Santa Cruz Biotechnology, Inc.; 1:300), rabbit
polyclonal anti-lefty (ab30955; Abcam, Cambridge, UK; 1:500),
rabbit polyclonal anti-smad2/smad3 (cat. no. 3102; Cell Signaling
Technology, Inc., Danvers, MA, USA; 1:600), rabbit monoclonal
anti-phospho-Smad2/Smad3 (cat. no. 8828; Cell Signaling Technology,
Inc.; 1:500), rabbit monoclonal anti-Erk1/2 (cat. no. 4695; Cell
Signaling Technology, Inc.; 1:800), rabbit monoclonal
anti-phospho-Erk1/2 (cat. no. 4370; Cell Signaling Technology,
Inc.; 1:600),and rabbit polyclonal anti-GAPDH (NBP1-47339; Novus
Biologicals, Littleton, CO, USA; 1:1,000) antibodies overnight at
4°C. Subsequently, goat anti-rabbit IgG secondary antibodies
conjugated with horseradish peroxidase (cat. no. 7074; Cell
Signaling Technology, Inc.; 1:7,000–8,000) were incubated with the
membranes for 1 h at room temperature. Protein bands were detected
using ECL color (Pierce Biotechnology, Inc.).
Cell proliferation
Cell proliferation was quantified using a
Bromodeoxyuridine colorimetric immunoassay kit (Cell Proliferation
ELISA, Roche Diagnostics, Basal, Switzerland), according to the
manufacturer’s instructions. Cell proliferation was expressed as
the mean percentage of cell proliferation of control cells (set at
100%).
Annexin-V-FLUOS apoptosis analysis
Following transfection for 72 h, cells were
collected. The translocation of phosphatidylserine, a positive cell
surface marker for apoptosis, was detected in treated cells using
the Annexin-V-FLUOS staining kit (Roche Applied Science, Penzberg,
Germany). Cells were suspended in 500 μl of binding buffer
(Roche Applied Science) and incubated at room temperature in
darkness for 15 min. Cells were then labeled with Annexin
V-fluorescein isothio-cyanate (5 μl; Roche Applied Science)
and propidium iodide (5 μl). The stained cells were then
analyzed using flow cytometry (Beckman Coulter, Inc., Brea, CA,
USA).
Transwell Matrigel™ invasion assay
Cell invasion was measured using a Transwell
Matrigel invasion assay (BD Biosciences, Shanghai, China).
Following transfection, cells (200 μl; 1×106/ml)
and complete medium (600 μl) were added to the upper and
lower compartments of the chamber respectively. Following 48 h of
incubation, cells that had migrated to the lower side of the filter
were fixed with 4% paraformaldehyde (BD Biosciences) for 15 min at
room temperature, washed with phosphate-buffered saline (BD
Biosciences), stained with crystal violet (BD Biosciences) and
observed under a confocal microscope (Olympus Corp., Beijing,
China).
Statistical analysis
Experiments were repeated at least three times and
results are expressed as the mean ± standard deviation. SPSS Inc.
(13.0; Chicago, IL, USA) was used for statistical analysis. The
differences between two groups were analyzed using two-tailed
Student’s t-test and the differences between three or more groups
were analyzed using one-way analysis of variance. In all cases
P<0.05 was considered to indicate a statistically significant
difference.
Results
Expression of lefty and nodal in RCC
cells
An RT-PCR analysis of lefty and nodal expression in
RCC tumor and adjacent non-tumor cells (45 pairs) was conducted.
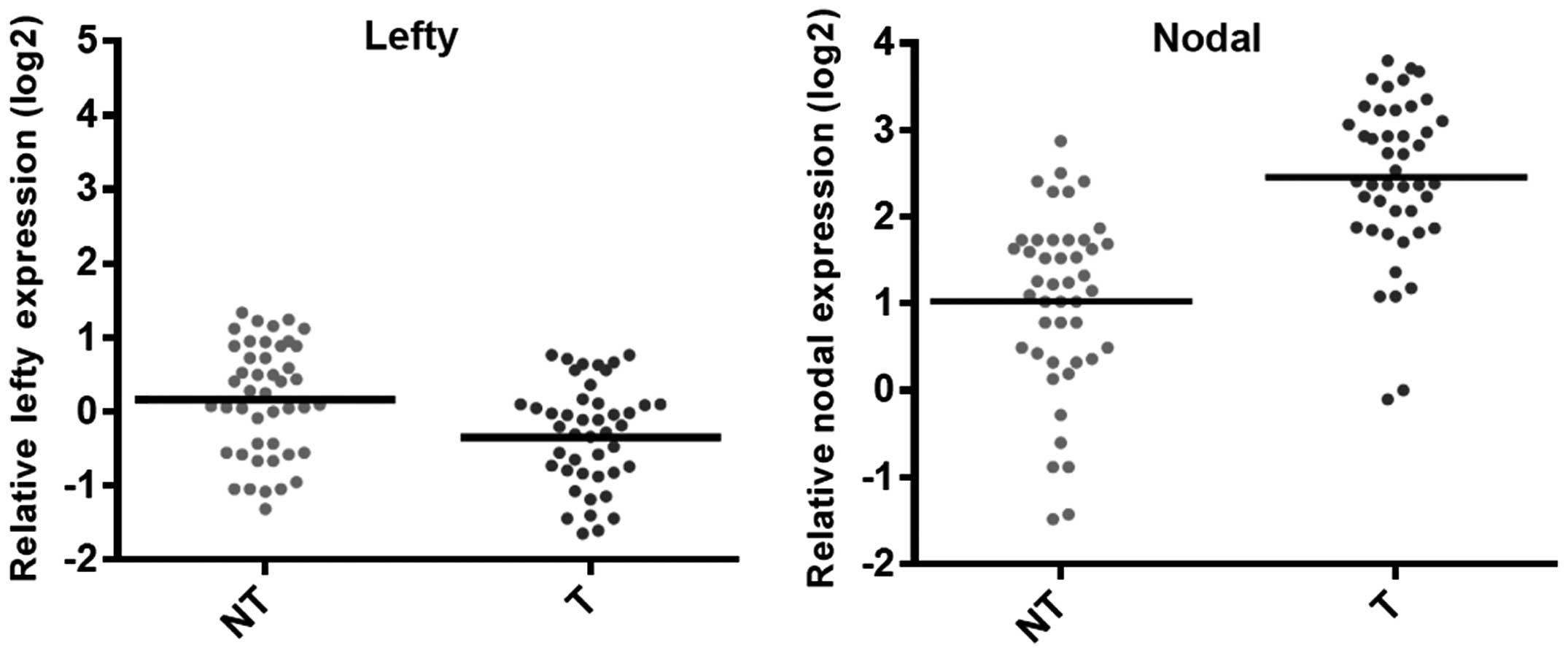
The results indicated that the expression of nodal in RCC cells was
high compared with that in adjacent non-tumor cells (Fig. 1). However, the expression of lefty
in RCC was significantly decreased compared with that of the
adjacent non-tumor cells (P<0.01) (Fig. 1). Expression of nodal was
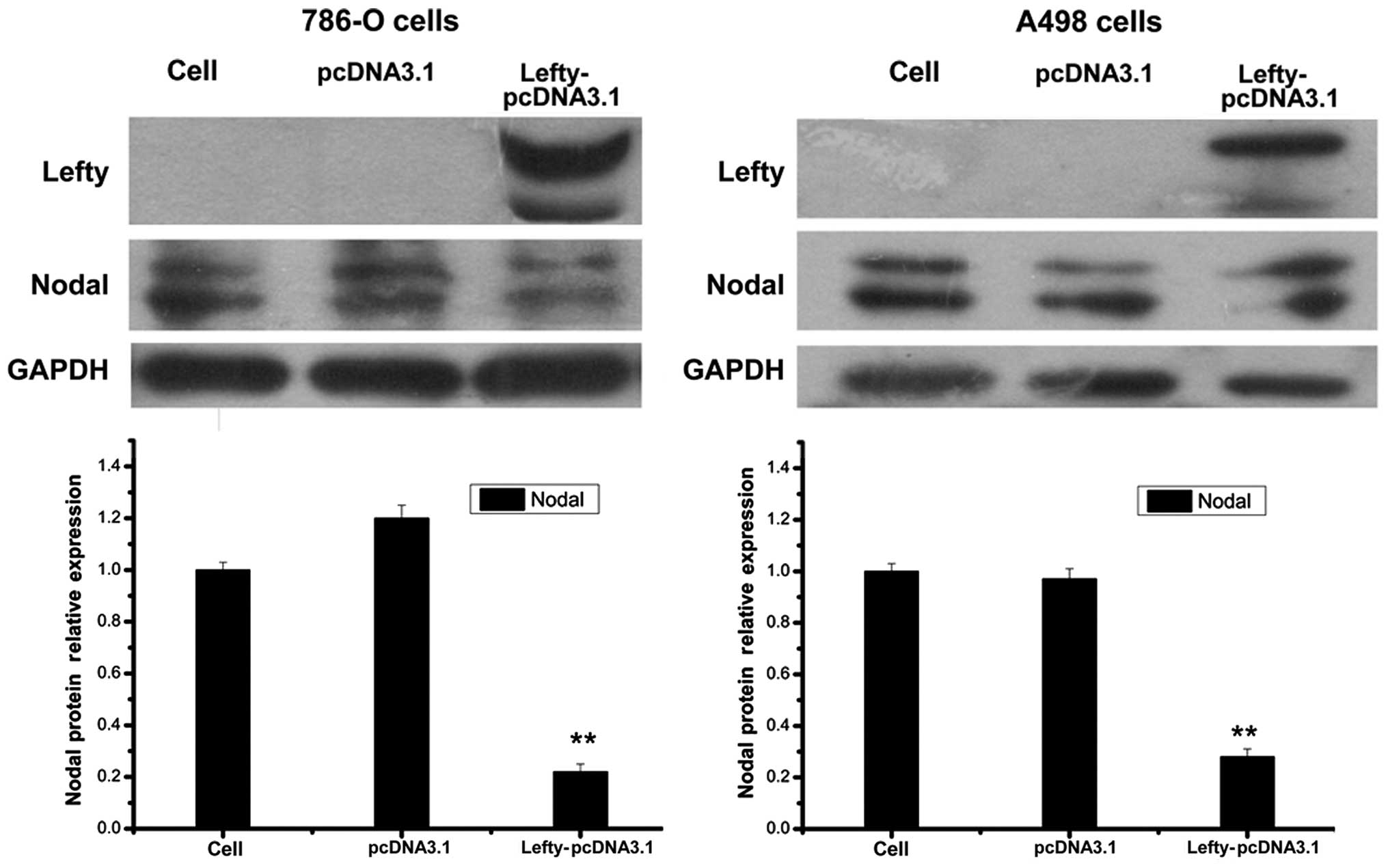
significantly lower in RCC cells overexpressing lefty compared with
that in the control cells (Fig.
2). These results suggested that lefty expression was lower in
RCC cells compared with that in control cells, which may result in
a loss of nodal regulation.
Effect of lefty and nodal expression on
cell proliferation and apoptosis
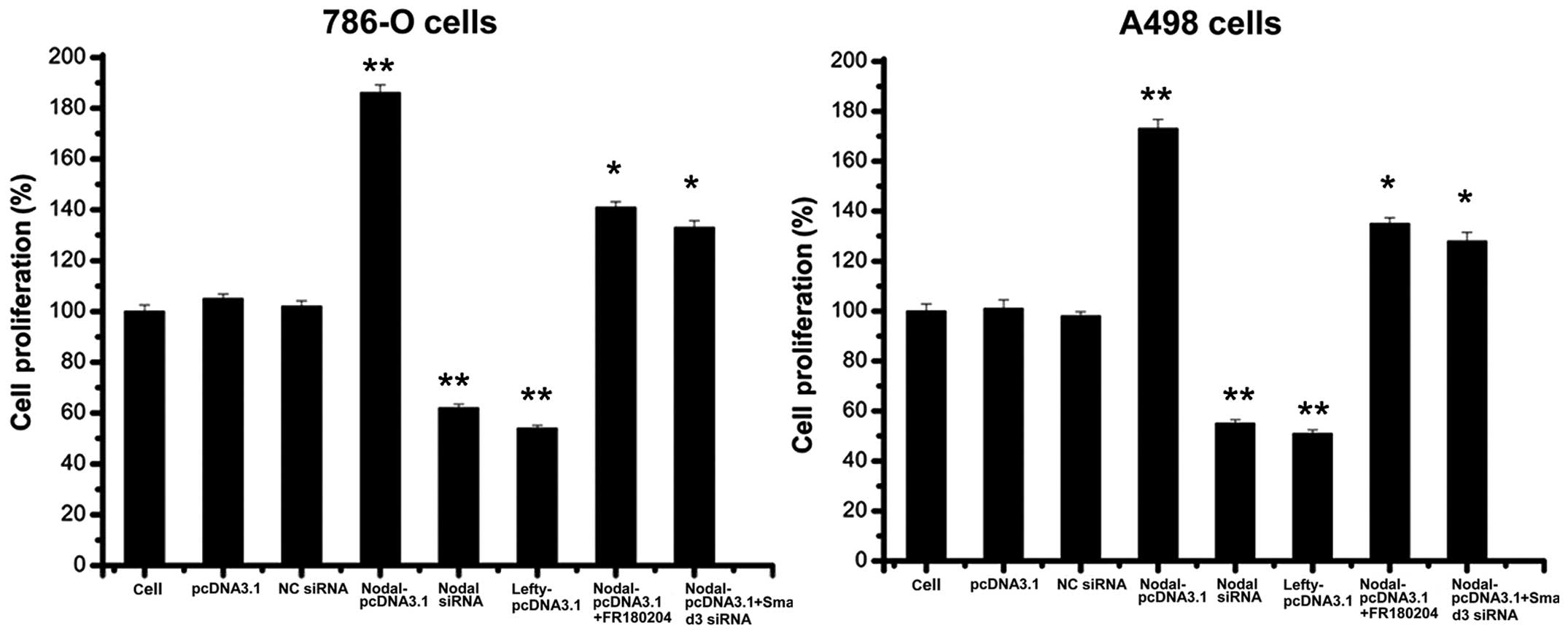
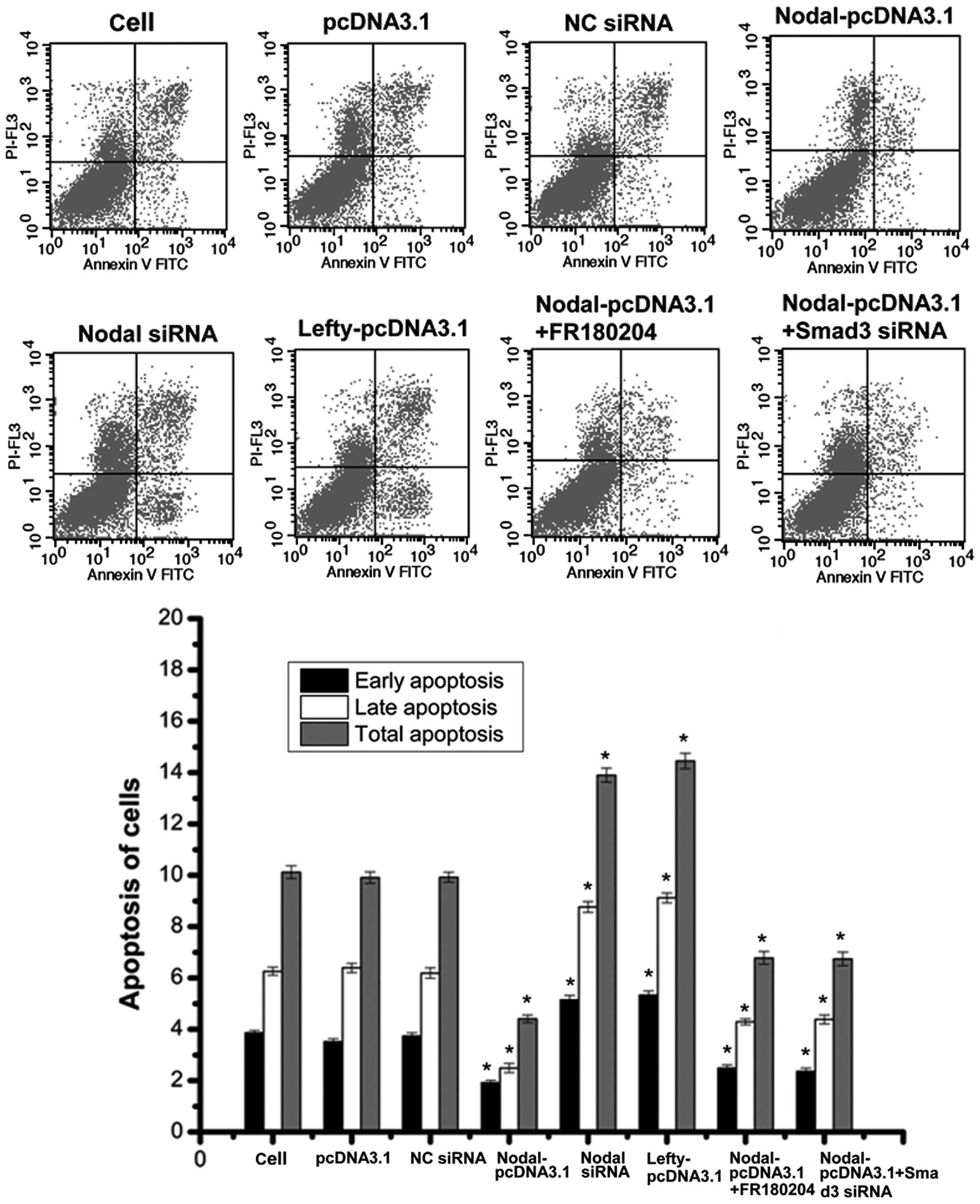
Nodal expression promotes cell proliferation and
inhibits apoptosis in different types of tumor cells (21–24),
and lefty expression results in the opposite effects (25–26).
The findings of the present study suggested that nodal
over-expression may promote RCC cell proliferation and inhibit
apoptosis (Figs. 3 and 4). The downregulation of nodal and the
overexpression of lefty led to similar observations: RCC cell
proliferation was inhibited and apoptosis was promoted (Figs. 3 and 4). Therefore, the growth of RCC cells may
be promoted by nodal expression and inhibited by lefty
expression.
Effect of lefty and nodal on cell
invasion
Studies have shown that nodal expression is high in
metastatic melanoma cell lines (C8131, WM278, and 1205Lu), whereas
that of a non-invasive melanoma cell line (C81-61) was shown to be
low or defective (27–28). Therefore, nodal expression may
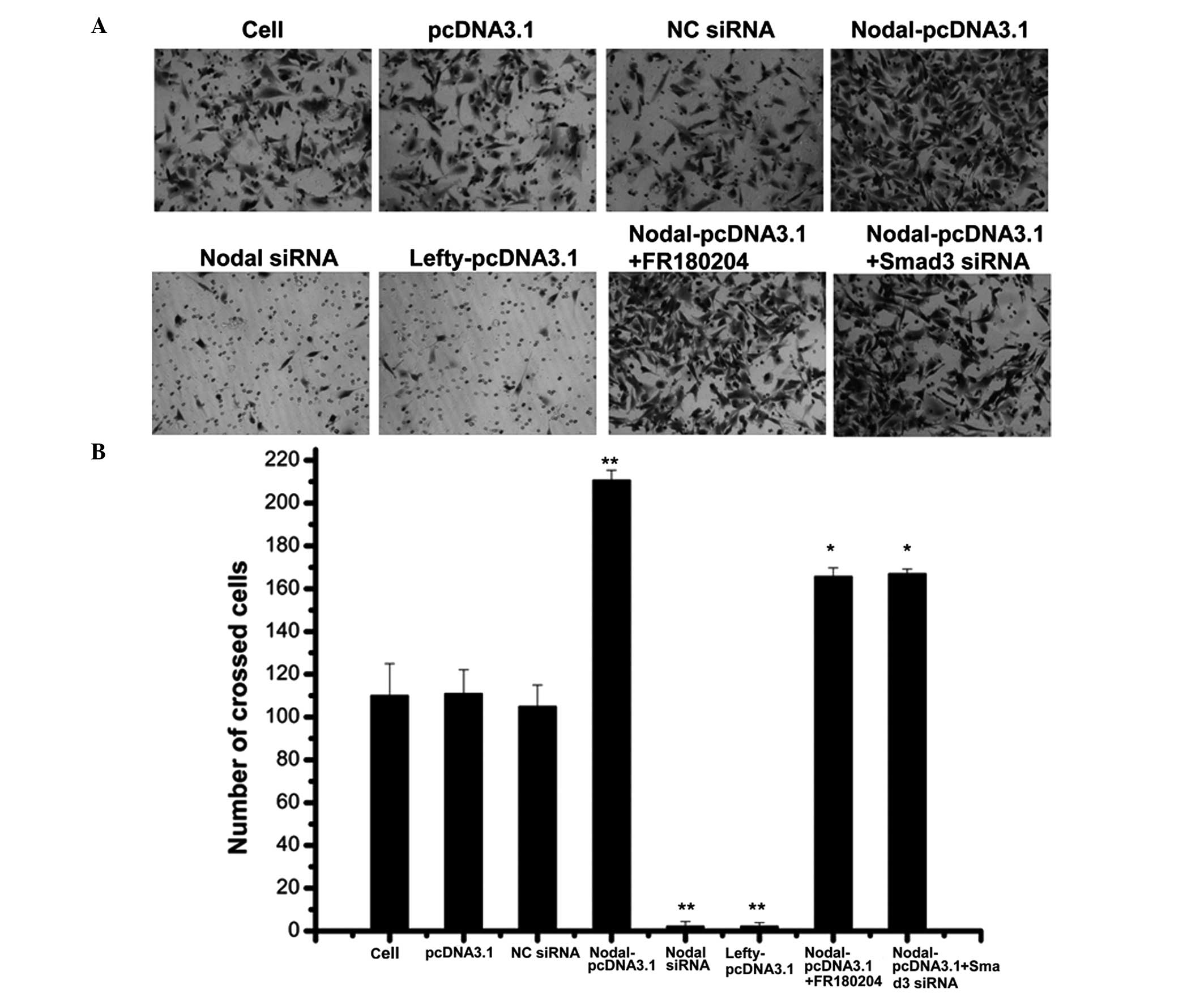
promote tumor cell invasion and metastasis. A transwell assay was
used to determine the effect of lefty and nodal on RCC cell
invasion. The results of the present study demonstrated that the
overexpression of nodal promoted RCC cell invasion, whereas RCC
cell invasion was inhibited through nodal gene knockdown or lefty
overexpression (Fig. 5).
Nodal expression activates smad and
ERK1/2 pathways, promoting RCC growth
Nodal is a member of the superfamily TGF-β, which
may activate the smad pathway (5).
Therefore, following the overexpression or downregulation of nodal,
the expression of a principle signal transduction molecule involved
in the smad pathway, smad2/3, was measured using western blotting.
The results demonstrated that nodal overexpression in 786-O RCC
cells induced smad2/3 phosphorylation (Fig. 6). By contrast, nodal expression
knockdown reduced smad2/3 phosphorylation in 786-O RCC cells
(Fig. 6). Therefore, nodal
expression may activate the smad pathway. However, following the
downregulation of smad3 in RCC cells overexpressing nodal, cell
proliferation and invasion was only partly reduced compared with
that of cells without smad downregulation. Furthermore, RCC cell
apoptosis was not significantly higher in RCC cells overexpressing
nodal with smad3 downregulation, compared with cells without smad
downregulation (Figs. 3Figure 4–5). These results suggested that nodal may
be involved in other pathways that promote the growth of RCC. In
pancreatic cancer cells, the ERK1/2 pathway may inhibit lefty
expression induced by TGF-β (29).
Therefore, nodal may be involved in the ERK1/2 pathway. In the
present study, overexpression of nodal promoted ERK1/2
phosphorylation, whereas the down-regulation of nodal reduced
ERK1/2 phosphorylation (Fig. 6),
indicating that nodal may activate the ERK1/2 pathway. Following
the addition of FR180204, an ERK inhibitor, RCC cells
overexpressing nodal did not exhibit significantly lower levels of
RCC cell proliferation and invasion compared with cells
overexpressing nodal that did not receive treatment with FR180204.
Furthermore, RCC cell apoptosis were not signifi-cantly higher in
cells overexpressing nodal that were treated with FR180204,
compared with those that were not treated with FR180204 (Figs. 3Figure 4–5). Therefore nodal expression may
activate the smad and ERK1/2 pathways, and promote RCC cell
proliferation and invasion, in addition to inhibiting cell
apoptosis. However, the influence of nodal expression on other
pathways involved in RCC growth requires investigation in order to
fully understand these mechanisms.
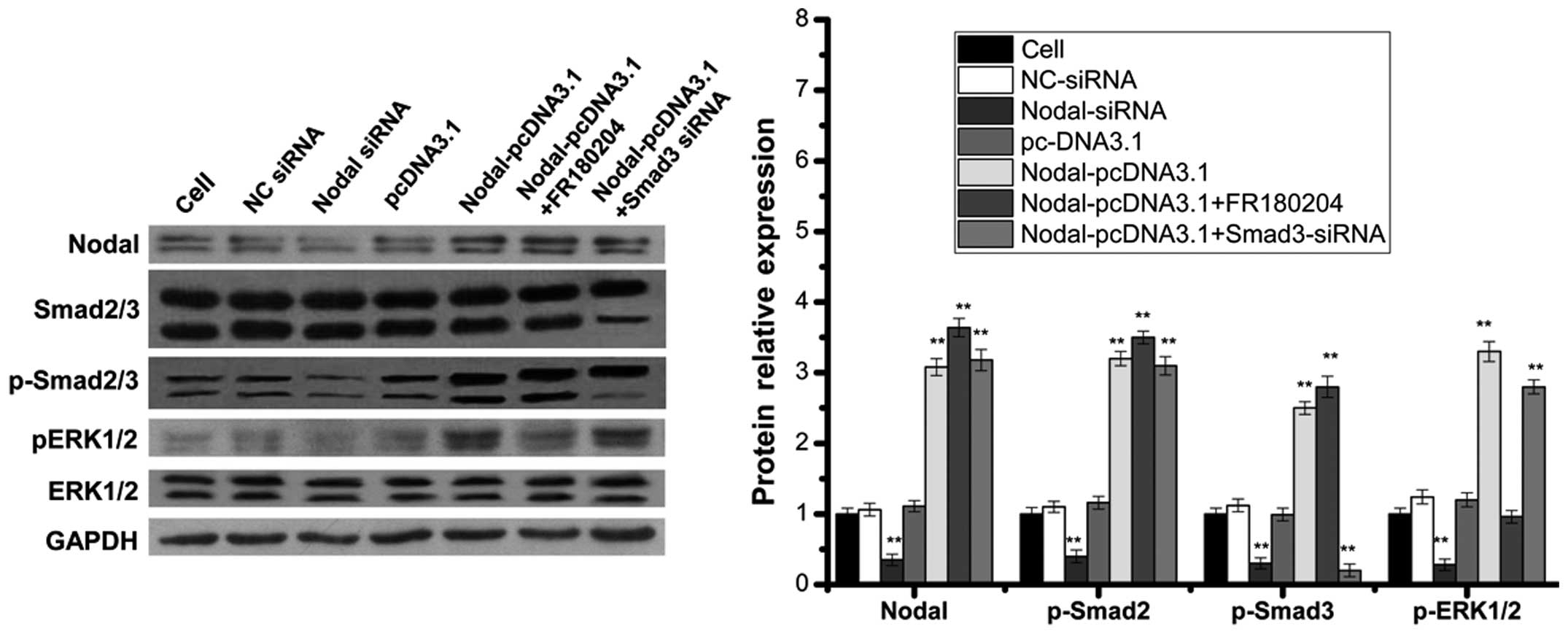 | Figure 6Nodal expression activated the smad
and ERK1/2 pathways. 786-O cells were transfected either with NC
siRNA, nodal siRNA, pcDNA3.1, nodal overexpression vector without
FR180204 (ERK Inhibitor II), nodal overexpression vector with
FR180204 or nodal overexpression vector with smad3 siRNA. The
expression of nodal, smad2/3, p-smad2/3, ERK1/2 and p-ERK1/2 was
measured using western blot analysis. Each bar represents the mean
± standard deviation from three samples. *P<0.05 vs.
control and **P<0.01 vs control. NC, non-cancerous;
siRNA, small interfering RNA; ERK; extracellular signal-regulated
kinase; GAPDH, glyceraldehyde 3-phosphate dehydrogenase. |
Discussion
The early symptoms of RCC are insidious, and
approximately 30–50% of RCC cases lack early clinical
manifestations (1). In the
majority of cases, by the time a patient exhibits three principle
symptoms (hematuria, flank pain and palpable abdominal mass), they
are in the advanced phase of RCC, and at this stage approximately
30% of patients will have developed tumor metastasis (2–3).
Multi-drug resistance genes may be expressed by RCC cells that are
insensitive to chemotherapy or radiotherapy. The efficacy of
immunotherapy for RCC remains unclear, and radical nephrectomy is
still the most common method of treatment for RCC. However, once
lymphatic metastasis occurs, the 5 year survival rate is extremely
low (5–15%), even with radical lymphatic node dissection (2–3).
Therefore, early diagnosis and treatment of RCC is important, and
the investigation of tumor biomarkers associated with RCC, which
have high specificity and high sensitivity has become a research
focus in urology.
hESCs and tumor cells express the morphogenetic
protein, nodal. Nodal may therefore be useful for determining
pluripotent phenotypes of tumor cells and hESCs, and for
controlling the differentiation of embryonic stem cells (12,27).
Increased nodal expression in human melanoma cells, breast, colon
and testicular cancer has been demonstrated (8–10).
hESCs express lefty, which inhibits nodal signaling pathways. In
metastatic tumor cells, nodal is expressed and lefty expression is
defective. Therefore, in metastatic tumor cells, the nodal
signaling pathway is unregulated. The uncontrolled overexpression
of the nodal signaling proteins may lead to the development of
malignant tumor cells (7,13–14).
A previous study demonstrated that inhibition of the nodal
signaling pathway in metastatic melanoma cells may reduce cell
colony formation and promote the development of the melanoma cell
low-plasticity phenotype (12),
reducing tumor inducibility to ~30% (27). In the present study, the expression
of nodal in RCC cells was high, compared with that of adjacent
non-tumor cells. However, the expression of lefty in RCC was
significantly decreased compared with that of the adjacent
non-tumor cells.
Numerous studies have demonstrated that nodal may
promote tumor growth, whereas lefty is capable of inhibiting tumor
growth (21–26). De Silva et al (30) demonstrated that nodal may promote
the tumorous growth of glioblastoma cells, which is mediated by
ALK4, ALK7 and smad3 proteins. Cavallari et al (25) demonstrated that lefty A is capable
of inhibiting the nodal signaling pathway in human liver stem
cells, thereby suppressing tumor cell growth in a similar manner to
that observed in hESCs.
The results of the present study suggested that
nodal expression may activate the smad and ERK1/2 pathways and
promote the growth of RCC. The inhibition of smad3 and the addition
of an ERK1/2 pathway inhibitor only partially reduced the
capability of nodal expression to promote RCC cell proliferation
and invasion, and inhibit cell apoptosis. Lawrence et al
(23) demonstrated that
recombinant human nodal expression triggered downstream smad2
phosphorylation in DU145 and LNCaP cell lines, and that the stable
transfection of pre-pro-nodal enhanced the growth of LNCaP cells in
Matrigel and soft agar. Nodal may inhibit androgen receptor
signaling, reducing the activity of a prostrate specific antigen
promoter and downregulating the endogenous expression of
androgen-regulated genes. To the best of our knowledge, there have
been limited studies on the influence of nodal and lefty expression
on tumor growth. Nodal may promote the growth of RCC by activating
the smad and ERK1/2 pathways. These findings provide a basis for
further investigations into the association between nodal
expression and tumor growth. The results of the present study may
therefore be useful for developing novel biomarkers for tumor
diagnosis and suggest a potential target gene for the treatment of
RCC.
References
|
1
|
Villa G and Hernández-Pastor LJ: Budget
impact analysis of first-line treatment with pazopanib for advanced
renal cell carcinoma in Spain. BMC Cancer. 13:3992013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nguyen MM, Gill IS and Ellison LM: The
evolving presentation of renal carcinoma in the United States:
trends from the Surveillance, Epidemiology, and End Results
program. J Urol. 176:2397–2400; discussion. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Stafford HS, Saltzstein SL, Shimasaki S,
Sanders C, Downs TM and Sadler GR: Racial/ethnic and gender
disparities in renal cell carcinoma incidence and survival. J Urol.
179:1704–1708. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Schier AF: Nodal signaling in vertebrate
development. Annu Rev Cell Dev Biol. 19:589–621. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Quail DF, Siegers GM, Jewer M and Postovit
LM: Nodal signalling in embryogenesis and tumourigenesis. Int J
Biochem Cell Biol. 45:885–898. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tabibzadeh S and Hemmati-Brivanlou A:
Lefty at the crossroads of ‘stemness’ and differentiative events.
Stem Cells. 24:1998–2006. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chen C and Shen MM: Two modes by which
Lefty proteins inhibit nodal signaling. Curr Biol. 14:618–624.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Strizzi L, Hardy KM, Kirsammer GT, Gerami
P and Hendrix MJ: Embryonic signaling in melanoma: potential for
diagnosis and therapy. Lab Invest. 91:819–824. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Strizzi L, Postovit LM, Margaryan NV, et
al: Nodal as a biomarker for melanoma progression and a new
therapeutic target for clinical intervention. Expert Rev Dermatol.
4:67–78. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Strizzi L, Hardy KM, Margaryan NV, et al:
Potential for the embryonic morphogen Nodal as a prognostic and
predictive biomarker in breast cancer. Breast Cancer Res.
14:R752012. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
McAllister JC, Zhan Q, Weishaupt C, Hsu MY
and Murphy GF: The embryonic morphogen, Nodal, is associated with
channel-like structures in human malignant melanoma xenografts. J
Cutan Pathol. 37(Suppl 1): 19–25. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Postovit LM, Margaryan NV, Seftor EA and
Hendrix MJ: Role of nodal signaling and the microenvironment
underlying melanoma plasticity. Pigment Cell Melanoma Res.
21:348–357. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hendrix MJ, Seftor EA, Seftor RE,
Kasemeier-Kulesa J, Kulesa PM and Postovit LM: Reprogramming
metastatic tumour cells with embryonic microenvironments. Nat Rev
Cancer. 7:246–255. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Postovit LM, Margaryan NV, Seftor EA, et
al: Human embryonic stem cell microenvironment suppresses the
tumorigenic phenotype of aggressive cancer cells. Proc Natl Acad
Sci USA. 105:4329–4334. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cucina A, Biava PM, D’Anselmi F, et al:
Zebrafish embryo proteins induce apoptosis in human colon cancer
cells (Caco2). Apoptosis. 11:1617–1628. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lee LM, Seftor EA, Bonde G, Cornell RA and
Hendrix MJ: The fate of human malignant melanoma cells transplanted
into zebrafish embryos: assessment of migration and cell division
in the absence of tumor formation. Dev Dyn. 233:1560–1570. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ebele JN, Sauter G, Epstein JI and
Sesterhenn IA: Pathology and Genetics of Tumours of the Urinary
System and Male Genital Organs. 1st. International Agency for
Research on Cancer Publications Collection; Lyon: 2004
|
|
18
|
Edge SB, Byrd DR, Compton CC, Fritz AG,
Greene FL and Trotti A: AJCC Cancer Staging Manual. 7th. Springer;
New York, NY: 2010
|
|
19
|
Nadeem L, Munir S, Fu G, et al: Nodal
signals through activin receptor-like kinase 7 to inhibit
trophoblast migration and invasion: implication in the pathogenesis
of preeclampsia. Am J Pathol. 178:1177–1189. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kobayashi T, Liu X, Wen FQ, et al: Smad3
mediates TGF-beta1-induced collagen gel contraction by human lung
fibroblasts. Biochem Biophys Res Commun. 339:290–295. 2006.
View Article : Google Scholar
|
|
21
|
Papageorgiou I, Nicholls PK, Wang F, et
al: Expression of nodal signalling components in cycling human
endometrium and in endometrial cancer. Reprod Biol Endocrinol.
7:1222009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lonardo E, Hermann PC, Mueller MT, et al:
Nodal/Activin signaling drives self-renewal and tumorigenicity of
pancreatic cancer stem cells and provides a target for combined
drug therapy. Cell Stem Cell. 9:433–446. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lawrence MG, Margaryan NV, Loessner D, et
al: Reactivation of embryonic nodal signaling is associated with
tumor progression and promotes the growth of prostate cancer cells.
Prostate. 71:1198–1209. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Strizzi L, Postovit LM, Margaryan NV, et
al: Emerging roles of nodal and Cripto-1: from embryogenesis to
breast cancer progression. Breast Dis. 29:91–103. 2008.PubMed/NCBI
|
|
25
|
Cavallari C, Fonsato V, Herrera MB, Bruno
S, Tetta C and Camussi G: Role of Lefty in the anti tumor activity
of human adult liver stem cells. Oncogene. 32:819–826. 2013.
View Article : Google Scholar
|
|
26
|
Saito A, Ochiai H, Okada S, Miyata N and
Azuma T: Suppression of Lefty expression in induced pluripotent
cancer cells. FASEB J. 27:2165–2174. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Topczewska JM, Postovit LM, Margaryan NV,
et al: Embryonic and tumorigenic pathways converge via Nodal
signaling: role in melanoma aggressiveness. Nat Med. 12:925–932.
2006. View
Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hardy KM, Kirschmann DA, Seftor EA, et al:
Regulation of the embryonic morphogen Nodal by Notch4 facilitates
manifestation of the aggressive melanoma phenotype. Cancer Res.
70:10340–10350. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Miyata N, Azuma T, Hozawa S, et al:
Transforming growth factor β and Ras/MEK/ERK signaling regulate the
expression level of a novel tumor suppressor Lefty. Pancreas.
41:745–752. 2012.PubMed/NCBI
|
|
30
|
De Silva T, Ye G, Liang YY, Fu G, Xu G and
Peng C: Nodal promotes glioblastoma cell growth. Front Endocrinol
(Lausanne). 3:592012.
|