Introduction
Lung cancer is the leading cause of
cancer-associated mortality and was responsible for 160,340
estimated mortalities in the United States in 2012, >80% of
which were diagnosed as non-small cell lung cancer (NSCLC)
(1). The treatment for NSCLC
includes stage-dependent therapy, surgery, radiotherapy and
chemotherapy. In addition, adjuvant methods, including
molecular-targeted therapy, are vital choices (2). As a therapeutic strategy for the
treatment of NSCLC, irradiation is the first choice under many
circumstances. However, only ~20% of patients achieve complete
pathological responses to irradiation, due to problems with
radioresistance and toxicity (3).
Efforts to improve this rate have focused on overcoming the
resistance of NSCLC to radiation therapy by increasing the
radiation dose, or by using radiosensitizers that may decrease its
toxic effect (4); however,
currently neither of these approaches has resulted in significantly
improved outcomes.
Certain types of Chinese drugs have been shown to
provide a rich resource for the identification of anticancer drugs.
Previous and ongoing studies by our group focused on observing the
anticancer activity of ginseng and related Chinese medicines. The
roots of Panax ginseng have been used in the treatment of
various disorders (5). Keum et
al (6) previously reported
that in HL-60 human pro-myelocytic leukemia cells, the antitumor
effects of ginsenoside Rg3 are caused by inhibition of
12-O-tetradecanoylphorbol-13-acetate-induced activation of
nuclear factor κB (NF-κB) (6).
Since NF-κB inhibitors have radiosensitizing potential (7), the present study hypothesized that
ginsenoside Rg3 may also radiosensitize NSCLC cells.
In the present study, the hypothesis that
ginsenoside Rg3 may be able to radiosensitize NSCLC cells was
investigated by measuring the effects of ginsenoside Rg3 on the
growth of cultured NSCLC cells and NSCLC xenografts in C57BL/6
mice, both of which were exposed to radiation. The present study
demonstrated that ginsenoside Rg3 sensitized NSCLC to radiation by
down-regulating NF-κB-regulated gene products, leading to
inhibition of tumor progression.
Materials and methods
Materials and cell culture
Raw ginseng (Jilin province, China) was steamed at
120°C using an autoclave (Techeng Machinery Equipment Ltd.,
Shanghai, China) for 2 h; the Rg3 content was then measured using
high performance liquid chromatography, as previously reported by
Kim et al (8). Primary
polyclonal antibodies against inhibitor of NF-κB (IκB; sc-371),
phosphorylated (p)-IκB (sc-52943), matrix metalloproteinase-9
(MMP-9; sc-10737), cyclooxygenase-2 (COX-2; sc-23984), vascular
endothelial growth factor (VEGF; sc-7269), B-cell lymphoma 2
(Bcl-2; sc-492) and β-actin (sc-8432) were diluted according to the
manufacturer’s instructions and were obtained from Santa Cruz
Biotechnology, Inc. (Dallas, TX, USA). Primary polyclonal
antibodies against cyclin D (#2922) and c-myc (#9402) were obtained
from Cell Signaling Technology, Inc. (Danvers, MA, USA).
Horseradish peroxidase-conjugated secondary antibodies (1:4,000)
were obtained from Santa Cruz Biotechnology, Inc. Parental A549 and
H1299 human lung carcinoma cells, and the Lewis lung carcinoma cell
line (LLC) were obtained from the Cell Center of the Chinese
Academy of Medical Sciences (Beijing, China). Dulbecco’s modified
Eagle’s medium (DMEM) and fetal bovine serum were purchased from
Gibco Life Technologies (Carlsbad, CA, USA). All of the cell lines
were cultured in DMEM supplemented with 10% fetal bovine serum, and
maintained at 37°C in a humidified atmosphere containing 5%
CO2. Cells in the exponential growth phase were used for
subsequent experiments. The NF-κB Electrophoretic Mobility Shift
assay (EMSA) kit was obtained from Viagene Biotech (Ningbo,
China).
Stable cell lines were generated by transfecting
A549 cells with the pNF-κB-TA-Luc reporter construct (Beyotime
Institute of Biotechnology, Haimen, China) using
GeneJuice® transfection reagent (Novagen®;
EMD Millipore, Billerica, MA, USA), according to the manufacturer’s
instructions. The plasmid pNF-κB-TA-Luc contains the luciferase
gene sequence driven by an artificial promoter element with four
NF-κB binding sites. Cells with genomic incorporation were selected
on the basis of antibiotic resistance (geneticin; Invitrogen Life
Technologies, Carlsbad, CA, USA). The newly generated cell line
A549-pN-κB-TA-Luc was cultured in DMEM supplemented with geneticin
(800 μg/ml) and was split (1:5) every seven days. The medium
was refreshed after a growth period of six days. Lipopolysaccharide
(1 μg/ml; Sigma-Aldrich, St. Louis, MO, USA) was added and
incubated for 4 h at room temperature. Luciferase activity was then
examined using a Luciferase Assay kit (Promega Corp., Madison, WI,
USA) to evaluate the reporter response.
Clonogenic survival assay
A549 and H1299 cells were treated with vehicle
(acetone; 2 μM) or 25 μM ginsenoside Rg3 for 24 h and
were then irradiated with a single dose of 0, 1, 2, 4, 6, 8 and 10
Gy using a 60Co unit (FHC50; Shanghai Medical Instrument
Factory, Shanghai, China) at room temperature. Ginsenoside Rg3 was
rinsed away by washing cells in sterile saline over 3 h, and the
cells were trypsinized and replated in 60-mm Petri dishes at an
appropriate cell density: 0 Gy, 40/ml; 1 Gy, 60/ml; 2 Gy,
1.5×102/ml; 4 Gy, 3.0×102/ml; 6 Gy,
8.0×102/ml, 8 Gy, 1.6×103/ml; 10 Gy,
5.0×103/ml. Colony formation was achieved by incubation
for 10–14 days. At the end of the experiment the cells were stained
with Giemsa (Beyotime Institute of Biotechnology) and the colonies
were counted. The plating efficiency (PE) and survival fraction
(SF) were calculated using the following equations: PE=(colony
number/inoculating cell number) ×100%; SF=PE (tested group)/PE (0
Gy group) ×100%. A dose-survival curve was obtained for each
experiment and used for calculating survival parameters. Parallel
samples were set at each radiation dosage. The survival curve was
plotted using Origin 7.5 software (OriginLab Corporation,
Northampton, MA, USA), using the following equation:
SF=1−(1−e−D/D0)N. The cellular
radiosensitivity (mean lethal dose, D0), the capacity
for sublethal damage repair (quasi-threshold dose, Dq) and the
extrapolation number (N) were calculated according to the
multitarget, single-hit model. The D0 values were used
to calculate sensitizer enhancement ratios (SER) (9).
In vivo antitumor effects of ginsenoside
Rg3 with or without radiation
Six-week-old male C57BL/6 mice provided by Zhengzhou
University (Zhengzhou, China) were used in the present study. All
experiments were approved by the Ethical Committee of Zhengzhou
University Health Science Center (Zhengzhou, China). The mice were
maintained in a climate-controlled room at 20°C with a 12 h
light/dark cycle and had free access to standard mice chow and
water. A total of 0.2 ml LLC cells (1×107 cells/ml) were
injected subcutaneously into the right hind leg of C57BL/6 mice.
Tumor volume was determined using caliper measurements of the tumor
length (L) and width (W), according to the following formula: Tumor
volume=0.5236× W2 × L (10). Treatment was initiated when the
tumors in each group had reached an average volume of 200
mm3, at ~6 days post innoculation. The mice were
randomly assigned to the following treatment groups (n=8):
Untreated controls; irradiation only; ginsenoside Rg3 only (10
mg/kg twice per week, orally); and combination of ginsenoside Rg3
and irradiation. Mice in the control and radiation-only groups were
administrated with the acetone vehicle. Tumors in the legs were
exposed to 8 Gy of γ-radiation using a 60Co irradiator
at a rate of 1 Gy/min immediately following treatment with the
drug. The time for the tumor volume to increase by five baseline
values was calculated for each mouse. The median times and standard
errors were calculated for each group.
EMSA
Protein (15 μg) was extracted from cell
nuclei using a nuclear extraction kit (Viagene Biotech); the
protein was then prepared, stored and qualified by methods
described previously (11). No
reducing agents were added to the EMSAs. EMSA was performed using a
DNA-Protein Binding Detection kit (Viagene Biotech), according to
the manufacturer’s instructions. Briefly, an NF-κB oligonucleotide
probe (5′-AGTTGAGGGGACTTTCCCAGGC-3′) was labeled with biotin. The
binding reaction was carried out in a 10-μl mixture
containing 1 μl 10X incubation buffer, 1 μl
poly(deoxyinosinic-deoxycytidylic) and nuclear extracts. Following
a 20-min incubation at room temperature, 0.5 μl
biotin-labeled probe was added to the samples. Following a further
incubation for 20 min at room temperature, the samples were
separated by 6% non-denaturing polyacrylamide gel (Applygen
Technologies, Inc., Beijing, China), at 150 V for 2 h at 4°C.
Subsequently, the gel was dried and exposed to X-ray film (Kodak,
Tokyo, Japan).
Assay of NF-κB transcription/promoter
activity
A total of 5×104 viable
A549-pNF-κB-TA-Luc cells were seeded into each well of a 24-well
plate. After 12 h, the cells were exposed to 60Co
radiation (8 Gy) and were then harvested 3 or 6 h later. In the
combination group, the cells were pretreated with 25 μM
ginsenoside Rg3 24 h prior to irradiation. Luciferase activity was
examined using a Luciferase Assay kit (Promega Corp.).
Western blot analysis
Whole cell lysate were collected and protein
concentrations were determined using a Bradford protein Assay kit
(P1511; Applygen Technologies, Inc.). Protein (40 mg) was denatured
and fractionated using 12% SDS-PAGE and then electrophoretically
transferred to polyvinylidene fluoride membranes (Pall Corporation,
Port Washington, NY, USA). Membranes were blocked using
Tris-buffered saline with Tween-20 (TBST) of 5% non-fat milk
(Applygen Technologies, Inc. Applygen Technologies, Inc.) for 1 h.
The membranes were subsequently probed with primary antibodies for
IκB, p-IκB, MMP-9, COX-2, VEGF, cyclin D, c-myc, Bcl-2 and β-actin.
The blots were then probed with appropriate horseradish
peroxidase-conjugated secondary antibodies (Santa Cruz
Biotechnology, Inc.). Blots were developed using a Supersignal
Chemiluminescent Detection kit (Pierce Biotechnology, Inc.,
Rockford, IL, USA) and protein bands were visualized using a Kodak
Image Station 400 and Kodak 1D 3.6 software (Kodak). β-actin was
used as an internal control.
Statistical analysis
Unless otherwise stated, values are expressed as the
mean ± standard deviation. For comparisons between groups, a
one-way analysis of variance was used. The significance of the
difference between the means of two variables was determined using
a paired Student’s t-test. SPSS 13.0 software (SPSS, Inc., Chicago,
IL, USA) was used for all statistical analyses. P<0.05 was
considered to indicate a difference, and P<0.01 was considered
to indicate a statistically significant difference.
Results
Ginsenoside Rg3 radiosensitizes NSCLC
cells in vitro
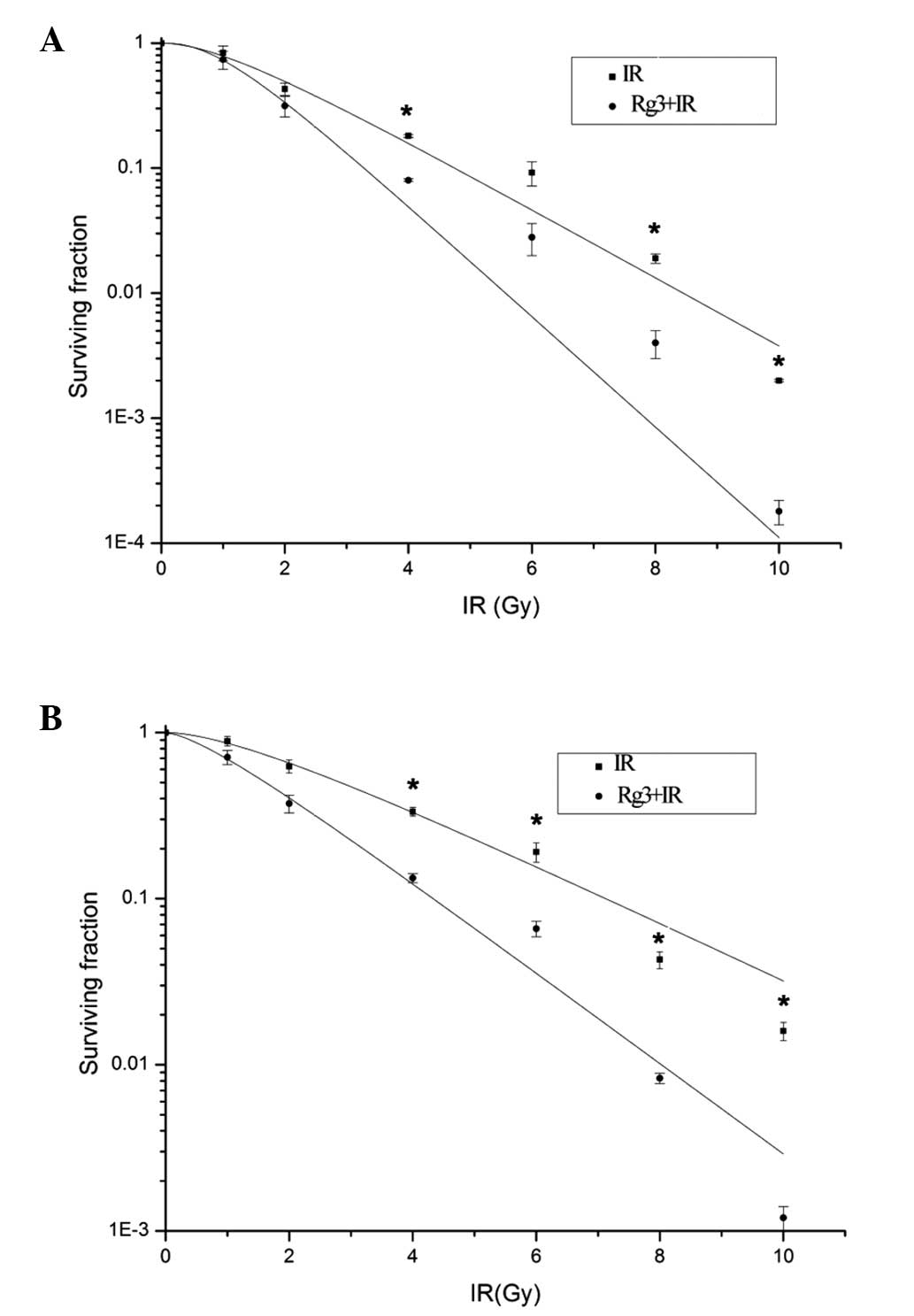
To examine the radiosensitizing effects of Rg3 on
NSCLC cells in vitro, a clonogenic survival assay was
conducted using A549 and H1299 cells. Prior to irradiation the
cells were pretreated with vehicle or ginsenoside Rg3. At the end
of the experiment the colonies were counted, and survival curves
were generated for the vehicle-treated and ginsenoside Rg3-treated
cell groups (Fig. 1). For the A549
cell line (Fig. 1A), the
radiobiological parameters of ginsenoside Rg3-treated cells were:
D0=0.971, Dq=1.021 and N=2.752; whereas the parameters
of the vehicle-treated cells were D0=1.612, Dq=1.135 and
N=2.124. The SER for the A549 cells was
SER=D0(vehicle)/D0(Rg3)=1.671. For the H1299
cell line, (Fig. 1B) the
radiobiological parameters of ginsenoside Rg3-treated cells were:
D0=1.583, Dq=0.674 and N=1.538, whereas the parameters
of the vehicle-treated cells were D0=2.598, Dq=1.463 and
N=1.793. The SER for the H1229 cells was SER=D0
(vehicle)/D0(Rg3)=1.639. These results indicated that
ginsenoside Rg3 may possess significant radio-sensitizing effects
in A549 and H1299 cells.
Ginsenoside Rg3 radiosensitizes NSCLC in
vivo
The radio-sensitizing effects of ginsenoside Rg3 on
NSCLC were also determined in vivo, using an LLC xenograft
model in C57BL/6 mice. Based on tumor volume measurements
calculated following tumor cell implantation, the mice were
randomized into four groups (n=8), as described in the Materials
and methods. Treatment with ginsenoside Rg3 was initiated after
randomization. In the untreated vehicle group, the time for the
normalized tumor volume to reach five times the original volume was
five days; for the ginsenoside Rg3-treated group the time was six
days; for the irradiation-treated group the time was 14 days; and
for the combined ginsenoside Rg3/irradiation-treated group the time
was 22 days (Fig. 2). The
enhancement factor was calculated as 1.78, as determined by
dividing the normalized tumor growth delay of the combined groups
(17 days) by the absolute tumor growth delay of the radiation-only
group (nine days).
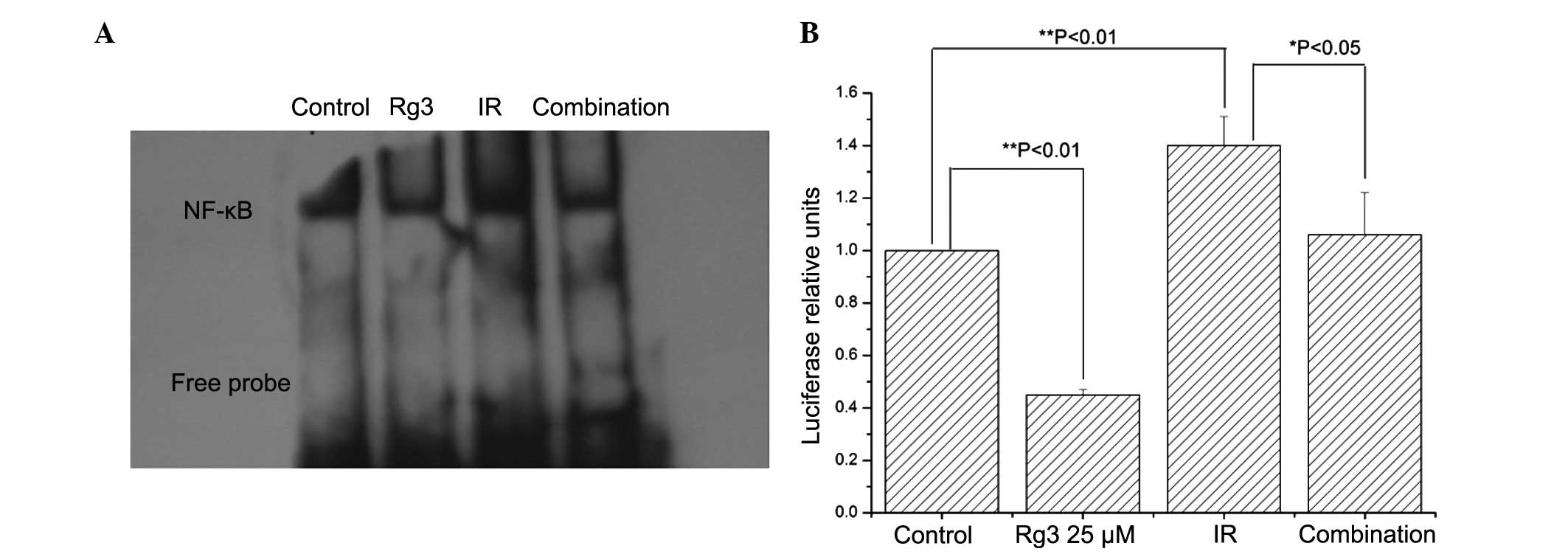
Ginsenoside Rg3 suppresses
radiation-induced NF-κB activation
Radiation induces cell death through DNA damage. In
order to prevent these injuries, numerous signaling pathways in
tumor cells are stimulated, among which the NF-κB pathway has been
shown to have a key role (12).
Furthermore, NF-κB has previously been identified as a direct
target of ginsenoside Rg3 (6).
Therefore, the present study investigated the effects of
ginsenoside Rg3 on radiation-induced NF-κB activation. Irradiation
increased NF-κB DNA binding activity, which was suppressed by
pretreatment with ginsenoside Rg3 (Fig. 3A). These results were confirmed by
NF-κB luciferase reporter assays (Fig.
3B). These data suggested that ginsenoside Rg3 may suppress
radiation-induced NF-κB activation in NSCLC cells.
Ginsenoside Rg3 inhibits
radiation-induced IκB phosphorylation and degradation
In order to investigate how ginsenoside Rg3 was able
to suppress radiation-induced NF-κB activation, the phosphorylation
status of IκB was determined by western blot analysis. IκB
phosphorylation leads to degradation of IκB and the release of
cytoplasmic NF-κB for nuclear translocation and DNA binding
(7). Irradiation decreased the
protein expression levels of IκB, which was most obvious 3 h after
radiation, whereas treatment with ginsenoside Rg3 (25 μM)
significantly inhibited this radiation-induced decrease in IκB
protein expression levels (Fig.
4).
Ginsenoside Rg3 downregulates the protein
expression levels of NF-κB-regulated genes
NF-κB regulates tumor radioresistance through
regulating the expression of Bcl-2, cyclin D1, c-myc, COX-2, VEGF
and MMP-9 (13). Therefore, the
present study examined the effects of radiation and ginsenoside Rg3
on the protein expression levels of these gene products by western
blot analysis in the A549 cell line (Fig. 5). Treatment with ginsenoside Rg3
downregulated radiation-induced expression of Bcl-2, cyclin D1,
c-myc, COX-2, VEGF and MMP-9. These results suggested that
ginsenoside Rg3 may sensitize NSCLC cells to radiation through
modulation of NF-κB-regulated gene products.
Discussion
Radiotherapy has an important role in NSCLC therapy;
however, radioresistance and toxicity are barriers to its
successful application. Radiosensitizers are designed to enhance
the destruction of tumor cells, whilst exhibiting reduced adverse
effects on normal tissues, which may partly solve the
radioresistance problem. Research regarding radiosensitizers has
focused on proteins associated with cell signaling and growth
receptors (14). As a key
transcription factor, NF-κB regulates numerous genes participating
in cell proliferation, invasion, angiogenesis, metastasis,
suppression of apoptosis and treatment resistance in tumors
(13). Numerous inhibitors of
NF-κB have been shown to possess radiosensitizing effects (15–19).
Therefore, targeting the NF-κB pathway may provide a novel method
for improving current radiosensitizing effects in NSCLC.
Numerous studies have investigated radiosensitizer
compounds; however, several of these compounds have been shown to
be too toxic (20). Due to their
immune-regulating effects, various Asian herbs have been reported
to exhibit radiosensitizing effects in vivo as well as in
vitro (21–23). Ginseng is widely used in numerous
cultures, particularly in China, for the prevention and treatment
of numerous types of disease, including cancer (24). Ginsenoside Rg3 is a major ginseng
saponin derived from heat-processed ginseng (Sun Ginseng), which
has been reported to possess anti-inflammatory and anti-tumor
promoting effects via inhibiting NF-κB activation (6). Due to the key role of NF-κB in
radio-resistance, the present study investigated the
radiosensitizing effects of ginsenoside Rg3.
The clonogenic assay is a common method used to
evaluate the effects of radiation on cell death. To study the
radiosensitizing effects of ginsenoside Rg3 on NSCLC, two lung
carcinoma cell lines, A549 and H1299, were selected. The SER in the
two cell lines was shown to be >1, which suggested that
ginsenoside Rg3 exhibits radiosensitizing effects on these cells.
Furthermore, the radiosensitizing effects of ginsenoside Rg3 were
observed in C57BL/6 mice bearing LLC xenografts. These results
indicated that ginsenoside Rg3 has the tendency to increase the
curative effects of radiation therapy without obvious toxic
effects, thus suggesting that ginsenoside Rg3 may possess vast
potential as a novel radiosensitizer.
To study the mechanisms underlying the
radiosensitizing effects of ginsenoside Rg3, EMSA and luciferase
reporter assays were performed. The radiosensitizing effects of
ginsenoside Rg3 were most obvious at 8 Gy (data not shown);
therefore, this dosage level was selected for the subsequent
studies. The EMSA demonstrated that NF-κB DNA binding activity was
markedly increased following 8 Gy irradiation, and this effect was
inhibited by pretreatment with ginsenoside Rg3. The luciferase
reporter assay also demonstrated that treatment with ginsenoside
Rg3 inhibited radiation-induced NF-κB expression.
NF-κB is a ubiquitous eukaryotic transcription
factor and is a dimer of Rel family proteins. NF-κB is inhibited by
IκB and is usually sequestered in the cytoplasm, where it loses its
DNA binding effect. However, when cells are exposed to
extracellular stimulation, IκB is degraded, following
phosphorylation, and NF-κB can subsequently translocate into the
nucleus, where it triggers the transcription of a wide array of
genes that are crucial for diverse physiological responses
(13). Numerous distinct NF-κB
activation pathways have been described (25). Among them, the classical pathway
has been the most-well studied. Following exposure to irradiation,
IKB kinase β, which is necessary and sufficient for phosphorylation
of IκB, is activated. IκB is then degraded following
phosphorylation, resulting in the release of NF-κB protein. NF-κB
may then translocate into the nucleus, where it binds DNA sites,
and various genes responsible for irradiation resistance are
stimulated (26). The results of
the present study demonstrated that by inhibiting phosphorylation
of IκB, ginsenoside Rg3 inhibited the radiation-induced activation
of NF-κB, resulting in a radio-sensitizing effect. However, there
may be other potential targets for the radiosensitizing effects of
ginsenoside Rg3, including its anti-angiogenic effect (27).
In conclusion, the present study demonstrated that
ginsenoside Rg3 exhibited a radiosensitizing effect in NSCLC cell
lines and a xenograft model. Furthermore, the clinical verification
of ginsenoside Rg3 as a radiosensitizer of NSCLC is of considerable
interest, due to its affordability, immune regulating effect, ease
of oral administration and lack of toxicity. All of these
properties make ginsenoside Rg3 a promising novel anti-tumor agent
for the treatment of NSCLC.
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2012. CA Cancer J Clin. 62:10–29. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lal R, Enting D and Kristeleit H: Systemic
treatment of non-small-cell lung cancer. Eur J Cancer.
47:S375–S377. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cole AJ, Hanna GG, Jain S and O’Sullivan
JM: Motion management for radical radiotherapy in non-small cell
lung cancer. Clin Oncol (R Coll Radiol). 26:67–80. 2014. View Article : Google Scholar
|
|
4
|
Wardman P: Chemical radiosensitizers for
use in radiotherapy. Clin Oncol (R Coll Radiol). 19:397–417. 2007.
View Article : Google Scholar
|
|
5
|
Jia L, Zhao Y and Liang XJ: Current
evaluation of the millennium phytomedicine- ginseng (II): Collected
chemical entities, modern pharmacology and clinical applications
emanated from traditional Chinese medicine. Curr Med Chem.
16:2924–2942. 2009. View Article : Google Scholar :
|
|
6
|
Keum YS, Han SS, Chun KS, et al:
Inhibitory effects of the ginsenoside Rg3 on phorbol ester-induced
cyclooxygenase-2 expression, NF-kappaB activation and tumor
promotion. Mutat Res. 523–524:75–85. 2003. View Article : Google Scholar
|
|
7
|
Munshi A, Kurland JF, Nishikawa T, et al:
Inhibition of constitutively activated nuclear factor-kappaB
radiosensitizes human melanoma cells. Mol Cancer Ther. 3:985–992.
2004.PubMed/NCBI
|
|
8
|
Kim WY, Kim JM, Han SB, et al: Steaming of
ginseng at high temperature enhances biological activity. J Nat
Prod. 63:1702–1704. 2000. View Article : Google Scholar
|
|
9
|
Wang Z, Cook T, Alber S, et al: Adenoviral
gene transfer of the human inducible nitric oxide synthase gene
enhances the radiation response of human colorectal cancer
associated with alterations in tumor vascularity. Cancer Res.
64:1386–1395. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Miyake H, Tolcher A and Gleave ME:
Antisense Bcl-2 oligodeoxynucleotides inhibit progression to
androgen-independence after castration in the Shionogi tumor model.
Cancer Res. 59:4030–4034. 1999.PubMed/NCBI
|
|
11
|
Takada Y, Khuri FR and Aggarwal BB:
Protein farnesyltransferase inhibitor (SCH 66336) abolishes
NF-kappaB activation induced by various carcinogens and
inflammatory stimuli leading to suppression of NF-kappaB-regulated
gene expression and up-regulation of apoptosis. J Biol Chem.
279:26287–26299. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kunnumakkara AB, Diagaradjane P, Guha S,
et al: Curcumin sensitizes human colorectal cancer xenografts in
nude mice to gamma-radiation by targeting nuclear
factor-kappaB-regulated gene products. Clin Cancer Res.
14:2128–2136. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shen HM and Tergaonkar V: NFkappaB
signaling in carcinogenesis and as a potential molecular target for
cancer therapy. Apoptosis. 14:348–363. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yu SD, Liu FY and Wang QR: Notch
inhibitor: a promising carcinoma radiosensitizer. Asian Pac J
Cancer Prev. 13:5345–5351. 2012. View Article : Google Scholar
|
|
15
|
Aravindan N, Madhusoodhanan R, Ahmad S,
Johnson D and Herman TS: Curcumin inhibits NFkappaB mediated
radio-protection and modulate apoptosis related genes in human
neuroblastoma cells. Cancer Biol Ther. 7:569–576. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Johnson GE, Ivanov VN and Hei TK:
Radiosensitization of melanoma cells through combined inhibition of
protein regulators of cell survival. Apoptosis. 13:790–802. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Raffoul JJ, Wang Y, Kucuk O, Forman JD,
Sarkar FH and Hillman GG: Genistein inhibits radiation-induced
activation of NF-kappaB in prostate cancer cells promoting
apoptosis and G2/M cell cycle arrest. BMC Cancer. 6:1072006.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sun Y, St Clair DK, Fang F, et al: The
radiosensitization effect of parthenolide in prostate cancer cells
is mediated by nuclear factor-kappaB inhibition and enhanced by the
presence of PTEN. Mol Cancer Ther. 6:2477–2486. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kamer S, Ren Q and Dicker AP: Differential
radiation sensitization of human cervical cancer cell lines by the
proteasome inhibitor velcade (bortezomib, PS-341). Arch Gynecol
Obstet. 279:41–46. 2009. View Article : Google Scholar
|
|
20
|
Eberhardt W, Pöttgen C and Stuschke M:
Chemoradiation paradigm for the treatment of lung cancer. Nat Clin
Pract Oncol. 3:188–199. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Block WD, Merkle D, Meek K and Lees-Miller
SP: Selective inhibition of the DNA-dependent protein kinase
(DNA-PK) by the radiosensitizing agent caffeine. Nucleic Acids Res.
32:1967–1972. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chendil D, Ranga RS, Meigooni D,
Sathishkumar S and Ahmed MM: Curcumin confers radiosensitizing
effect in prostate cancer cell line PC-3. Oncogene. 23:1599–1607.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Raffoul JJ, Banerjee S, Singh-Gupta V, et
al: Down-regulation of apurinic/apyrimidinic endonuclease 1/redox
factor-1 expression by soy isoflavones enhances prostate cancer
radiotherapy in vitro and in vivo. Cancer Res. 67:2141–2149. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shibata S: Chemistry and cancer preventing
activities of ginseng saponins and some related triterpenoid
compounds. J Korean Med Sci. 16:S28–S37. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Van Waes C: Nuclear factor-κB in
development, prevention, and threapy of cancer. Clin Cancer Res.
13:1076–1082. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li N and Karin M: Ionizing radiation and
short wavelength UV activate NF-kappaB through two distinct
mechanisms. Proc Natl Acad Sci USA. 95:13012–13017. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kim JW, Jung SY, Kwon YH, et al:
Ginsenoside Rg3 attenuates tumor angiogenesis via inhibiting
bioactivities of endothelial progenitor cells. Cancer Biol Ther.
13:504–515. 2012. View Article : Google Scholar : PubMed/NCBI
|