Introduction
Breast cancer is the most common cancer in female
adults worldwide and its incidence is rising (1). Although significant advances have
been achieved in therapeutic strategies for breast cancer, it
remains to be the cancer type with the second highest rate of
mortality (1). Breast cancer is a
complex process with a long latency; thus, chemoprevention aiming
to prevent, reverse or delay carcinogenesis may benefit patients by
reducing cancer risk and the need for invasive interventions
(2).
In clinical practice, erbB2 is amplified and
overexpressed in ~30% of human breast primary tumors, and these
tumors tend to have poor prognosis (3). Following several clinical trials, the
US Food and Drug Administration (FDA) approved tamoxifen in 1997
and raloxifene in 2007 for breast cancer chemoprevention (4–6).
However, to date, the use of tamoxifen and raloxifene for primary
breast cancer chemoprevention in the US has remained low due to
their side effects (2). Thus, it
is important to investigate novel chemopreventive agents and
intervention strategies for patients with breast cancer,
particularly for those with erbB2-positive cancer.
In the etiology and progression of breast cancer,
the protein kinase B (AKT) and c-Jun N-terminal kinase (JNK)
signaling pathways have been suggested to participate in cell
proliferation, migration, angiogenesis and protection against
apoptosis (7,8). It is widely accepted that activation
of AKT through phosphorylation can inhibit proliferation in tumor
cells (9). Previous studies have
demonstrated that copper (Cu) is a strong inducer of reactive
oxygen species (ROS) and high ROS activity can damage DNA, protein
and lipids, leading to apoptosis (10,11).
In addition, breast cancer tumors have been reported to have high
tissue levels of copper (12–15).
Thus, copper and the AKT and JNK signaling pathways are suggested
as potential therapeutic targets in breast cancer chemoprevention
and chemotherapy. Disulfiram (DS) has been used as the first-line
anti-alcoholism drug for over 70 years with no clear toxicity
(16). Previous studies indicated
that DS can penetrate and intracellularly chelate Cu forming a new
complex, which in turn inhibits cell proliferation and induces
apoptosis via AKT, JNK and ROS regulation (15,17–20).
However, whether the DS/Cu complex exerts chemopreventive effects
on breast cancer remains elusive. Thus, the present study aimed to
investigate the potential chemopreventive effects of DS/Cu on
breast cancer.
The MMTV-erbB2 transgenic mouse model, which
overexpresses the wild-type erbB2/neu proto-oncogene, is able to
spontaneously produce erbB2-positive mammary cancer tumors due to
the MMTV promoter-induced overexpressed erbB2 gene in their mammary
glands (21). Thus far, this mouse
model has been widely used to evaluate the efficacy of potential
chemopreventive drugs and investigate relative underlying
mechanisms (22,23). In a previous study, it was observed
that high doses of dietary soy isoflavones delay tumor development
in MMTV-erbB2 transgenic mice (24). In the present study, in order to
investigate the potential of using DS/Cu in erbB2-positive breast
cancer chemoprevention, the effect of DS/Cu treatment on the
development of mammary tumors was examined in MMTV-erbB2 transgenic
mice. In the present study, DS/Cu was used at a low dose (50 mg/kg
body weight for eight weeks) with minimum toxicity risk, good
medication adherence and thereby acceptable risk-benefit ratio,
which are all factors critical to the success of a cancer risk
reduction intervention in clinical practice. The use of DS/Cu was
hypothesized to delay the development of mammary tumors in
MMTV-erbB2 transgenic mice.
Materials and methods
Reagents and antibodies
DS, copper (II) chloride (CuCl2) and
dimethyl sulfoxide were obtained from Sigma-Aldrich (St. Louis, MO,
USA). The following antibodies were purchased from Cell Signaling
Technology (Danvers, MA, USA): Rabbit monoclonal Akt (cat. no.
4685; 11E7), p-Akt (Ser 473; cat. no. 4058; 193H12), JNK (cat. no.
9258; 56G8) and p-JNK (Thr183/Tyr185; cat. no. 4668; 81E11). The
following antibodies were purchased from Santa Cruz Biotechnology,
Inc. (Dallas, TX, USA): Rabbit polyclonal immunoglobulin (Ig)G
cyclin D1 (sc-718; M-20), pP38 (Tyr182; sc-101759), nuclear factor
κB (NF-κB p65; sc-7151; H-286) and mouse monoclonal IgG1 β-actin
(sc-8432; C-2). Horseradish peroxidase (HRP)-labeled goat
anti-rabbit (cat. no. 31466) and goat anti-mouse (#31431) secondary
antibodies were obtained from Thermo Fisher Scientific (Waltham,
MA, USA). erbB2 was obtained from Neomarkers, Inc. (Fremont, CA,
USA). The following reagents were obtained from Maixin Biotech Co.,
(Fuzhou, China): 10% neutral formalin, ethanol, citrate buffer,
xylene, H2O2, paraffin, methanol, normal
serum, eosin, hematoxylin, chloroform, glacial acetic acid and
carmine alum. Tris-buffered saline/Tween 20 (TBST) was purchased
from BD Chemical (Greenwood Village, CO, USA) and DMEM/F12, fetal
bovine serum, penicillin and streptomycin were ordered from
Gibco-BRL (Carlsbad, CA, USA).
Animals
The present study was approved by the ethics
committee of The First Affiliated Hospital of Henan University of
Science and Technology (Luoyang, China). All animal experiments
were conducted in accordance with National Institutes of Health
guidelines. A total of 50 six-week-old female FVB/N-Tg (MMTV-erbB2)
202Mul/J mice were purchased from The Jackson Laboratory (Stock no.
002376; Bar Harbor, ME, USA). Average weight at 20 weeks was 20±2
g. Mice were kept under a 12-h reversed light/dark cycle and the
temperature was maintained at 22°C in the animal house facility,
which was located in the Molecular Biology Core Laboratory (The
First Affiliated Hospital of Henan University of Science and
Technology, Luoyang, China). The mice were fed an AIN-93G pellet
diet (D10012G; Research Diets, New Brunswick, NJ, USA) with free
access to water. Based on previous studies and human therapeutic
doses (16,18), mice were treated with DS (50
mg/kg/day)/Cu (0.1 mg/kg/day) or vehicle (100 μl normal
saline) for 6 days/week from the age of 20–28 weeks via
subcutaneous injection (n=25/group). Subsequent to eight weeks of
treatment (28 weeks of age), five mice from each group were
sacrificed through cervical vertebra dislocation following
intraperitoneal anaesthesia with propofol (100 mg/kg; GuoRui
Pharmaceutical Co., Ltd, Chengdu, China), the right abdominal
mammary gland was whole mounted and the right thoracic mammary
gland was fixed in 10% neutral formalin for immunohistochemistry,
whereas the rest of the tissue was immediately frozen in liquid
nitrogen for protein analyses. The remaining mice in each group
were monitored twice a week to the end of the experiment. Tumor
development and tumor volume were recorded. The first sign of tumor
development was a palpable solid tumor with a volume of 100
mm3. The tumor-free interval was defined as the time
from birth until the first tumor occurred. In order to alleviate
suffering, the mice were sacrificed by cervical dislocation under
intraperitoneal anaesthesia with propofol (100 mg/kg) prior to the
tumor diameter reaching 1.5 cm, which occurred ~6 weeks following
the development of palpable tumors. Following 6 weeks the mice were
sacrificed and the tumors were harvested and weighed.
Cell culture
The human breast cancer cells BT474
(erbB2high), human microvascular endothelial cells
(HMECs) and normal breast epithelial cells (MCF10A) were purchased
from the China Center for Type Cell Culture Collection (Wuhan
University; Wuhan, China), while the EM01 cell line derived from
MMTV-erbB2 mammary tumors was established in The Molecular Biology
Core Laboratory of the First Affiliated Hospital of Henan
University of Science and Technology. All cell lines were
maintained in DMEM/F12 culture medium supplemented with 10% fetal
bovine serum, penicillin (100 U/ml) and streptomycin (100
μg/ml) in a humidified Thermo Forma Series II Water Jacket
incubator (37°C, 5% carbon dioxide; Thermo Fisher Scientific).
MTS assay
In vitro cell proliferation was determined by
the MTS assay using the Cell Titer 96 AQueous One Solution Cell
Proliferation Assay System (Promega Corporation, Madison, WI, USA).
Briefly, cells were seeded into 96-well plates (500 cells/well).
After 24 h, cells were treated with DS (0–10 μmol/l)/Cu (0.2
μmol/l) for 24 h. Control cells were treated with 0.1%
dimethyl sulfoxide in culture medium. Subsequently, the MTS assay
was conducted in order to examine the growth of different cell
groups and absorbance was read at 540 nm using a 96-well Thermo
Scientific Multiskan Ascent Microplate reader (Thermo Fisher
Scientific) according to the manufacturer’s instructions.
Histology and immunohistochemistry
Mammary glands were fixed in 10% neutral formalin,
dehydrated with a graded ethanol series and embedded in paraffin.
Sections (5 μm) were stained with hematoxylin and eosin for
histological analysis or processed for immunohistochemistry.
Immunohistochemistry was performed using the Vectastain Elite
avidin-biotin complex (ABC) kit (Vector Laboratories, Inc.,
Burlingame, CA, USA) in accordance with the manufacturer’s
instructions. Mammary gland sections were deparaffinized and
rehydrated by successive washes with xylene and a graded ethanol
series. Antigen retrieval was conducted in citrate buffer (pH 6.0)
at 100°C for 30 min in a steamer, endogenous peroxidase activity
was quenched with 3% H2O2 in methanol for 10
min at room temperature and nonspecific binding was blocked with
10% normal serum. The sections were incubated at 4°C overnight with
the primary antibodies, then subsequently with the biotinylated
secondary antibody for 1 h, followed by ABC reagent (Vector
Laboratories, Inc.) and diaminobenzidine. Slides were
counterstained with hematoxylin.
Bromodeoxyuridine (BrdU) and terminal
deoxynucleotidyl transferase-mediated dUTP nick end labeling
(TUNEL) assay
The BrdU assay was conducted using the BrdU Cell
Proliferation Assay kit (BD Biosciences, San Jose, CA, USA). Mice
were injected intraperitoneally with 200 μl (3 mg/ml) BrdU
solution 2 h prior to scarification. Subsequently, cellular
incorporation of BrdU was detected by immunohistochemistry using
anti-BrdU-specific antibodies. The TUNEL assay was performed using
the ApopTag Peroxidase In situ Apoptosis Detection kit
(Merck Millipore, Darmstadt, Germany). Briefly, following
deparaffinization and hydration, the tissue was incubated with
Working Strength TdT enzyme, Working Strength Stop/Wash buffer,
conjugated with antidigoxigenenin, and then stained with peroxidase
substrate. The tissue was then mounted under a glass coverslip in
Permount and viewed under a Nikon 80i microscope (Nikon Corp.
Tokyo, Japan).
Mammary whole-mount preparation
Mammary tissue was mounted onto a glass slide and
fixed in Carnoy’s fix solution (100% ethanol/chloroform/glacial
acetic acid 6:3:1) overnight. The sample was then rehydrated in 70,
50, 30 and 10% ethanol for 30 min each, stained overnight in
carmine alum stain, then dehydrated with 70, 95 and 100% ethanol
and cleared in xylene overnight. Following this, the glass slide
was analyzed under a DTM 1200 digital camera (Nikon Corp.) mounted
on a Nikon C-LEDS microscope (Nikon Corp.).
Western blot analysis
Cells or tissues were homogenized at 4°C in
radioimmunoprecipitation assay lysis buffer (Thermo Fisher
Scientific) for extracting total protein. The protein was
quantitated using a bicinchoninic acid protein assay kit (Pierce
Biotechnology, Inc., Rockford, IL, USA). Equal samples (50
μg protein) were electrophoresed through 10–12% SDS-PAGE
gels and transferred to nitrocellulose membranes (Millipore,
Boston, MA, USA). The membranes were then blocked in 5% non-fat
powdered milk/TBST for 1 h and then incubated overnight with the
relevant primary antibodies at 4°C overnight (Akt, 1:1,000; p-Akt,
1:1,000; JNK, 1:1,000; p-JNK, 1:1,000; Cyclin D1, 1:2,000; pP38,
1:500; NF-κB p65, 1:500; p65, 1:500; and β-actin, 1:3,000). The
membranes were then washed with TBST and incubated with goat
anti-mouse/rabbit HRP-labeled secondary antibodies (1:5,000) for
1.5 h at room temperature. The membranes were then washed again
with TBST and visualized by using electrochemiluminescence (Thermo
Fisher Scientific).
Statistical analysis
Statistical analyses were performed using GraphPad
Prism 5.0 software (GraphPad, Inc., La Jolla, CA, USA). Tumor-free
intervals for survival curves were calculated using the
Kaplan-Meier method. The significance of the differences between
the groups was determined using a two-sided Student’s t-test.
P≤0.05 was considered to indicate a statistically significant
difference.
Results
DS/Cu inhibits growth of BC cells but not
of normal cells
In cancer chemoprevention, the ability to exert
cytotoxicity in tumor cells but not in normal cells is an important
criterion for novel chemopreventative drugs (25,26).
As breast cancer cells and tissues accumulate high levels of copper
(12), this can be utilized for
the enhancement of copper in breast cancer cells as a novel
chemopreventive strategy. DS is known to be able to bind copper,
and the DS/Cu complex has been reported to be toxic to various
cancer cells and xenografts (11,18).
However, whether the DS/Cu complex selectively targets breast
cancer cells while sparing normal mammary cells remains to be
investigated.
In the present study, normal cells (HMECs),
immortalized normal cells [normal breast epithelial cells (MCF10A)]
(27), malignant cells [human
breast cancer cells BT474 (erbB2high)] and EM01
(erbB2high) breast cancer primary culture cells from
MMTV-erbB2 transgenic mice were used to determine this issue. These
cell lines were incubated with the DS (0, 0.1, 1 or 10
μmol/l)/Cu (0.2 μmol/l) complex for 24 h and were
then examined using the MTS assay. As demonstrated in Fig. 1, the effects of DS-Cu treatment on
the proliferation of the BT474 cells and EM01 cells were observed
to be dose-dependent. Compared with these two cell lines, the
normal HMECs and MCF10A cells are significantly more resistant to
the DS/Cu complex. These results suggested that the DS/Cu complex
has anti-cancer activity selectively in breast cancer cells over
normal cells.
The DS/Cu complex inhibits cell growth
and induces apoptosis via activating the JNK pathway and inhibiting
AKT, cyclin D1 and NF-κB signaling
Tumor inhibition is known to commonly occur via two
mechanisms: i) Proliferation inhibition and ii) apoptosis promotion
(28). Whether these two
mechanisms serve a role in DS/Cu complex-induced tumor inhibition
remains elusive. Regulation of AKT, cyclin D1, NF-κB and JNK
signaling has been reported to be the predominant mechanism
underlying DS/Cu complex activity (15,17,19,20,29).
Thus, in the present study, BT474 cells were incubated with 0, 5 or
10 μmol/l DS/Cu (0.2 μmol/l) complex for 24 h, and
the treated cells were then analyzed using western blot analysis
and TUNEL staining. Consistent with previous studies (10,11,18),
TUNEL staining identified a significantly greater number of
apoptotic cells in DS (5 and 10 μmol/l)/Cu (0.2
μmol/l) complex-treated cells. DS/Cu complex treatment
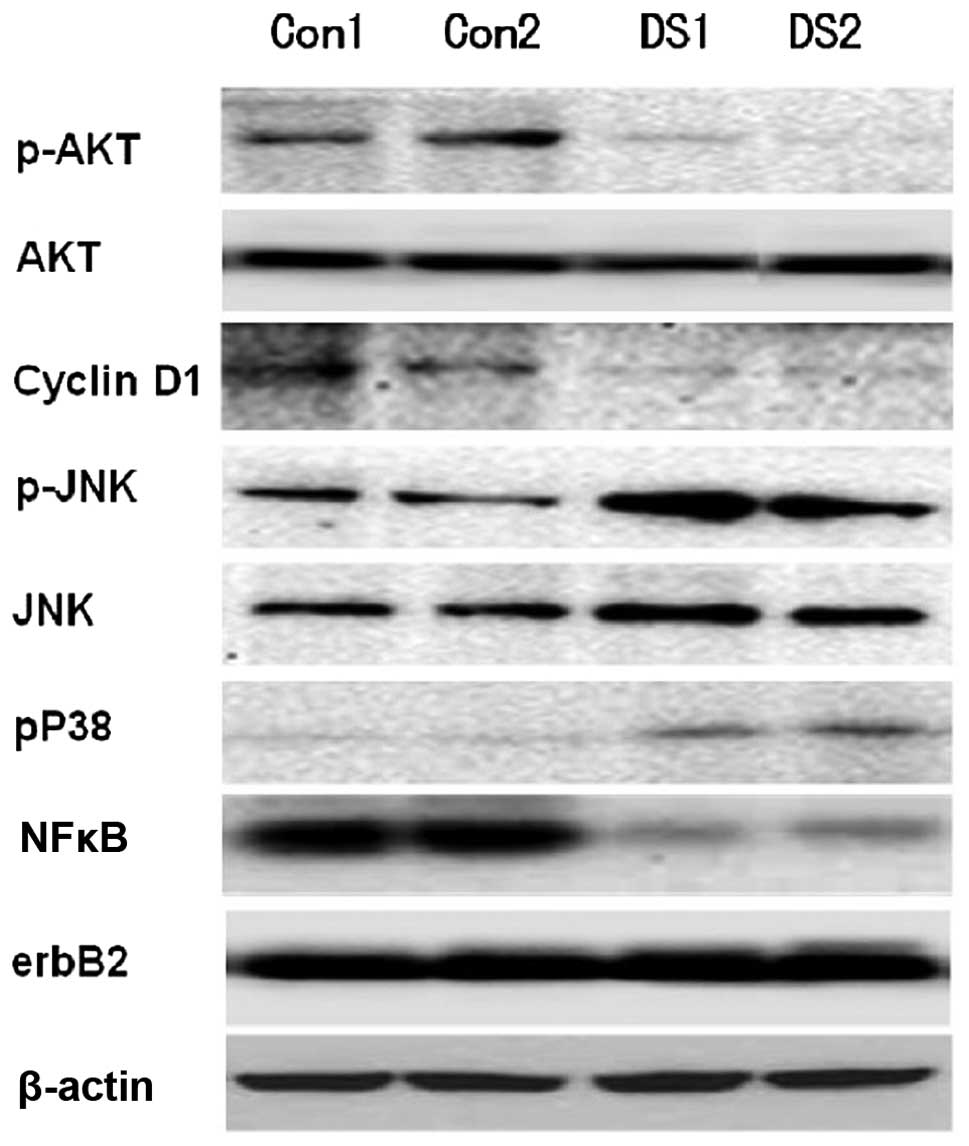
resulted in a significant upregulation of pJNK and pP38 expression
and a downregulation of p-AKT, cell proliferation marker cyclin D1
and NF-κB expression (Fig. 2).
Furthermore, the effect of the DS/Cu complex on these protein
expression levels in the normal HMECs and MCF10A cells was also
measured, and no significant results were observed (data not
shown). These observations suggested that the DS/Cu complex may
upregulate JNK and inhibit AKT, cyclin D1 and NF-κB signaling,
which in turn activate apoptosis and suppress proliferation in
breast cancer cells.
DS/Cu delays the development of mammary
tumors in MMTV-erbB2 mice
To determine the cancer-preventive effect of the
DS/Cu complex, 50 MMTV-erbB2 mice were equally randomized into two
groups. One group of mice were treated with the vehicle, while the
other one was treated with DS (50 mg/kg/day)/Cu (0.1 mg/kg/day) for
6 days/week from age 20 weeks to 28 weeks by subcutaneous injection
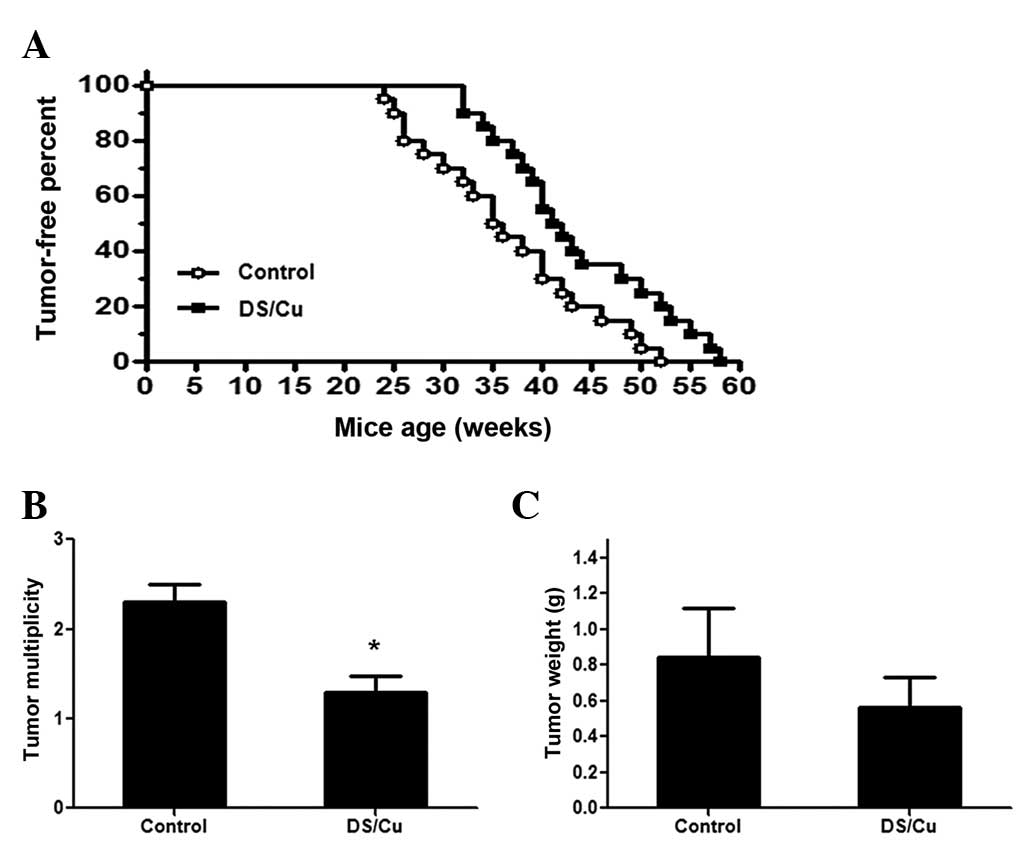
(n=25/group). Data for the development of mammary tumors was
collected, and it was observed that the DS/Cu-treated mice took
32–58 weeks to begin to develop palpable tumors, with a median time
of 292 days, while the mice in the vehicle group began to develop
tumors after 24–52 weeks, with a median time of 265 days. There was
a significant delay in tumor development between the two groups
(P=0.0191) (Fig. 3A). The tumor
multiplicity and tumor weight of the mice between the two groups
was also measured, and it was identified that DS/Cu treatment
significantly reduced the average tumor multiplicity and
nonsignificantly reduced tumor weight. As presented in Fig. 3B, the tumor multiplicity (number of
tumors in each mouse) in the DS/Cu treatment group (1.5±0.19) was
significantly lower than that in control group (2.3±0.18)
(P<0.05). The tumor weight in the DS/Cu treatment group
(0.51±0.19 g) was nonsignificantly lower than that of the control
group (0.84±0.28 g) (Fig. 3C).
Compared with the untreated MMTV-erbB2 mice, common toxicities
(rash, dry skin or diarrhea) nor weight loss were observed in mice
treated with either DS/Cu or vehicle. These results suggested that
short term exposure of DS/Cu at low doses may delay the development
of mammary tumors in MMTV-erbB2 mice.
DS/Cu delays mammary gland development,
inhibits proliferation and induces apoptosis of mammary tumors in
MMTV-erbB2 mice
DS/Cu has been previously demonstrated to exert its
biological activity primarily through proliferation inhibition and
apoptosis promotion (18,30); thus, the present study aimed to
further verify this in vivo. To investigate this, following
8 weeks of treatment, the mammary glands at age 28 weeks were
examined for the ductal architecture, proliferation and apoptosis
using whole mount analysis, BrdU assay and TUNEL staining,
respectively. As presented in Fig.
4, compared with the vehicle-treated mammary glands, those
treated with DS/Cu displayed significantly reduced lateral
branching and alveolar structures. As expected, the mammary glands
and tumors from the DS/Cu-treated group demonstrated significantly
lower proliferation and higher apoptotic rates than those of
vehicle-treated group (Fig. 5).
These data indicated that DS/Cu suppresses mammary tumorigenesis
and mammary tumor growth in MMTV-erbB2 mice through proliferation
inhibition and apoptosis promotion.
DS/Cu activates JNK signaling while
suppressing AKT, cyclin D1 and NF-κB signaling in the mammary
glands of MMTV-erbB2 mice
Having verified the inhibitory effect and underlying
mechanism of DS/Cu on breast cancer cells in vitro, it was
hypothesized that DS/Cu may exert similar effects on the mammary
glands in MMTV-erbB2 mice. To clarify this, the mammary glands
obtained from the mice receiving 8 weeks of DS/Cu treatment were
analyzed for the expression of JNK, AKT and NF-κB signaling
pathways using western blot analysis. The expression levels of the
cell proliferation marker cyclin D1 in mammary glands were also
measured. Compared with the control group, increased pJNK and pP38
expression levels, and reduced p-AKT, cyclin D1 and NF-κB protein
expression levels were observed in the mammary glands of mice
treated with DS/Cu (Fig. 6). These
data are consistent with the in vitro results. These
observations are also in support of the hypothesis that DS/Cu may
exert chemopreventive effects through two ways: Proliferation
inhibition via regulating AKT and cell cycle signaling, and
apoptosis promotion via activating JNK while inhibiting NF-κB
signaling.
DS/Cu does not interfere with MMTV
promoter-driven erbB2 expression
To exclude the possibility that the suppressing
effect of DS/Cu was facilitated by the reduced expression of the
erbB2 transgene, the erbB2 protein expression levels were further
investigated in mammary glands treated with DS/Cu or the vehicle
using western blot analysis. It was observed that erbB2 protein
expression remained unaltered in the presence of DS/Cu in
vivo (Fig. 6), which indicated
that the effects of DS/Cu are not due to a reduction in erbB2
transgene expression.
Discussion
In the present study, the potential chemopreventive
effects of DS/Cu exposure on the development of erbB2 positive
tumors were investigated in MMTV-erbB2 transgenic mice. An age of
20 weeks is considered as the premalignant phase in mice (21), and the results of the present study
demonstrated that short term DS/Cu exposure at the premalignant
phase delayed the mammary tumor development in MMTV-erbB2
transgenic mice. This is suggested to be based on growth inhibition
and apoptosis activation in mammary glands. The results indicated
that DS may suppress erbB2-positive mammary tumorigenesis in
MMTV-erbB2 mice by preventing premalignant lesions, via
proliferation inhibition and apoptosis promotion. The underlying
mechanisms of this may include suppression of AKT, cyclin D1 and
NF-κB signaling and activation of the JNK pathway.
Short-term, low-dose chemoprevention can improve
patient compliance, in addition to minimizing chronic side effects,
making it a suitable chemopreventive strategy (26). It was hypothesized that short-term,
low-dose DS/Cu intervention in the premalignant phase may delay the
onset of mammary tumor development. To verify this hypothesis, the
MMTV-erbB2 transgenic mouse model was used, which mimicked
carcinogenesis of human erbB2-positive breast cancer. The mice were
treated with DS/Cu or vehicle for 2 months from the age of 20–28
weeks. As the mice usually develop tumors from 6 months of age, the
treatment phase was considered to be in the premalignant phase in
the mice. It was observed that 8 weeks of DS treatment delayed the
development of mammary tumors by 4 weeks (mean age of tumor onset,
38 and 42 weeks in DS-treated and vehicle-treated mice,
respectively), and all vehicle-treated and DS-treated mice had
developed tumors at the conclusion of the experiment (58
weeks).
It is of note that the delay time (4 weeks) in tumor
onset is shorter than the treatment time (8 weeks). The following
reasons are suggested to explain this disparity. Firstly, the dose
of DS/Cu used was relatively low, which may not completely prevent
premalignant lesions. Secondly, DS/Cu itself may not have the
capacity to completely suppress mammary tumorigenesis, which is
supported by the observation that MMTV-erbB2 mice treated with
DS/Cu did develop tumors. Additionally, these mice may have
developed resistance following constant exposure to low doses of
DS/Cu. Carrying the inactivated neu/c-erbB2 proto-oncogene under
the transcriptional control of the MMTV, the MMTV-erbB2 mice have a
constant tendency to develop tumors following puberty (21). For these reasons, although all
vehicle- and DS/Cu-treated mice had developed tumors by the
conclusion of the experiment (58 weeks), the median 4-week delay
following the 8-week DS treatment indicates that DS exposure may in
part be chemopreventive via the suppression of premalignant
lesions.
To verify this, the effect of DS/Cu was investigated
in normal or malignant mammary cells in vitro and by
measuring mammary gland development in vivo. Since
proliferative and apoptotic promotion are the two mechanisms
involved in tumor delay (28),
alterations in cell proliferation and apoptosis were examined in
the present study. As expected, significant growth inhibition and
elevated apoptosis were observed in mammary gland tissue and breast
cancer cells. Due to the fact that DS/Cu has no apparent effect on
normal human mammary epithelial cells, the results suggested that
DS/Cu may suppress mammary tumor development by the inhibition of
cell proliferation and apoptotic activation of premalignant cells
in the mammary gland. In agreement with this hypothesis, further
experiments identified that mammary glands treated with DS/Cu
displayed fewer premalignant lesions than those treated with the
vehicle.
The NF-κB- and AKT-mediated promotion of apoptosis
and the activation of JNK-mediated inhibition of proliferation have
been previously reported in various studies to be involved in the
biological activity of DS/Cu (11,15,17,18,20,30,31).
Since these signaling pathways have been reported to be regulated
at the level of protein-protein interaction (11,17,18),
the relative protein expression levels but not mRNA levels were
measured in mammary gland tissues in vivo and in normal or
malignant mammary cells in vitro. In agreement with previous
studies, the present study observed that DS/Cu suppressed
proliferation and promoted apoptosis in the breast cancer cells,
which was associated with the reduction of the AKT, cyclin D1 and
NF-κB signaling pathways and the activation of the JNK pathway.
This suggested that DS/Cu may inhibit proliferation via inhibition
of AKT and cyclin D1 signaling and promote apoptosis via JNK
activation and NF-κB signaling suppression.
There are several limitations in the present study:
The dose of DS/Cu administrated was not high enough to exhibit
significant chemopreventive effects. However, since good patient
compliance and minimal side effects are two basic requirements for
cancer chemoprevention strategy (25,26,32,33),
high dose level intervention will likely limit its clinical
applicability. Additional protocols in which DS/Cu is administered
at a variety of dose levels and for various phases are required in
order to achieve an ideal benefit risk ratio in future studies.
However, the present study does suggest that short-term, low-dose
DS/Cu exposure at the premalignant phase may be a novel strategy to
prevent the development of erbB2-positive breast cancer, which
warrants further investigation.
References
|
1
|
DeSantis C, Ma J, Bryan L and Jemal A:
Breast cancer statistics, 2013. CA Cancer J Clin. 64:52–62. 2014.
View Article : Google Scholar
|
|
2
|
Green VL: Breast cancer risk assessment,
prevention and the future. Obstet Gynecol Clin North Am.
40:525–549. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Allen-Petersen BL, Carter CJ, Ohm AM and
Reyland ME: Protein kinase Cdelta is required for ErbB2-driven
mammary gland tumorigenesis and negatively correlates with
prognosis in human breast cancer. Oncogene. 33:1306–1315. 2014.
View Article : Google Scholar
|
|
4
|
Chlebowski RT, Col N, Winer EP, Collyar
DE, Cummings SR, Vogel VG III, Burstein HJ, Eisen A, Lipkus I and
Pfister DG: American Society of clinical oncology technology
assessment of pharmacologic interventions for breast cancer risk
reduction including tamoxifen, raloxifene and aromatase inhibition.
J Clin Oncol. 20:3328–3343. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Visvanathan K, Hurley P and Bantug E: Use
of pharmacologic interventions for breast cancer risk reduction:
american society of clinical oncology clinical practice guideline.
J Clin Oncol. 31:2942–2962. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jordan VC: Tamoxifen as the first targeted
long-term adjuvant therapy for breast cancer. Endocr Relat Cancer.
21:R235–R246. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sukawa Y, Yamamoto H, Nosho K, et al: HER2
expression and PI3K-Akt pathway alterations in gastric cancer.
Digestion. 89:12–17. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chiang CT, Way TD, Tsai SJ and Lin JK:
Diosgenin, a naturally occurring steroid, suppresses fatty acid
synthase expression in HER2-overexpressing breast cancer cells
through modulating Akt, mTOR and JNK phosphorylation. FEBS Lett.
581:5735–5742. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang MH, Man HT, Zhao XD, Dong N and Ma
SL: Estrogen receptor-positive breast cancer molecular signatures
and therapeutic potentials (Review). Biomed Rep. 2:41–52.
2014.PubMed/NCBI
|
|
10
|
Daniel KG, Gupta P, Harbach RH, Guida WC
and Dou QP: Organic copper complexes as a new class of proteasome
inhibitors and apoptosis inducers in human cancer cells. Biochem
Pharmacol. 67:1139–1151. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cen D, Brayton D, Shahandeh B, Meyskens FL
Jr and Farmer PJ: Disulfiram facilitates intracellular Cu uptake
and induces apoptosis in human melanoma cells. J Med Chem.
47:6914–6920. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rizk SL and Sky-Peck HH: Comparison
between concentrations of trace elements in normal and neoplastic
human breast tissue. Cancer Res. 44:5390–5394. 1984.PubMed/NCBI
|
|
13
|
Daniel KG, Harbach RH, Guida WC and Dou
QP: Copper storage diseases: Menkes, Wilsons and cancer. Front
Biosci. 9:2652–2662. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Huang YL, Sheu JY and Lin TH: Association
between oxidative stress and changes of trace elements in patients
with breast cancer. Clin Biochem. 32:131–136. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang H, Chen D, Ringler J, Chen W, Cui
QC, Ethier SP, Dou QP and Wu G: Disulfiram treatment facilitates
phosphoinositide 3-kinase inhibition in human breast cancer cells
in vitro and in vivo. Cancer Res. 70:3996–4004. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jørgensen CH, Pedersen B and Tønnesen H:
The efficacy of disulfiram for the treatment of alcohol use
disorder. Alcohol Clin Exp Res. 35:1749–1758. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang W, McLeod HL and Cassidy J:
Disulfiram-mediated inhibition of NF-kappaB activity enhances
cytotoxicity of 5-fluorouracil in human colorectal cancer cell
lines. Int J Cancer. 104:504–511. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen D, Cui QC, Yang H and Dou QP:
Disulfiram, a clinically used anti-alcoholism drug and
copper-binding agent, induces apoptotic cell death in breast cancer
cultures and xenografts via inhibition of the proteasome activity.
Cancer Res. 66:10425–10433. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Doyon G, Zerbato J, Mellors JW and
Sluis-Cremer N: Disulfiram reactivates latent HIV-1 expression
through depletion of the phosphatase and tensin homolog. AIDS.
27:F7–F11. 2013. View Article : Google Scholar
|
|
20
|
Conticello C, Martinetti D, Adamo L, et
al: Disulfiram, an old drug with new potential therapeutic uses for
human hematological malignancies. Int J Cancer. 131:2197–2203.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ursini-Siegel J, Schade B, Cardiff RD and
Muller WJ: Insights from transgenic mouse models of ERBB2-induced
breast cancer. Nat Rev Cancer. 7:389–397. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li Y, Zhang Y, Hill J, Shen Q, Kim HT, Xu
X, Hilsenbeck SG, Bissonnette RP, Lamph WW and Brown PH: The
Rexinoid LG100268 prevents the development of preinvasive and
invasive estrogen receptor negative tumors in MMTV-erbB2 mice. Clin
Cancer Res. 13:6224–6231. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lee HJ, So JY, DeCastro A, Smolarek A,
Paul S, Maehr H, Uskokovic M and Suh N: Gemini vitamin D analog
suppresses ErbB2-positive mammary tumor growth via inhibition of
ErbB2/AKT/ERK signaling. J Steroid Biochem Mol Biol. 121:408–412.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang GP, Han D, Liu G, Gao SG, Cai XQ,
Duan RH and Feng XS: Effects of soy isoflavone and endogenous
oestrogen on breast cancer in MMTV-erbB2 transgenic mice. J Int Med
Res. 40:2073–2082. 2012. View Article : Google Scholar
|
|
25
|
Wattenberg LW: Chemoprevention of cancer.
Cancer Res. 45:1–8. 1985.PubMed/NCBI
|
|
26
|
Bertram JS, Kolonel LN and Meyskens FL Jr:
Rationale and strategies for chemoprevention of cancer in humans.
Cancer Res. 47:3012–3031. 1987.PubMed/NCBI
|
|
27
|
Santner SJ, Dawson PJ, Tait L, Soule HD,
Eliason J, Mohamed AN, Wolman SR, Heppner GH and Miller FR:
Malignant MCF10CA1 cell lines derived from premalignant human
breast epithelial MCF10AT cells. Breast Cancer Res Treat.
65:101–110. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhu J, Xiong G, Trinkle C and Xu R:
Integrated extracellular matrix signaling in mammary gland
development and breast cancer progression. Histol Histopathol.
2014.
|
|
29
|
Lövborg H, Oberg F, Rickardson L, Gullbo
J, Nygren P and Larsson R: Inhibition of proteasome activity,
nuclear factor-KappaB translocation and cell survival by the
antialcoholism drug disulfiram. Int J Cancer. 118:1577–1580. 2006.
View Article : Google Scholar
|
|
30
|
Li L, Yang H, Chen D, Cui C and Dou QP:
Disulfiram promotes the conversion of carcinogenic cadmium to a
proteasome inhibitor with pro-apoptotic activity in human cancer
cells. Toxicol Appl Pharmacol. 229:206–214. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Brar SS, Grigg C and Wilson KS: Disulfiram
inhibits activating transcription factor/cyclic AMP-responsive
element binding protein and human melanoma growth in a
metal-dependent manner in vitro, in mice and in a patient with
metastatic disease. Mol Cancer Ther. 3:1049–1060. 2004.PubMed/NCBI
|
|
32
|
den Hollander P, Savage MI and Brown PH:
Targeted therapy for breast cancer prevention. Front Oncol.
3:2502013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Brown P: Prevention: targeted
therapy-anastrozole prevents breast cancer. Nat Rev Clin Oncol.
11:127–128. 2014. View Article : Google Scholar : PubMed/NCBI
|