Introduction
Endocrine therapy has facilitated a significant
survival advantage for patients with hormone receptor (HR)-positive
breast cancer, with approximately two-thirds of patients with
HR-positive breast cancer benefiting from endocrine therapies
(1). Aromatase inhibitors (AIs),
for example anastrozole, are currently considered to be the
standard treatment for post-menopausal breast cancer patients with
estrogen receptor (ER)-positive cancer subtypes, and they have been
demonstrated to be more effective than the selective ER modulator
tamoxifen (2). However, over the
past several years, an increasing body of evidence has reported
that drug resistance may still develop with anastrozole treatment
(3–5). Treatment efficacy is limited through
intrinsic and acquired therapeutic resistance (6). To date, studies have shown that ~40%
of primary resistance to endocrine therapy existed in ER-positive
breast cancer and that almost all patients would lose sensitivity
to endocrine drugs during the period of therapy (7). Macedo et al identified
drug-resistance to anastrozole in vivo using mouse xenograft
models of aromatase-overexpressing human ER1 breast cancer cells,
which were in receipt of anastrozole treatment for several weeks.
Resistant tumors exhibited high expression levels of insulin-like
growth factor receptor 1b, mammalian target of rapamycin (mTOR) and
phosphorylated-mTOR, as well as decreased expression of ERa and
aromatase activity (8). The
results of clinical trials conducted on patients with metastatic
breast cancer additionally revealed that even tumors that initially
respond to AI treatment later develop resistance, leading to
disease progression and recurrence. These reasons indicate the
requirement for the development of novel treatments for HR-positive
breast cancer.
A previous study revealed that the AR is expressed
in 60–70% of breast cancers, regardless of ER status (9). Hu et al observed that among
1467 cases of breast cancer, 78.7% were AR positive; and that among
1164 ER-positive cases of breast cancer, 88.0% were AR positive. AR
positivity was associated with a significant reduction in breast
cancer mortality and overall mortality (10). Previous studies have suggested that
the majority (95%) of ER-positive tumors are also AR positive
(11–13). Amongst the ER-negative tumors, AR
reactivity was observed in 10% of triple-negative cases [ER
negative, progesterone receptor (PR) negative and human epidermal
receptor 2 negative]. Certain studies have also demonstrated that
dehydroepiandrosterone (Dhea) has growth inhibitory effects on ER-
and PR-negative breast cancer cell lines with AR expression
(14,15). Morris et al reported that
Dhea was compatible for the treatment of ER-negative and
AR-positive breast cancer when combined with aromatase inhibitors
(16).
Testosterone undecanoate, which is characterized by
high safety and few side-effects, is the only existing oral form of
testosterone replacement therapy, and is one of the most widely
used androgens in clinical therapies (17–19).
To the best of our knowledge, there are few reports evaluating the
effects of androgen treatment for HR-positive breast cancer. The
present study was therefore performed in order to evaluate the
effects of combined treatment with testosterone undecanoate and
anastrozole on HR-positive breast cancer cell proliferation, and to
observe the mechanism of drug action.
Materials and methods
Main experimental materials
Testosterone undecanoate, Dulbecco’s modified
Eagle’s medium (DMEM) and fetal bovine serum (FBS) were purchased
from Sigma-Aldrich (St. Louis, MO, USA) and anastrozole tablets
were bought from AstraZeneca (London, UK). Cell counting kit
(CCK-8; WST-8, cat. no. C0038) was obtained from Dojindo Molecular
Technologies, Inc. (Kumamoto, Japan). A cell apoptosis kit with
Annexin-V (fluorescein isothiocyanate; FITC) and propidium iodide
(PI) for flow cytometry was supplied by Life Technologies (Grand
Island, NY, USA). AR (KGA21105), rabbit anti-human GAPDH monoclonal
(1:1,000) and goat anti-rabbit secondary monoclonal (1:1,000)
antibodies were purchased from Keygen Biotech Co. Ltd (Nanjing,
China).
Drug preparation
The stock solutions of drugs were prepared in 0.1%
dimethyl sulfoxide (DMSO) and stored at room temperature. The stock
solutions of anastrozole and testosterone undecanoate were diluted
in DMEM supplemented with 5% FBS prior to use. The final stock
solution of anastrozole was 1,000 μg/ml and in the in
vitro studies, cells were treated with 0.1 or 0.01
μg/ml. The trial concentrations of testosterone undecanoate
used were 20 and 200 μg/ml. The combinations of anastrozole
and testosterone undecanoate used for the in vitro
investigations were: 0.1 μg/ml anastrozole + 20 or 200
μg/ml testosterone undecanoate and 0.01 μg/ml
anastrozole + 20 or 200 μg/ml testosterone undecanoate.
Cell culture
The MCF-7 [ER+, progesterone receptor (PR)+ and AR+]
breast cancer cells used were obtained from the American Type
Culture Collection (ATCC; Manassas, VA, USA) and cultured according
to the ATCC protocol. The MCF-7 ER+ line was grown in DMEM
supplemented with 10% FBS and cultured in a 5% CO2
enriched atmosphere at 37°C. Images of the cells were captured
using a BX51 microscope (Olympus, Tokyo, Japan). Briefly,
~5×104 MCF7 cells were seeded into 6-well plates in 2 ml
DMEM, containing 10% FBS and 5×103 MCF7 cells were
seeded into 96-well plates in 0.2 ml DMEM, containing 10% FBS. The
cells were cultured in a 5% CO2 enriched atmosphere at
37°C. The cell density was between 60 and 70% confluency on the day
of the experiment and the old media was replaced. Following
treatment with different drugs for 24, 48 and 72 h, the cells in
the 6-well plate were harvested for western blotting and flow
cytometric analysis, and the cells in the 96-well plate were
harvested for CCK-8 assessment.
Cell cytotoxicity assays
Cell cytotoxicity assays on MCF-7 cells following
anastrozole and/or testosterone undecanoate treatment were
performed using the CCK8 assay according to the manufacturer’s
instructions in 96-well plates. Exponentially growing cells were
seeded at a density of 10,000 cells/well and allowed to grow for 24
h. Following removal of the cell culture medium, MCF-7 cells were
incubated for 0, 24, 48 and 72 h in medium containing various
concentrations of anastrozole and testosterone undecanoate.
Subsequently, MCF-7 cells were treated with CCK8 reagent at 37°C
for 1 h and the solution was converted to a quantifiable yellow dye
by mitochondrial dehydrogenases present in viable cells. Absorbency
was measured at 450 nm using a microplate reader (RT-6500; Rayto
Life and Analytical Sciences Co., Ltd, Shenzhen, China).
Flow cytometric analysis
In order to elucidate the effects of the combination
of anastrozole and testosterone undecanoate, MCF-7 cells were
stained with Annexin-V and PI using an Annexin-V/PI staining kit
according to the manufacturer’s instructions to detect the level of
apoptosis. MCF-7 cells were harvested through trypsinization and
washed twice with cold phosphate-buffered saline. The cells were
centrifuged at 1006.2 × g for 5 min and then the supernatant was
discarded and the pellet was resuspended in 1X binding buffer at a
density of 1.0×105−1.0×l06 cells/ml. Sample
solution (100 μl) was transferred to a 5 ml culture tube and
incubated with 5 μl of FITC-conjugated annexin V (BD
Biosciences, San Jose, CA, USA) and 5 μl of PI (BD
Biosciences) for 15 min at room temperature in the dark.
Subsequently, 1X binding buffer (400 μl) was added to each
sample tube and the samples were analyzed using a flow cytometer
(Gallios™; Beckman Coulter, Inc., Pasadena, CA, USA).
Western blot analysis
The four control groups comprised
anastrozole-treated (0.1 and 0.01 μg/ml) and testosterone
undecanoate-treated (20 and 200 μg/ml) cells, the two
experimental groups were the combination-treated cells (0.1
μg/ml + 20 μg/ml and 0.1 μg/ml + 200
μg/ml). Cell lysates were prepared using modified
radioimmunoprecipitation assay buffer (Keygen Biotech Co., Ltd)
containing a tablet of complete protease inhibitors (Keygen Biotech
Co., Ltd). The whole cell lysates were separated by 10% SDS-PAGE
(Life Technologies) and the gels were transferred onto
polyvinylidine difluoride membranes, blocked with 5% skim milk
powder (Life Technologies) and incubated with rabbit anti-human AR
monoclonal antibody (1:1,000; cat. no. 21105; Keygen Biotech Co.,
Ltd., Nanjing, China). The band was visualized with enhanced
chemiluminescent substrate (Life Technologies) following incubation
at 4°C for 12 h with the appropriate horseradish
peroxidase-conjugated secondary antibodies. Images were captured
using an image analyzer (G:BOX chemiXR5; Synoptics Ltd, Cambridge,
UK).
Statistical analysis
The results are presented as the mean ± standard
deviation. Statistical analysis was performed using SPSS 13.0
software (SPSS, Inc., Chicago, IL, USA). The results of the cell
cytotoxicity and western blot assays were analyzed by one-way
analysis of variance to compare data from cell cultures treated
with various drug concentrations and incubation times of
anastrozole and testosterone undecanoate. P<0.05 was considered
to indicate a statistically significant difference between
values.
Results
Combined treatment with anastrozole and
testosterone undecanoate enhances cytotoxity in MCF-7 cells
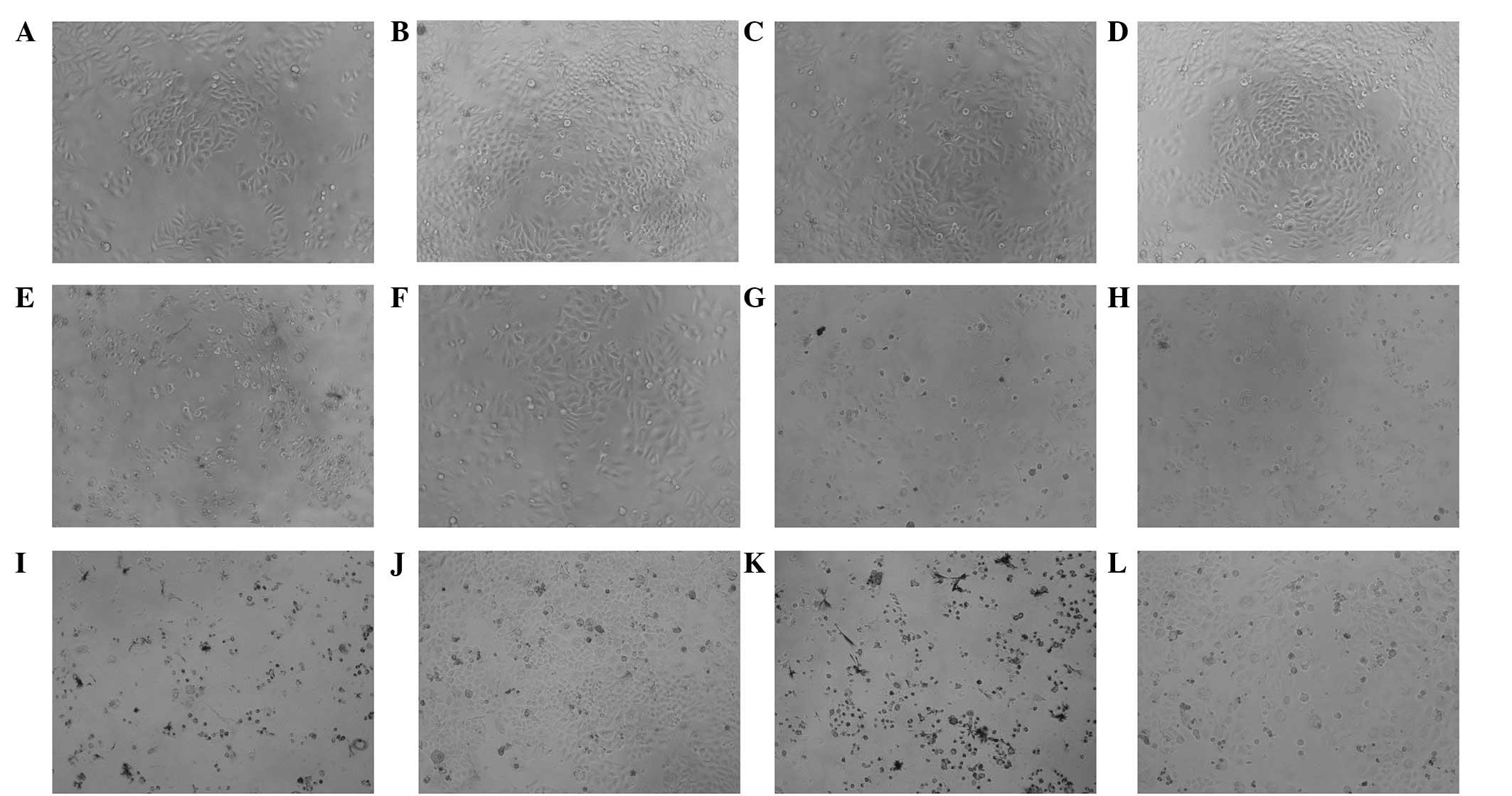
A cytotoxity assay was performed in order to analyze
MCF-7 cell viability following treatments with anastrozole and/or
testosterone undecanoate for 0, 24, 48 and 72 h. The main
cytotoxicities of the drugs included reducing the number of MCF-7
cells and inducing cell lysis (Fig.
1). A microplate reader was subsequently used to measure the
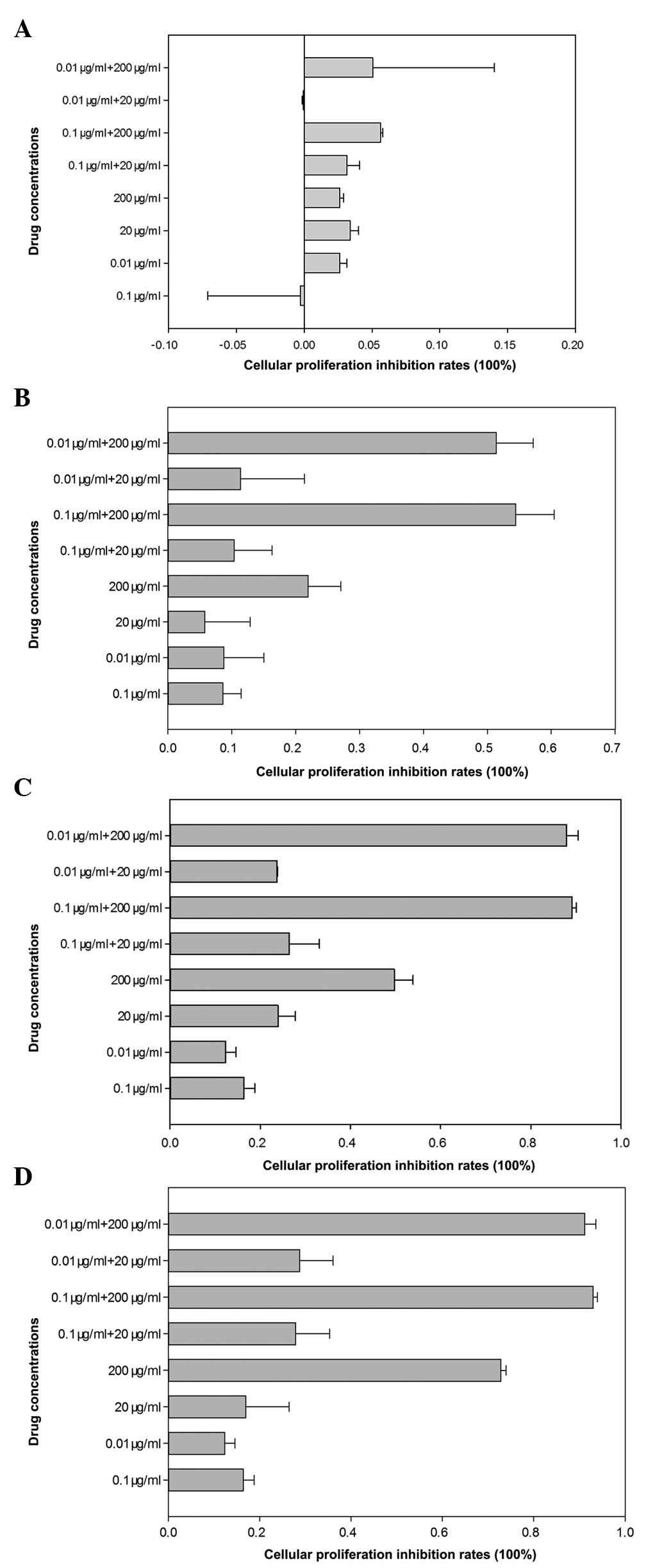
absorbency of the solution in each well (Table 1). The cellular proliferation
inhibition rates following different drugs within 0–24 h were low
and no significant differences among different groups were
identified (Fig. 2A). As indicated
in Fig. 2, the results revealed
that compared with anastrozole-treated (0.1 or 0.01 μg/ml)
cells, the cellular proliferation inhibition rates of
combination-treated (0.1 μg/ml + 200 μg/ml or 0.01
μg/ml + 200 μg/ml) cells were significantly higher
following 24 h of treatment (P<0.05; Fig. 2B). However, there was no
significant decrease in MCF-7 cell viability among other
concentration combinations (0.1 μg/ml + 20 μg/ml and
0.01 μg/ml + 20 μg/ml; P>0.05). Following 48 h of
treatment, the testosterone undecanoate and all drug combination
groups had significantly fewer viable cells than those of the
anastrozole-treated group (P<0.05; Fig. 2C). Following treating for 72 h, the
cellular proliferation inhibition rates in all groups were higher
than at 0–24 h and the results revealed that the drug combination
groups eradicated more cancer cells than the anastrozole-treated
group (Fig. 2D).
 | Table IA450 optical density values of MCF-7
cells following treatment with various drug concentrations at 0,
24, 48 and 72 h. |
Table I
A450 optical density values of MCF-7
cells following treatment with various drug concentrations at 0,
24, 48 and 72 h.
| Treatment | 0 h | 24 h | 48 h | 72 h |
|---|
| Anastrozole |
| 0.1
μg/ml | 0.7003±0.0524 | 0.6766±0.0695 | 1.2504±0.1943 | 1.5202±0.0647 |
| 0.01
μg/ml | 0.6810±0.0655 | 0.6748±0.0725 | 1.3049±0.0570 | 1.4475±0.1843 |
| TU |
| 20
μg/ml | 0.6818±0.7815 | 0.6947±0.0481 | 0.9674±0.1699 | 1.2871±0.1876 |
| 200
μg/ml | 0.6743±0.0266 | 0.5756±0.0361 | 0.7052±0.0860 | 0.1244±0.0132 |
| Combined
(anastrozole + TU) |
| 0.1
μg/ml+20 μg/ml | 0.6445±0.0517 | 0.6621±0.0592 | 1.0259±0.0608 | 0.9764±0.1031 |
| 0.1
μg/ml+200 μg/ml | 0.6765±0.0321 | 0.3345±0.0248 | 0.1525±0.0238 | 0.1221±0.0039 |
| 0.01
μg/ml+20 μg/ml | 0.6986±0.0633 | 0.6548±0.0886 | 1.1009±0.1167 | 1.1073±0.1394 |
| 0.01
μg/ml+200 μg/ml | 0.6639±0.4530 | 0.3591±0.0454 | 0.1726±0.0525 | 0.1485±0.0330 |
| Control group | 0.6989±0.0361 | 0.7390±0.0535 | 1.4018±0.1182 | 1.6516±0.1107 |
Combined treatment enhances apoptosis in
MCF-7 cells
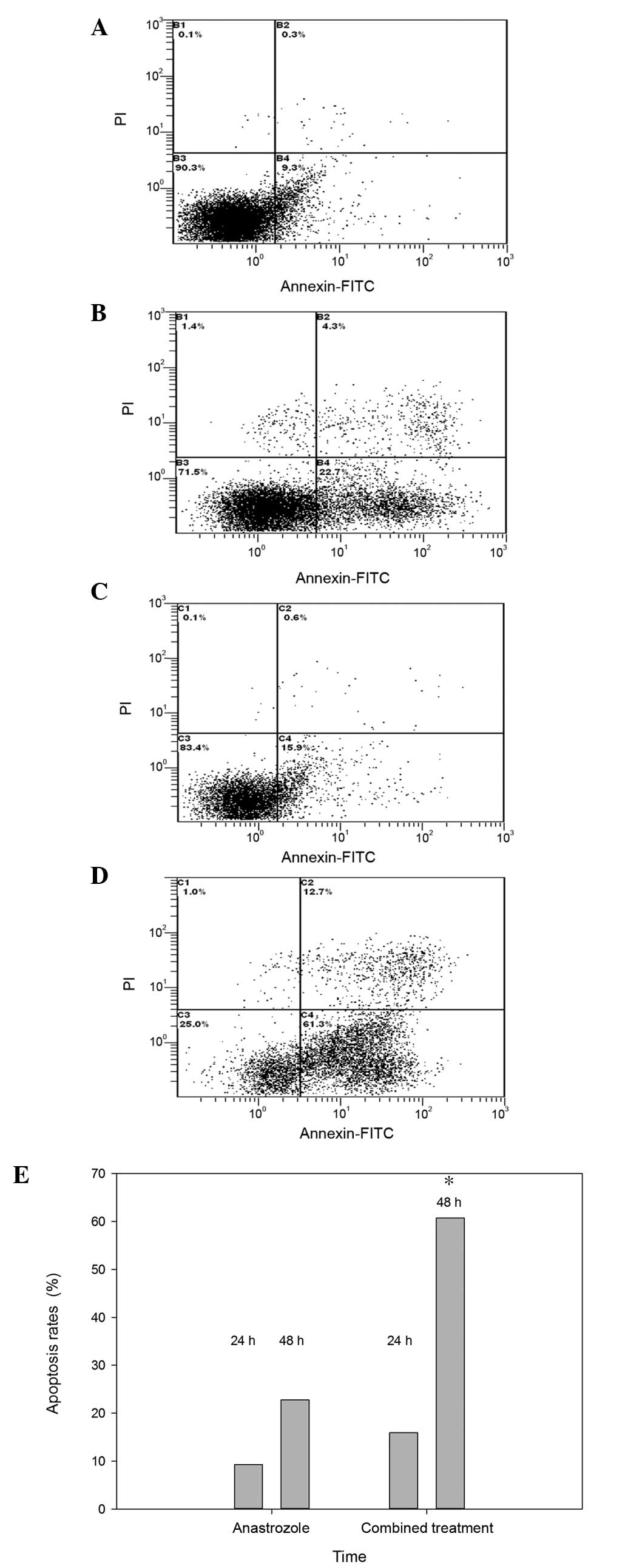
Fig. 3 exhibits
representative flow cytometric scatter plots of MCF-7 cells
double-stained with Annexin V-FITC and PI, revealing the number of
apoptotic cells 24 and 48 h following treatment with anastrozole
(0.1 μg/ml; Fig. 3A and B)
and combined drug treatment (0.1 μg/ml anastrozole + 20
μg/ml testosterone undecanoate; Fig. 3C and D). There were significant
differences in the level of apoptosis between the various
concentrations of drugs at 24 and 48 h. Data analysis revealed that
the average percentage of apoptotic cells was significantly higher
at 48 h in MCF-7 cells treated with the drug combination, compared
with anastrozole treatment alone (60.73±0.81% vs. 22.73±0.35%;
P<0.05; Fig. 3E).
AR protein expression is decreased in the
combined treatment groups
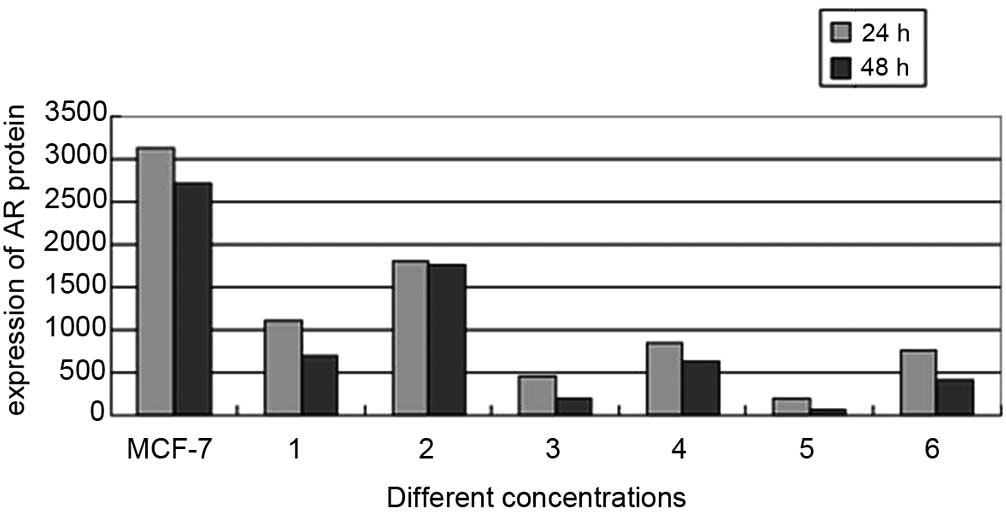
To elucidate whether the AR signaling pathway
functioned via direct interaction with the combined treatment drug
pathway, the expression of AR protein in MCF-7 cells was evaluated
via western blot analysis in the various concentration treatment
groups following 24 and 48 h of incubation. A significant decrease
in AR protein expression was observed in the 0.1 μg/ml + 20
μg/ml and 0.1 μg/ml + 200 μg/ml co-treated
MCF-7 cells, compared with that of the untreated cells (Fig. 4). Quantification of the bands
indicated that the AR protein expression levels were significantly
reduced in the 0.1 μg/ml + 20 μg/ml combined
treatment group, compared with those of the 20 μg/ml
testosterone undecanoate-treated group (755.88±0.95 vs.
854.51±2.17; P<0.05). In addition, the AR expression levels in
the 0.1 μg/ml + 200 μg/ml experimental group were
significantly lower than those is the 200 μg/ml testosterone
undecanoate-treated group (194.35±1.01 vs. 453.74±2.07; P<0.05;
Fig. 5).
Discussion
Breast cancer is one of the most common types of
cancer observed among females. Endocrine therapies targeting
hormone receptors or aromatase have successfully improved the
overall survival and markedly reduced the risk of recurrence of
patients with ER positive breast cancer. Despite such developments,
certain patients with ER-positive or -negative types of breast
cancer still lose sensitivity to endocrine therapy, reducing its
effectiveness. The mechanisms underlying this effect have remained
to be elucidated. To date, studies have revealed that the androgen
signaling pathway may have key functions in normal and malignant
breast tissue (20). ARs are the
most ubiquitously expressed sex-steroid receptors amongst malignant
breast tumors, and are expressed in up to 90% of primary tumors and
75% of metastases (21). Previous
studies have indicated that AR expression is positively correlated
with ERα and PR expression, as well as low proliferative activity
(22–24). Prostate cancer and breast cancer
share similar biological features and common components (25). Previous studies have suggested that
AR may contribute to prostate cancer growth during its recurrence
and that endocrine therapy targeting AR may delay prostate cancer
progression by inhibiting AR activity via androgen ablation and
regulation of signal transduction pathways (26). As a result, AR dysregulation and
its potential therapeutic value have been investigated in this
group of breast neoplasms (27).
Ni et al demonstrated that AR functioned as an
antiproliferative effector in ER-positive breast cancer, but that
it facilitated tumor cell growth in AR-positive and ER-negative
cell line models of breast cancer in an androgen-dependent manner
(28). A retrospective study,
which followed 508 postmenopausal females in South Australia
receiving testosterone treatment in addition to normal hormone
therapy, hypothesized that the inclusion of testosterone with
conventional hormone therapy for postmenopausal females did not
increase, and may reduce, the risk of hormone therapy-associated
breast cancer; therefore, returning the levels of incidence to
those observed in the general untreated population (29). The results of the present study
revealed when MCF-7 ER-positive breast cancer cells were co-treated
with anastrozole and testosterone undecanoate, the
anti-proliferative effects were enhanced and the levels of
apoptosis were more than two-fold greater in cells treated with
anastrozole and various concentrations of testosterone undecanoate,
compared with those of cells receiving anastrozole treatment
alone.
Notably, it was also demonstrated that the AR
signaling pathway was suppressed and the AR protein level was
significantly reduced following anastrozole and testosterone
undecanoate combined treatment, compared with the two drugs alone,
respectively. Previous studies have evaluated the association
between AR expression in breast cancer and the effectiveness of
hormone therapies, including tamoxifen and AI treatments, using
ER-positive breast cancer cell lines. The results indicated that AR
overexpression may induce tamoxifen resistance (30–32);
and therefore, if AR expression influences the activity of
tamoxifen (33), then tamoxifen
should only be used in the treatment of AR-negative subtypes of
breast cancer (13). Further
studies have also demonstrated that the cytotoxic effects of
anastrozole on breast cancer cells could be enhanced by treating
with androgens simultaneously (34–36).
However, based on the results of the present study, various
concentrations of testosterone undecanoate were utilized to reduce
the expression of AR protein, as previously reported (37), and therefore significantly enhance
the cytotoxic effects of anastrozole. Further studies are required
to evaluate these hypotheses and confirm the present findings.
In conclusion, the antiproliferative effects of
anastrozole on MCF-7 human breast cancer cells were significantly
enhanced by combined treatment with testosterone undecanoate, and
the AR signaling pathway may represent a novel target for the
development of breast cancer therapies.
Acknowledgments
The research described in the present study was
supported by the Shenzhen Science and Research Innovation
Foundation (no. JCYJ20130402114702122) and the Shenzhen Science and
Technology Plan Projects (no. 201103010). The authors would like to
thank the Pharmacy Department and Central Laboratory of Peking
University Shenzhen Hospital (Shenzhen, China) for their technical
support.
Abbreviations:
|
HR
|
hormone receptor
|
|
AIs
|
aromatase inhibitors
|
|
ER
|
estrogen receptor
|
|
AR
|
androgen receptor
|
|
Dhea
|
dehydroepiandrosterone
|
|
TU
|
testosterone undecanoate
|
|
FBS
|
fetal bovine serum
|
|
DMSO
|
dimethyl sulfoxide
|
|
ATCC
|
American Type Culture Collection
|
References
|
1
|
Burstein HJ, Prestrud AA, Seidenfeld J, et
al: American Society of Clinical Oncology: American Society of
Clinical Oncology clinical practice guideline: update on adjuvant
endocrine therapy for women with hormone receptor-positive breast
cancer. J Clin Oncol. 28:3784–3796. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Geisler J, King N, Anker G, et al: In vivo
inhibition of aromatization by exemestane, a novel irreversible
aromatase inhibitor, in postmenopausal breast cancer patients. Clin
Cancer Res. 4:2089–2093. 1998.PubMed/NCBI
|
|
3
|
Wong ST and Goodin S: Overcoming drug
resistance in patients with metastatic breast cancer.
Pharmacotherapy. 29:954–965. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Moy I, Lin Z, Rademaker AW, Reierstad S,
Khan SA and Bulun SE: Expression of estrogen-related gene markers
in breast cancer tissue predicts aromatase inhibitor
responsiveness. PLoS One. 8:e775432013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Madeira M, Mattar A, Logullo AF, Soares FA
and Gebrim LH: Estrogen receptor alpha/beta ratio and estrogen
receptor beta as predictors of endocrine therapy responsiveness-a
randomized neoadjuvant trial comparison between anastrozole and
tamoxifen for the treatment of postmenopausal breast cancer. BMC
Cancer. 13:4252013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lønning PE and Eikesdal HP: Aromatase
inhibition 2013: clinical state of the art and questions that
remain to be solved. Endocr Relat Cancer. 20:R183–R201. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Normanno N, Di Maio M, De Maio E, et al:
Mechanisms of endocrine resistance and novel therapeutic strategies
in breast cancer. Endocr Relat Cancer. 12:721–747. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Macedo LF, Sabnis GJ, Goloubeva OG and
Brodie A: Combination of anastrozole with fulvestrant in the
intratumoral aromatase xenograft model. Cancer Res. 68:3516–3522.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Castellano I, Allia E, Accortanzo V, et
al: Androgen receptor expression is a significant prognostic factor
in estrogen receptor positive breast cancers. Breast Cancer Res
Treat. 124:607–617. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hu R, Dawood S, Holmes MD, et al: Androgen
receptor expression and breast cancer survival in postmenopausal
women. Clin Cancer Res. 17:1867–1874. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Agoff SN, Swanson PE, Linden H, Hawes SE
and Lawton TJ: Androgen receptor expression in estrogen
receptor-negative breast cancer Immunohistochemical, clinical, and
prognostic associations. Am J Clin Pathol. 120:725–731. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Moinfar F, Okcu M, Tsybrovskyy O, et al:
Androgen receptors frequently are expressed in breast carcinomas:
potential relevance to new therapeutic strategies. Cancer.
98:703–711. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Niemeier LA, Dabbs DJ, Beriwal S, Striebel
JM and Bhargava R: Androgen receptor in breast cancer: expression
in estrogen receptor-positive tumors and in estrogen
receptor-negative tumors with apocrine differentiation. Mod Pathol.
23:205–212. 2010. View Article : Google Scholar
|
|
14
|
Somboonporn W and Davis SR; National
Health and Medical Research Council: Testosterone effects on the
breast: implications for testosterone therapy for women. Endocr
Rev. 25:374–388. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nahleh Z: Androgen receptor as a target
for the treatment of hormone receptor-negative breast cancer: an
unchartered territory. Future Oncol. 4:15–21. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Morris KT, Toth-Fejel S, Schmidt J,
Fletcher WS and Pommier RF: High dehydroepiandrosterone-sulfate
predicts breast cancer progression during new aromatase inhibitor
therapy and stimulates breast cancer cell growth in tissue culture:
a renewed role for adrenalectomy. Surgery. 130:947–953. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gooren LJ: A ten-year safety study of the
oral androgen testosterone undecanoate. J Androl. 15:212–215.
1994.PubMed/NCBI
|
|
18
|
Schubert M, Minnemann T, Hübler D, et al:
Intramuscular testosterone undecanoate: pharmacokinetic aspects of
a novel testosterone formulation during long-term treatment of men
with hypogonadism. J Clin Endocrinol Metab. 89:5429–5434. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Saad F, Haider A and Gooren L: Effects of
long-term treatment of hypogonadal men with testosterone
undecanoate on blood pressure, fasting glucose, HbA1c and
C-reactive protein. Endocrine Abstracts. 29:3152012.
|
|
20
|
Peters AA, Buchanan G, Ricciardelli C, et
al: Androgen receptor inhibits estrogen receptor-alpha activity and
is prognostic in breast cancer. Cancer Res. 69:6131–6140. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gonzalez LO, Corte MD, Vazquez J, et al:
Androgen receptor expresion in breast cancer: relationship with
clinicopathological characteristics of the tumors, prognosis and
expression of metalloproteases and their inhibitors. BMC Cancer.
8:1492008. View Article : Google Scholar
|
|
22
|
Labrie F, Simard J, de Launoit Y, et al:
Androgens and breast cancer. Cancer Detect Prev. 16:31–38.
1992.PubMed/NCBI
|
|
23
|
Weigelt B, Mackay A, A’hern R, et al:
Breast cancer molecular profiling with single sample predictors: a
retrospective analysis. Lancet Oncol. 11:339–349. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hugh J, Hanson J, Cheang MC, et al: Breast
cancer subtypes and response to docetaxel in node-positive breast
cancer: use of an immunohistochemical definition in the BCIRG 001
trial. J Clin Oncol. 27:1168–1176. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Robinson JL, MacArthur S, Ross-Innes CS,
et al: Androgen receptor driven transcription in molecular apocrine
breast cancer is mediated by FoxA1. EMBO J. 30:3019–3027. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang Q, Li W, Zhang Y, et al: Androgen
receptor regulates a distinct transcription program in
androgen-independent prostate cancer. Cell. 138:245–256. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hu R, Dawood S, Holmes MD, et al: Androgen
receptor expression and breast cancer survival in postmenopausal
women. Clin Cancer Res. 17:1867–1874. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ni M, Chen Y, Lim E, et al: Targeting
androgen receptor in estrogen receptor-negative breast cancer.
Cancer Cell. 20:119–131. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Dimitrakakis C, Jones RA, Liu A and Bondy
CA: Breast cancer incidence in postmenopausal women using
testosterone in addition to usual hormone therapy. Menopause.
11:531–535. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Santoni G and Farfariello V: TRP channels
and cancer: new targets for diagnosis and chemotherapy. Endocr
Metab Immune Disord Drug Targets. 11:54–67. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kuenen-Boumeester V, Van der Kwast TH, van
Putten W, Claassen C, Van Ooijen B and Henzen-Logmans SC:
Immunohistochemical determination of androgen receptors in relation
to oestrogen and progesterone receptors in female breast cancer.
Int J Cancer. 52:581–584. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hickey TE, Robinson JL, Carroll JS and
Tilley WD: Minireview: The androgen receptor in breast tissues:
growth inhibitor, tumorsuppressor, oncogene? Mol Endocrinol.
26:1252–1267. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tokunaga E, Hisamatsu Y, Taketani K, et
al: Differential impact of the expression of the androgen receptor
by age in estrogen receptor-positive breast cancer. Cancer Med.
2:763–773. 2013. View
Article : Google Scholar
|
|
34
|
Doane AS, Danso M, Lal P, Donaton M, Zhang
L, Hudis C and Gerald WL: An estrogen receptor-negative breast
cancer subset characterized by a hormonally regulated
transcriptional program and response to androgen. Oncogene.
25:3994–4008. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yu Q, Niu Y, Liu N, et al: Expression of
androgen receptor in breast cancer and its significance as a
prognostic factor. Ann Oncol. 22:1288–1294. 2011. View Article : Google Scholar
|
|
36
|
Campagnoli C, Pasanisi P, Castellano I,
Abba C, Brucato T and Berrino F: Postmenopausal breast cancer,
androgens, and aromatase inhibitors. Breast Cancer Res Treat.
139:1–11. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Liu CQ, Wu SZ, Wang ZD, Lai WY and Sun F:
Effect of testosterone on expression of androgen receptor in human
monocytic cell line THP-1. Di Yi Jun Yi Da Xue Xue Bao. 24:389–391.
2004.In Chinese. PubMed/NCBI
|